Abstract
Introduction
In South Africa (SA), steroid-resistant nephrotic syndrome (SRNS) is more frequent in black than in Indian children.
Methods
Seeking a genetic basis for this disparity, we enrolled 33 Indian and 31 black children with steroid-sensitive nephrotic syndrome (SSNS) and SRNS from KwaZulu-Natal, SA; SRNS children underwent kidney biopsy. We sequenced NPHS2 and genotyped APOL1 in 15 SSNS and 64 SRNS unrelated patients and 104 controls and replicated results in 18 black patients with steroid-resistant focal segmental glomerulosclerosis (SR-FSGS). Known FSGS genes (n = 21) were sequenced in a subset of patients.
Results
Homozygosity for NPHS2 V260E was found in 8 of 30 black children with SRNS (27%); all 260E/E carriers had SR-FSGS. Combining SR-FSGS patients from the 2 groups, 14 of 42 (33%) were homozygous for V260E. One black control was heterozygous for V260E; no Indian patients or controls were carriers. Haplotype analysis indicated that homozygosity for V260E was not explained by cryptic consanguinity. Children with NPHS2 260E/E developed SRNS at earlier age than noncarriers (34 vs. 78 months, P = 0.01), and none achieved partial or complete remission (0% vs. 47%, P = 0.002). APOL1 variants did not associate with NS. Sequencing FSGS genes identified a CD2AP predicted pathogenic variant in the heterozygous state in 1 Indian case with SR-FSGS.
Conclusion
NPHS2 260E/E was present in one-third of black FSGS patients, was absent in black controls and Indian patients, and affected patients were unresponsive to therapy. Genotyping V260E in black children from South Africa with NS will identify a substantial group with SR-FSGS, potentially sparing these children biopsy and ineffective steroid treatment.
Keywords: APOL1, CD2AP, FSGS, nephrotic syndrome, NPHS2, steroid resistance
Nephrotic syndrome (NS), the major cause kidney failure in children, is the consequence of damage to the glomerular filtration barrier, leading to proteinuria, hypoalbuminemia, hyperlipidemia, and edema. NS is classified by its response to steroid therapy as SSNS or SRNS.1 Although most children with idiopathic NS respond to glucocorticoids and have a favorable prognosis, approximately 20% are steroid resistant, which is associated with hospital admissions, treatment with toxic immunosuppressive drugs, and often progressive loss of kidney function.2 Among SA children with primary NS, the most common histological diagnosis in black children is FSGS, whereas minimal change disease is more frequently diagnosed in white and Indian children.3, 4, 5 SA black children with SRNS are less likely to achieve complete remission with oral cyclophosphamide treatment than Indian children (20% vs. 69%).6, 7 The basis for these disparities has not been explained, but has been hypothesized to be due in part to genetic differences predisposing to SR-FSGS.8
Among mutations causing monogenic NS in families, variants in NPHS2 are the most frequent cause of SRNS.9, 10, 11, 12 In a global study of 430 families with NS, 18% were homozygous or compound heterozygous for deleterious NPHS2 mutations; of these, the most common mutation was the European founder R138Q mutation, found in 7% of families.13 A study of 1783 families found that monogenic causes of SRNS were highest in those with early age of onset and among geographic regions with high rates of consanguinity. Mutations in NPHS2 were the most frequent cause of SRNS in children aged 1 to 18 (6% to 13% for different age groups).14 Among families with SRNS and congenital NS enrolled in the PodoNet registry cohort, recessive mutations in NPHS2 were identified 138 of 1088 children (12.6%) undergoing targeted mutational analysis.15 In other targeted screening studies, monogenic causes of nonfamilial FSGS were identified in only 6% of pediatric FSGS patients and rarely in African American children.16, 17
Coding variants in APOL1, encoding apolipoprotein L1, are recessively associated with FSGS and a spectrum of chronic kidney disease in African Americans.18, 19, 20 In SA adults, APOL1 variants are strongly associated with HIV-asssociated nephropathy, but not with FSGS.21 Among African American children with CKD, APOL1 high-risk status was associated with glomerular disease and with more rapid decline in estimated glomerular filtration rate,22 and among African American children with FSGS, 78% carried high-risk APOL1 genotypes.23 The association of APOL1 with NS in children in sub-Saharan Africa has not been investigated.
The genetic basis for the racial disparity in SRNS and FSGS histology for black children with NS from SA is unknown, and no genetic studies have been reported from sub-Saharan Africa. Considering the increased risk of black children for SRNS in SA, we hypothesized that APOL1 renal risk variants, which are found only on African ancestry haplotypes, or founder mutations in NPHS2, might be responsible for the higher rate of SR in black children compared to Indian or white SA children with NS.
Methods
Subjects
Unrelated Indian and black children (N = 64) with sporadic SRNS (n = 49) or SSNS (n = 15) treated between January 2005 and December 2011 at 2 referral hospitals in Durban, KwaZulu Province, SA, were invited to participate in the study. The majority of black Africans in KwaZulu-Natal Province identify as Zulu (>95%). All children with primary NS were given oral prednisone (2 mg/kg, maximum 60 mg) for 6 weeks, followed by the same dose on alternate days for another 6 weeks, and reduced to none over 2.5 months. Failure to respond to oral steroids after 6 weeks was taken as SR in accordance with standard criteria.2, 24 Second-line treatment included oral cyclophosphamide (2 mg/kg with a maximum dose of 100 mg daily) given as a daily dose for 8 to 12 weeks with an angiotensin-converting enzyme inhibitor (0.5 mg/kg, maximum 5 mg) given daily, and oral prednisone (1 mg/kg, maximum 60 mg) given on alternate days. Children with SRNS who did not respond to oral cyclophosphamide plus low-dose oral prednisone received intensive treatment with i.v. methylprednisolone (n = 11) or i.v. cyclophosphamide (n = 7) or both methylprednisolone and i.v. cyclophosphamide (n = 2) or tacrolimus (n = 14), or both i.v. cyclophosphamide plus tacrolimus (n = 2) together with low-dose prednisone on alternate days. Only children not responding to oral steroids underwent kidney biopsy. Kidney biopsies were interpreted using light microscopy, immunofluorescence, and electron microscopy. Children with primary SSNS or SRNS and with an estimated glomerular filtration rate >60 ml/min per 1.73 m2 using the modified Schwartz formula25 were eligible for entry into the study. Children with estimated glomerular filtration rate <60 ml/min per 1.73 m2 at presentation were excluded because they were not given immunosuppression therapy. Other exclusion criteria included those for whom histology was indeterminate or who were lost to follow-up or refused to participate in the study. The following tests were performed to exclude secondary forms of NS: antistreptolysin O titer (ASOT), hepatitis B and C screen, blood culture, Widal tests for enteric fever, Wasserman reaction for syphilis, antinuclear factor, and testing for Epstein-Barr virus, HIV, parvovirus B19, and cytomegalovirus. Black (n = 55) and Indian (n = 49) healthy blood donors were enrolled as control groups. Proteinuric remission was defined as a protein to creatinine ratio (PCR) <0.2 mg/mg and serum albumin >30g/dl; partial remission was defined as a PCR<1.9 mg/mg and serum albumin >25g/dl, but not meeting full remission criteria.
For replication of NPHS2 results, we enrolled an additional 18 black patients with biopsy-proven sporadic SR-FSGS, and 18 age-matched black controls, with normal kidney function and no proteinuria.
Written informed consent was obtained from the parents of children, and children over 7 years provided assent. The Biomedical Research Ethics Committee of the University of KwaZulu-Natal, SA, approved this study.
DNA Sequencing and Genotyping
DNA sequencing and genotyping methods are described in the Supplementary Methods. Briefly, APOL1 single-nucleotide polymorphisms (SNPs) defining G1 (rs73885319 and rs60910145) and G2 (rs717185313) were genotyped by ABI Custom TaqMan SNP Genotyping Assays (ABI, Foster City, CA). NPHS2 exons 1 through 8 were Sanger sequenced using the ABI 3700 Analyzer under standard conditions.9 Targeted amplification of 21 nephrotic syndrome genes followed by next-generation sequencing was performed in 21 NS patients (not carrying the V260E mutation) and 19 controls to identify additional causal variants as described in the Supplementary Methods and in Sampson et al. and Crawford et al.16, 26 To determine relatedness and the age of the NPHS2 V260E mutation, DNA from 9 of 14 V260E homozygous patients (those with remaining DNA) and 71 black patients and controls who did not carry the mutation were genotyped using the Human Exome Chip (HumanExome-12 v1.2, Illumina, San Diego, CA).
Statistical and Bioinformatics Methods
The Fisher exact test was used for categorical tests for APOL1 and NPHS2 variants, including aggregation tests comparing the number of singleton NPHS2 missense variants in patients and controls.27 We compared the age distributions between Indian and black SRNS patients, and between black SRNS patients with and without V260E homozygosity, with the Mann−Whitney test. All statistical tests and simulations were done in R (http://www/R-project.org).
We determined relatedness among NPHS2 V260E homozygotes by estimating the age of the most recent common ancestor for the V260E mutation among subjects using coalescence (Supplementary Methods).28 Briefly, we determined the length of homozygosity of chr 1 SNPs typed on the Illumina Exome chip 12 v1.2, by plotting heterozygous and homozygous SNPs for each subject in the region around NPHS2. We compared the observed lengths of homozygosity with simulation results for different numbers of generations since the common ancestor using the Mann−Whitney test. We tested for consanguinity among V260E homozygotes by the coefficient of relatedness test implemented in PLINK.29
Results
A total of 64 unrelated children with sporadic idiopathic NS were enrolled between 2005 and 2011 (Table 1) from 2 tertiary hospitals in Durban, KwaZulu-Natal Province, SA. Mothers of the affected children reported no affected siblings and no family history of kidney disease. SRNS was much more frequent among black children with NS; 97% of black (all but 1 child) versus 58% of Indian children were SR (odds ratio = 21; 95% confidence interval = 2.8−960; P = 0.0002). For children with SRNS, the median age of presentation was similar for Indian (86 months) and black (83 months) children. Among 49 children with SRNS undergoing biopsy, FSGS was the most common histopathology in both black (80%) and Indian (74%) children. Children responding to oral steroids did not undergo biopsy.
Table 1.
Demographic and clinical characteristics of children with nephrotic syndrome in the discovery and replication cohorts
| Disease and patient groups | Discovery |
Replication |
||
|---|---|---|---|---|
| Indian NS patients n = 33 | Black NS patients n = 31 | Statistics: black versus Indian | Black SR-FSGS patients n = 18 | |
| SSNS | 14 (42.4%) | 1 (3.2) | OR = 21 95% CI = 2.8−960 P = 0.0002 |
0 |
| SSRS | 19 (57.6%) | 30 (96.8%) | 18 | |
| Age range, mo | 37−169 | 24−168 | P = 0.34 | 26−165 |
| Median age, mo | 86 | 83 | 93 | |
| Males | 13 (31.6%) | 13 (43.3%) | OR = 2.8 95% CI = 0.7−11.5 P = 0.14 |
11 (62%) |
| Females | 6 (68.4%) | 17 (56.7%) | 7 (38%) | |
| Histology of SRNS | ||||
| FSGS | 14 (73.7%) | 24 (80.0%) | OR = 1.4 95% CI = 0.3−6.8 P = 0.7 |
18 (100%) |
| Interstitial nephritis | 1 (5.3%) | 2 (6.7%) | ND | 0 |
| Mesangioproliferative glomerulonephritis | 1 (5.3%) | 2 (6.7%) | ND | 0 |
| Mesangial sclerosis | 0 | 1 (3.3%) | ND | 0 |
| Proliferative glomerulonephritis | 0 | 1 (3.3%) | ND | 0 |
| Glomerulonephritis | 2 (10.5%) | 0 | ND | 0 |
| Minimal change disease | 1 (5.3%) | 0 | ND | 0 |
CI, confidence interval; FSGS, focal segmental glomerulosclerosis; ND, not determined; NS, nephrotic syndrome; OR, odds ratio; SR-FSGS, steroid-resistant focal segmental glomerulosclerosis; SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome.
Data are numbers (percentages) of patients with SSNS and SRNS. Black children were much more likely than South Asian Indian children to have SRNS.
As previous studies identified NPHS2 autosomal recessive mutations as the most frequent Mendelian cause of SRNS in children with both familial and sporadic disease, we Sanger sequenced all NPHS2 exons in SSNS and SRNS patients and controls. Supplementary Table S1 lists the NPHS2 variants identified in Indian and black patients and controls. We observed 7 missense variants, namely P20L, G42R, A61V, R229Q, A242V, and V260E, all of which have been previously reported, and a novel variant, P369S, which has not been reported in the 1000 Genomes or gnomAD databases and is predicted to be benign. As A242V has been observed with minor allele frequency of >5% in African populations (Supplementary Table S1), this mutation is unlikely to have severe phenotypic consequences.
Notably, NPHS2 V260E was present in the homozygous state in 8 of 30 (27%) black SRNS patients. V260E homozygosity was specifically associated with biopsy-confirmed FSGS (Table 2), accounting for 33% of black children (8 of 24) with SR-FSGS, but none of the Indian children. One black healthy control was heterozygous for NPHS2 V260E; no black SRNS case was heterozygous for NPHS2 V260E, and the variant was not observed in any of the Indian NS patients or controls (Table 2, Table 3). NPHS2 V260E (Hg19 coordinate,1:179523626 A/T) is a known recessive pathogenic mutation previously observed in several consanguineous families from regions associated with the former Omani Empire.11, 30 The NPHS2 V260E mutation disrupts podocin trafficking to the podocyte plasma membrane by producing a protein that is retained in the endoplasmic reticulum.31
Table 2.
NPHS2 V260E in 64 black and Indian SSNS and SRNS patients in the discovery cohort
| NPHS2 genotype | Black patients n = 31 |
Indian patients n = 33 |
||
|---|---|---|---|---|
| 260V/V n = 23 | 260E/E n = 8 | 260V/V n = 33 | 260E/E n = 0 | |
| SSNS | 1 | 0 | 14 | 0 |
| SRNS | 22 | 8 | 19 | 0 |
| FSGS | 16 (73%) | 8 (100%) | 14 (74%) | 0 |
| Other histologies | 6 (27%) | 0 | 5 (26%) | 0 |
FSGS, focal segmental glomerulosclerosis; SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome.
Only children who showed no remission in proteinuria after a 4-week course of glucocorticoid treatment underwent renal biopsy. Data are the number of NPHS2 V260E genotypes in the nephrotic syndrome discovery cohort. No steroid-sensitive case carried the NPHS2 260E mutation, and the mutation was not observed in Indian patients. NPHS2 V260E homozygosity was specifically associated with FSGS histology.
Table 3.
Association of NPHS2 V260E restricted to the subset of black children with SR-FSGS
| Discovery cohort | |||
|---|---|---|---|
| NPHS2 genotype | Controls | SR-FSGS | OR (95% CI), PFET |
| p. 260 V/V | 54 (98%) | 16 (67%) | Reference |
| p. 260 E/V | 1 (2%) | 0 | Not significant |
| p. 260 E/E | 0 | 8 (33%) | ∞ (4.9−∞) 3 × 10−5 |
| Replication cohort | |||
| p. 260 V/V | 18 (100%) | 12 (67%) | Reference |
| p. 260 V/E | 0 | 0 | − |
| p. 260 E/E | 0 | 6 (33%) | ∞ (1.4−∞) 0.02 |
| Combined cohorts (n=42) | |||
| p. 260 V/V | 72 (99%) | 28 (67%) | Reference |
| p. 260 V/E | 1 (0.7%) | 0 | Not significant |
| p. 260 E/E | 0 | 14 (33%) | ∞ (7.7−∞) 2 × 10−7 |
CI, confidence interval; FET, Fisher exact test; OR, odds ratio; SR-FSGS, steroid-resistant focal segmental glomerulosclerosis.
NPHS2 genotypes are summarized for individuals with SR-FSGS and controls. NPHS2 sequencing results were available for 42 FSGS patients and 73 controls. NPHS2 260 E/E were compared to NPHS2 260V/V.
Several of the other NPHS2 mutations observed are predicted to be pathogenic (Supplementary Table S1) and have been associated with FSGS, but only in the homozygous or compound heterozygous state. In particular, NPHS2 R229Q is a relatively frequent pathogenic polymorphism for FSGS, but causes FSGS only with certain other NPHS2 pathogenic mutations in trans configuration.32 However, outside of the children homozygous for NPHS2 V260E, no black or Indian SSNS or SRNS patients carried more than 1 missense variant. NPHS2 mutations in the heterozygous state have not been associated with childhood FSGS, and gene burden tests for these variants did not reveal an excess of variants in SSNS or SRNS patients versus controls (P > 0.5).
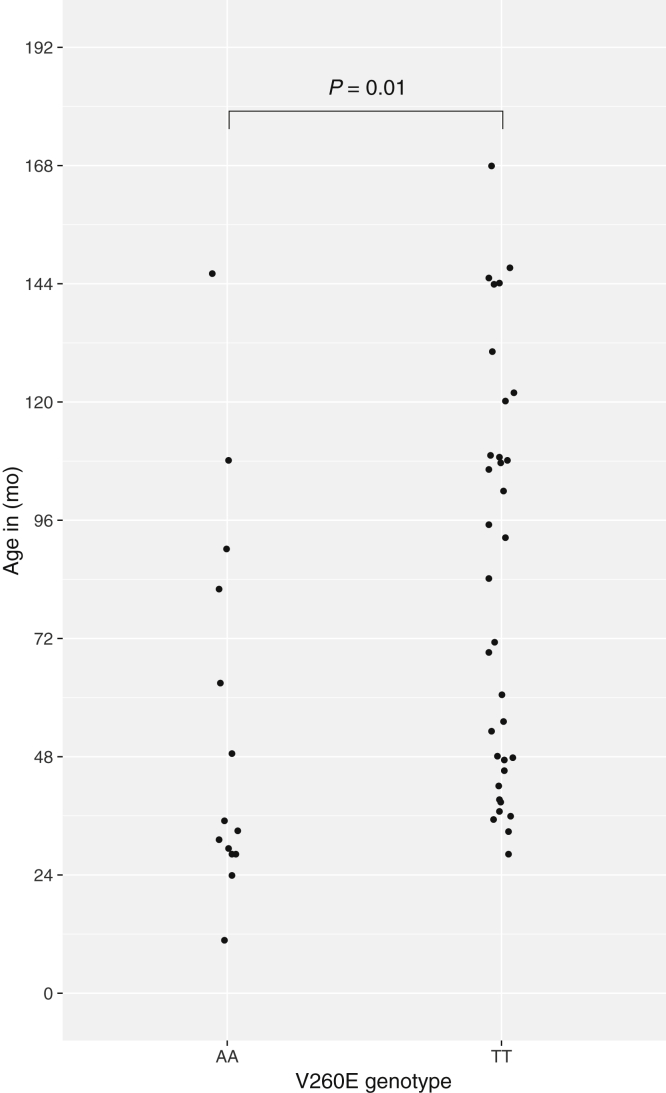
To replicate the 260E/E association with SR-FSGS, we sequenced DNA from a second group of 18 unrelated black children with SR-FSGS and 18 race- and age-matched controls. Of the 18 children with SR-FSGS, 6 (33%) were homozygous for V260E, whereas none of the controls in this cohort carried the mutation. Combining the discovery and replication SR-FSGS patients, V260E homozygosity accounted for 14 of 42 (33%) black children with SR-FSGS (P < 10−6) (Table 3). Black SR-FSGS patients homozygous for V260E had an earlier age of onset compared to SRNS patients homozygous for the V260 reference allele (median onset age, 34 months vs. 78 months, P = 0.01) (Figure 1, Supplementary Figure S1). One of 73 black controls was heterozygous for V260E (nominal allele frequency = 0.7%; 95% binomial confidence interval = 0.02%−4%) (Table 3). The ExAC Browser (exac.broadinstitute.org) reports a population frequency of 0.02% (2 of 10,384) in Africans and 0 in Asians, Europeans, Latinos, and South Asians.
Figure 1.
Comparison of distributions of ages of onset of steroid-resistant nephrotic syndrome (SRNS) between carriers of 2 copies of the reference allele, 260V (n = 34), and carriers of 2 copies of the mutant allele, 260E (n = 14) at NPHS2 V260E. The P value was calculated using the Mann–Whitney test. There were no heterozygotes for this locus in the SRNS group. Data are from the combined discovery and replication cohorts.
Response to Therapy
We assessed response to therapy by urinary PCR and serum albumin levels in black children with SRNS. No subjects homozygous for V260 E (n = 14) had either complete or partial remission in response to additional immunosuppressive therapy (Table 4). In contrast, among 32 black NS subjects homozygous for the V260 reference allele, 9 had complete remission, 6 had partial remission, and 17 had no treatment response. Six children carrying 260E/E were further treated with tacrolimus (n = 3) or i.v. cyclophosphamide (n = 3), but none showed complete or partial remission. The differences in treatment response between NPHS2 260 EE and 260VV were statistically significant for complete remission (P = 0.04) and combined complete and partial remission (P = 0.002). Five of the 14 children (35%) homozygous for NPHS2 V260E progressed to end-stage kidney disease (estimated glomerular filtration rate <15 ml/min per 1.73 m2), with times from NS diagnosis of between 38 and 61 months, whereas 2 of 28 children (7%) not carrying the variant progressed to end-stage kidney disease, at 44 and 51 months.
Table 4.
Response to therapy by NPHS2 genotype for black SRNS and SSNS patients
| NPHS2 genotype | Treatment response |
Any remission n (%) | ||
|---|---|---|---|---|
| No response N (%) | Partial remission n (%) | Full remission n (%) | ||
| p. 260 VV | 17 (53) | 6 (19) | 9 (28) | 15 (47) |
| p. 260 EE | 14 (100) | 0 | 0 | 0 |
| P value | 0.16 | 0.04 | 0.002 | |
SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome.
In contrast to patients with NPHS2 260 V/V, patients with NPHS2 E/E showed no response to glucorticoid or second-line therapies. Complete remission was defined as urinary protein to creatinine ratio <0.2 g/g and serum albumin >30 g/dl. Partial remission was defined as not meeting the criteria for full remission, with protein to creatinine ratio <1.9 g/g and serum albumin >25 g/dl. Three subjects had insufficient clinical data to determine remission status.
Founder Effect and Age of Variant
We tested for identity by descent by further genotyping 9 patients homozygous for NPHS2 V260E (all that had sufficient DNA) and 71 patients and controls lacking the mutation. We used the Illumina Exome array; this provides good coverage of the region (1674 SNPs within 10 Mb of NPHS2). Inbreeding coefficient tests in PLINK showed no evidence of consanguinity among the 9 children.29 To test for identity by descent from a more distant founder we determined the length of homozygosity surrounding NPHS2 (Supplementary Figures S2 and S3). Two of the 9 V260E homozygous children had runs of homozygosity around NPHS2 of 13 and 14 Mb, whereas the remaining 7 had runs of homozygosity ranging from 1.9 to 3.6 Mb. The longer runs likely result from more recent common ancestors of the respective parents. We took the shorter runs to be indicative of time to the overall common ancestor, and by comparing these lengths to results of a coalescent simulation, we arrived at an estimate of 31 generations as the most likely distance to a common ancestor (95% confidence interval = 19−47).
Search for Additional Genetic Associations
We performed targeted amplification paired with next-generation sequencing of 21 genes implicated in monogenic NS or proteinuria in 21 patients and 19 controls lacking NPHS2 260E/E, as previously described.16 A predicted pathogenic variant, CD2AP K346N, in a known FSGS gene, was observed in the heterozygote state in an Indian child diagnosed with SR-FSGS at 7.5 years (Supplementary Table S2).33, 34
We also tested for association between APOL1 and NS. No Indian patients or controls carried either the G1 or G2 variant. Among the black controls, the allele frequencies for the G1 and G2 alleles were 8.9% and 10.1%, respectively. The APOL1 association with kidney disease is largely recessive, with carriage of 2 risk alleles required for APOL1-associated FSGS.18, 19 When we limited analysis to the subset of black individuals not carrying 2 copies of NPHS2 V260E, 2 of 73 controls and 1 of 28 FSGS patients carried 2 APOL1 risk alleles (odds ratio = 1.3, 95% confidence interval = 0.02−26.2, P = 1, Fisher exact test) (Supplementary Table S3). Two black FSGS patients homozygous for NPHS2 V260E also carried 2 APOL1 risk alleles.
Discussion
We found that 1 autosomal recessive mutation, NPHS2 V260E, accounted for 33% of SR-FSGS among unrelated black children with NS. This was an unexpected result, as published data suggest that monogenic mutations are infrequently detected in children with nonfamilial SRNS.16, 17, 35 Children homozygous for NPHS2 V260E were steroid resistant with FSGS, did not respond to intensive treatment, and developed disease at a younger age compared to noncarriers, consistent with the severity of disease previously reported for the NPHS2 V260E pathogenic mutation.11 NPHS2 V260E, like the European founder mutation NPHS2 R138Q, disrupts the trafficking of the altered podocin protein to the plasma membrane by its retention in the endoplasmic reticulum. Podocin localization is essential for recruitment of nephrin to podocyte lipid rafts and for the structural integrity of the podocyte slit diaphragm.31, 36
To elucidate the history of this mutation and to determine whether consanguinity accounted for the high rate of V260E homozygosity, we genotyped 9 subjects homozygous for V260E, and 71 black patients and controls without the mutation, with a genome-wide array. Overall, parents of children carrying the V260E mutation showed no sign of recent consanguinity, but rather the length of segments homozygous due to identity by descent around NPHS2 showed evidence of descent from a common ancestor estimated to be 31 generations removed from the present. This suggests that V260E may be more widely dispersed among the Zulu population, comprising the largest ethnic group in SA.
Previous observations of SRNS caused by NPHS2 V260E have been in consanguineous families from regions in and around the Indian Ocean, notably in regions associated with the Omani Empire of the late 17th to 19th centuries, prompting the hypothesis that the mutation spread with travel and migrations associated with this empire.37 The Omani Empire was a power on the East African coast from the 1690s to the mid 19th century, and in the early 19th century traded extensively with the African Great Lakes region, where Bantu ancestors of the Zulu population lived.38 However, the age of the mutation in our population suggests an appearance prior to this time and makes it more likely that it was introduced to the Omani Empire by Africans. It remains to be determined whether NPHS2 V260E in our population is related by descent to previously observed V260E or is an independent mutation.
APOL1 was not significantly associated with increased risk for FSGS in black SA children, in agreement with our previous finding that APOL1 did not associate with FSGS in SA adults; however, the prevalence of APOL1 high-risk genotypes is much lower in southern Africa (∼3%) compared to either western Africa (∼25%) or in African Americans (∼13%).21 We did, however, have 96% power to detect significant associations for odds ratio >4.5 for the recessive model, suggesting that if APOL1 HR status is associated with pediatric FSGS in this population, its penetrance is much less than we see in children or adult African Americans with FSGS.18, 19, 23
To identify additional variants associated with nephrotic syndrome, we performed targeted sequencing of 21 genes implicated in FSGS or proteinuria in 21 patients and 19 controls, which revealed 1 predicted pathogenic variant of unknown significance.16 An Indian child with SR-FSGS was heterozygote for CD2AP K346N; haploinsufficiency of CD2AP has been implicated in early and late onset of FSGS.39, 40, 41 Unfortunately, the family was not available to assess causality by segregation analysis.
The results for the studied population, black Africans of the Zulu ethnic group of the KwaZulu-Natal Province, may not be generalizable to other black ethnic groups. However, the frequency of the association in our study and the age of the mutation suggest that the mutation may be present in the broader Zulu population, and possibly in other Bantu populations that share ancestry with the Zulu. This study reaffirms the importance of sequencing diverse, endogamous populations to detect disease-causing variants that may account for population-specific disparities in disease prevalence, presentation, or treatment response.
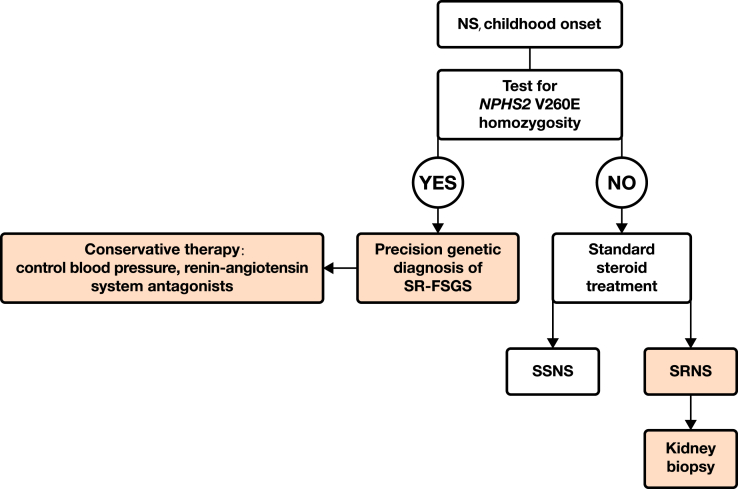
Screening for the NPHS2 V260E mutation has the potential to inform the differential diagnosis, prognosis, and treatment in black African children presenting with NS. Identification of this mutation as a part of differential diagnosis would be a cost-effective alternative to kidney biopsy in homozygous carriers, and would identify a large subset of patients who are unresponsive to immunosuppressive agents (specifically, oral glucocortiocoid treatment, cyclophosphamide, and calcineurin inhibitors). This would spare children with NPHS2 V260E homozygosity the adverse effects of toxic agents while at the same time reducing health care costs for an overburdened health care system (Figure 2). In view of the lack of response to immunosuppressive treatment in patients with V260E homozygosity, the focus of treatment for this group of patients should shift to other nonimmunosuppressive agents, such as maximal renoprotection using angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers.42 With such a large burden of homozygous NPHS2 V260E SRNS patients, sufficiently powered clinical trials may be possible to determine optimal treatment and, particularly, responsiveness to treatment with nonglucocorticoid immunosuppressive agents.
Figure 2.
Proposed targeted genetic approach for black African children presenting with nephrotic syndrome to identify those who could be managed conservatively and those who might benefit from a course of glucocorticoid therapy. NS, nephrotic syndrome; SR-FSGS, steroid-resistant focal segmental glomerulosclerosis; SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome.
In summary, the high frequency of NPHS2 V260E homozygosity makes genetic testing for this mutation in black African children presenting with NS a potential application of precision medicine; for individuals carrying 2 copies of NPHS2 V260E, a targeted genetic test may replace an invasive kidney biopsy requiring hospitalization, and an unhelpful and potentially toxic course of treatment. The applicability of this approach may extend beyond the more than 12 million Zulu individuals living in southern Africa, as the variant is likely to be present in other Bantu populations sharing ancestry with Zulu.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank the families and children for their participation in this study. We thank Elizabeth Binns-Roemer for excellent technical assistance. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health (NIH), under contract HHSN26120080001E and the Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, NIH. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Supplementary Methods. Sequencing and genotyping and bioinformatics.
Figure S1. Kaplan−Meier plot of age of onset of SRNS for subjects homozygous for the variant or for the wild-type allele for NPHS2 V260E. The Kaplan−Meier log-rank statistic was calculated by the r function survdiff.
Figure S2. Heterozygous and homozygous loci around NPHS2 V260E from genotyping by the Illumina Exome V2.1 chip, for 9 individuals carrying p.260 E/E (homozygous for the variant allele). The region shown is 10 mB before and after V260E; x axis coordinates are base positions relative to this locus. Top dots represent homozygous loci, bottom heterozygous; the region lacking heterozygous loci indicates the region where the subjects’ 2 parental chromosomes carry identical haplotypes.
Figure S3. Heterozygous and homozygous loci around NPHS2 V260E from genotyping by the Illumina Exome V2.1 chip, for 40 individuals (representative of 71 total) carrying p.260 V/V (homozygous for the wild-type allele), for comparison with Supplementary Figure S1. In contrast to the individuals homozygous for the V260E mutant allele, for most individuals here there is no visible extent of homozygosity around the locus.
Figure S4. Histograms of the extent of homozygosity around NPHS2 V260E (A) for individuals homozygous for the mutant allele and (B) for individuals homozygous for the wild-type allele, as shown in Supplementary Figures S2 and S3.
Table S1. NPHS2 variants detected in Indian and black African children with nephrotic syndrome and controls.
Table S2. Pathogenic variants identified in 21 nephrotic syndrome patients, lacking NPHS2 V260E, and 19 controls, detected by next-generation sequencing 21 monogenic nephrotic syndrome genes.
Table S3. APOL1 risk alleles in SR-FSGS patients and controls.
Supplementary References.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Sequencing and genotyping and bioinformatics.
Kaplan−Meier plot of age of onset of SRNS for subjects homozygous for the variant or for the wild-type allele for NPHS2 V260E. The Kaplan−Meier log-rank statistic was calculated by the r function survdiff.
Heterozygous and homozygous loci around NPHS2 V260E from genotyping by the Illumina Exome V2.1 chip, for 9 individuals carrying p.260 E/E (homozygous for the variant allele). The region shown is 10 mB before and after V260E; x axis coordinates are base positions relative to this locus. Top dots represent homozygous loci, bottom heterozygous; the region lacking heterozygous loci indicates the region where the subjects’ 2 parental chromosomes carry identical haplotypes.
Heterozygous and homozygous loci around NPHS2 V260E from genotyping by the Illumina Exome V2.1 chip, for 40 individuals (representative of 71 total) carrying p.260 V/V (homozygous for the wild-type allele), for comparison with Supplementary Figure S1. In contrast to the individuals homozygous for the V260E mutant allele, for most individuals here there is no visible extent of homozygosity around the locus.
Histograms of the extent of homozygosity around NPHS2 V260E (A) for individuals homozygous for the mutant allele and (B) for individuals homozygous for the wild-type allele, as shown in Supplementary Figures S2 and S3.
NPHS2 variants detected in Indian and black African children with nephrotic syndrome and controls.
Pathogenic variants identified in 21 nephrotic syndrome patients, lacking NPHS2 V260E, and 19 controls, detected by next-generation sequencing 21 monogenic nephrotic syndrome genes.
APOL1 risk alleles in SR-FSGS patients and controls.
References
- 1.Eddy A.A., Symons J.M. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. doi: 10.1016/S0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- 2.Gipson D.S., Massengill S.F., Yao L. Management of childhood onset nephrotic syndrome. Pediatrics. 2009;124:747–757. doi: 10.1542/peds.2008-1559. [DOI] [PubMed] [Google Scholar]
- 3.Adhikari M., Bhimma R., Coovadia H.M. Focal segmental glomerulosclerosis in children from KwaZulu/Natal, South Africa. Clin Nephrol. 2001;55:16–24. [PubMed] [Google Scholar]
- 4.Bhimma R., Coovadia H.M., Adhikari M. Nephrotic syndrome in South African children: changing perspectives over 20 years. Pediatr Nephrol. 1997;11:429–434. doi: 10.1007/s004670050310. [DOI] [PubMed] [Google Scholar]
- 5.Coovadia HM, Adhikari M, Morel-Maroger L. Clinico-pathological features of the nephrotic syndrome in South African children. Q J Med. 19719;48:77–91. [PubMed]
- 6.Bhimma R., Adhikari M., Asharam K. Steroid-resistant nephrotic syndrome: the influence of race on cyclophosphamide sensitivity. Pediatr Nephrol. 2006;21:1847–1853. doi: 10.1007/s00467-006-0276-2. [DOI] [PubMed] [Google Scholar]
- 7.Bhimma R., Adhikari M., Asharam K. Management of steroid-resistant focal segmental glomerulosclerosis in children using tacrolimus. Am J Nephrol. 2006;26:544–551. doi: 10.1159/000097864. [DOI] [PubMed] [Google Scholar]
- 8.Chanchlani R., Parekh R.S. Ethnic differences in childhood nephrotic syndrome. Front Pediatr. 2016;4:39. doi: 10.3389/fped.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boute N., Gribouval O., Roselli S. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 10.Laurin L.P., Lu M., Mottl A.K. Podocyte-associated gene mutation screening in a heterogeneous cohort of patients with sporadic focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2014;29:2062–2069. doi: 10.1093/ndt/gft532. [DOI] [PubMed] [Google Scholar]
- 11.Machuca E., Benoit G., Nevo F. Genotype-phenotype correlations in non-Finnish congenital nephrotic syndrome. J Am Soc Nephrol. 2010;21:1209–1217. doi: 10.1681/ASN.2009121309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleem M.A. New developments in steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2013;28:699–709. doi: 10.1007/s00467-012-2239-0. [DOI] [PubMed] [Google Scholar]
- 13.Hinkes B., Vlangos C., Heeringa S. Specific podocin mutations correlate with age of onset in steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2008;19:365–371. doi: 10.1681/ASN.2007040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadowski C.E., Lovric S., Ashraf S. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26:1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trautmann A., Bodria M., Ozaltin F. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin J Am Soc Nephrol. 2015;10:592–600. doi: 10.2215/CJN.06260614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson M.G., Gillies C.E., Robertson C.C. Using population genetics to interrogate the monogenic nephrotic syndrome diagnosis in a case cohort. J Am Soc Nephrol. 2016;27:1970–1983. doi: 10.1681/ASN.2015050504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chernin G., Heeringa S.F., Gbadegesin R. Low prevalence of NPHS2 mutations in African American children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2008;23:1455–1460. doi: 10.1007/s00467-008-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genovese G., Friedman D.J., Ross M.D. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp J.B., Nelson G.W., Sampath K. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limou S., Nelson G.W., Kopp J.B. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis. 2014;21:426–433. doi: 10.1053/j.ackd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasembeli A.N., Duarte R., Ramsay M. APOL1 risk variants are strongly associated with HIV-associated nephropathy in black South Africans. J Am Soc Nephrol. 2015;26:2882–2890. doi: 10.1681/ASN.2014050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng D.K., Robertson C.C., Woroniecki R.P. APOL1-associated glomerular disease among African-American children: a collaboration of the Chronic Kidney Disease in Children (CKiD) and Nephrotic Syndrome Study Network (NEPTUNE) cohorts. Nephrol Dial Transplant. 2017;32:983–990. doi: 10.1093/ndt/gfw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woroniecki R.P., Ng D.K., Limou S. Renal and cardiovascular morbidities associated with APOL1 status among African-American and non-African-American children with focal segmental glomerulosclerosis. Front Pediatr. 2016;4:122. doi: 10.3389/fped.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson A. Consensus statement on management and audit potential for steroid responsive nephrotic syndrome: report of a workshop by the British Association for Paediatric Nephrology and Research Unit, Royal College of Physicians. Arch Dis Child. 1994;70:151–157. doi: 10.1136/adc.70.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz G.J., Munoz A., Schneider M.F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford B.D., Gillies C.E., Robertson C.C. Evaluating Mendelian nephrotic syndrome genes for evidence for risk alleles or oligogenicity that explain heritability. Pediatr Nephrol. 2017;32:467–476. doi: 10.1007/s00467-016-3513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B., Leal S.M. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet. 2008;83:311–321. doi: 10.1016/j.ajhg.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens J.C., Reich D.E., Goldstein D.B. Dating the origin of the CCR5-Delta32 AIDS-resistance allele by the coalescence of haplotypes. Am J Hum Genet. 1998;62:1507–1515. doi: 10.1086/301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell S., Neale B., Todd-Brown K. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber S., Gribouval O., Esquivel E.L. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 2004;66:571–579. doi: 10.1111/j.1523-1755.2004.00776.x. [DOI] [PubMed] [Google Scholar]
- 31.Roselli S., Moutkine I., Gribouval O. Plasma membrane targeting of podocin through the classical exocytic pathway: effect of NPHS2 mutations. Traffic. 2004;5:37–44. doi: 10.1046/j.1600-0854.2003.00148.x. [DOI] [PubMed] [Google Scholar]
- 32.Machuca E., Hummel A., Nevo F. Clinical and epidemiological assessment of steroid-resistant nephrotic syndrome associated with the NPHS2 R229Q variant. Kidney Int. 2009;75:727–735. doi: 10.1038/ki.2008.650. [DOI] [PubMed] [Google Scholar]
- 33.Ovunc B., Otto E.A., Vega-Warner V. Exome sequencing reveals cubilin mutation as a single-gene cause of proteinuria. J Am Soc Nephrol. 2011;22:1815–1820. doi: 10.1681/ASN.2011040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowik M.M., Groenen P.J., Pronk I. Focal segmental glomerulosclerosis in a patient homozygous for a CD2AP mutation. Kidney Int. 2007;72:1198–1203. doi: 10.1038/sj.ki.5002469. [DOI] [PubMed] [Google Scholar]
- 35.Lovric S., Fang H., Vega-Warner V. Rapid detection of monogenic causes of childhood-onset steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2014;9:1109–1116. doi: 10.2215/CJN.09010813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roselli S., Gribouval O., Boute N. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131–139. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouchireb K., Boyer O., Gribouval O. NPHS2 mutations in steroid-resistant nephrotic syndrome: a mutation update and the associated phenotypic spectrum. Hum Mutat. 2014;35:178–186. doi: 10.1002/humu.22485. [DOI] [PubMed] [Google Scholar]
- 38.Bennett N.R. The Arab influence. In: Orgot B.A., Kieran J.A., editors. A Survey of East African History. East African Publishing House and Longmans of Kenya; Nairobi: 1968. [Google Scholar]
- 39.Akilesh S., Koziell A., Shaw A.S. Basic science meets clinical medicine: identification of a CD2AP-deficient patient. Kidney Int. 2007;72:1181–1183. doi: 10.1038/sj.ki.5002575. [DOI] [PubMed] [Google Scholar]
- 40.Gigante M., Pontrelli P., Montemurno E. CD2AP mutations are associated with sporadic nephrotic syndrome and focal segmental glomerulosclerosis (FSGS) Nephrol Dial Transplant. 2009;24:1858–1864. doi: 10.1093/ndt/gfn712. [DOI] [PubMed] [Google Scholar]
- 41.Tsvetkov D., Hohmann M., Anistan Y.M. A CD2AP mutation associated with focal segmental glomerulosclerosis in young adulthood. Clin Med Insights Case Rep. 2016;9:15–19. doi: 10.4137/CCRep.S30867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kangovi S., Edwards M., Woloszynek S. Renin-angiotensin-aldosterone system inhibitors in pediatric focal segmental glomerulosclerosis. Pediatr Nephrol. 2012;27:813–819. doi: 10.1007/s00467-011-2056-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequencing and genotyping and bioinformatics.
Kaplan−Meier plot of age of onset of SRNS for subjects homozygous for the variant or for the wild-type allele for NPHS2 V260E. The Kaplan−Meier log-rank statistic was calculated by the r function survdiff.
Heterozygous and homozygous loci around NPHS2 V260E from genotyping by the Illumina Exome V2.1 chip, for 9 individuals carrying p.260 E/E (homozygous for the variant allele). The region shown is 10 mB before and after V260E; x axis coordinates are base positions relative to this locus. Top dots represent homozygous loci, bottom heterozygous; the region lacking heterozygous loci indicates the region where the subjects’ 2 parental chromosomes carry identical haplotypes.
Heterozygous and homozygous loci around NPHS2 V260E from genotyping by the Illumina Exome V2.1 chip, for 40 individuals (representative of 71 total) carrying p.260 V/V (homozygous for the wild-type allele), for comparison with Supplementary Figure S1. In contrast to the individuals homozygous for the V260E mutant allele, for most individuals here there is no visible extent of homozygosity around the locus.
Histograms of the extent of homozygosity around NPHS2 V260E (A) for individuals homozygous for the mutant allele and (B) for individuals homozygous for the wild-type allele, as shown in Supplementary Figures S2 and S3.
NPHS2 variants detected in Indian and black African children with nephrotic syndrome and controls.
Pathogenic variants identified in 21 nephrotic syndrome patients, lacking NPHS2 V260E, and 19 controls, detected by next-generation sequencing 21 monogenic nephrotic syndrome genes.
APOL1 risk alleles in SR-FSGS patients and controls.