Abstract
Viral pathogens are a common cause of severe lower respiratory tract infection in adults. Our ability to rapidly and accurately identify viral infections has dramatically improved as slow culture-based techniques have been largely replaced by multiplex high-throughput systems. Given these advances, reevaluation of the role of respiratory viral testing in adults presenting with lower respiratory tract infection is important. This article reviews the potential benefits of testing, provides an overview of the most commonly used diagnostic techniques, and considers whether current evidence supports routine testing.
Key Words: diagnostic testing, pneumonia, viral disease
Abbreviations: CAP, community-acquired pneumonia; CDC, Centers for Disease Control and Prevention; LRTI, lower respiratory tract infection; PCR, polymerase chain reaction; PCT, procalcitonin; RSV, respiratory syncytial virus; RT-PCR, reverse-transcriptase polymerase chain reaction
Lower respiratory tract infection (LRTI) encompasses a range of infections from acute bronchitis and bronchiolitis to pneumonia.1 LRTIs are a major cause of morbidity and mortality in adults.2 In the United States, billions of dollars are spent annually on the diagnosis and treatment of LRTIs.3, 4
Viruses are the most frequently identified pathogens in severe community-acquired respiratory infections.5 Diagnostic testing for respiratory viruses has dramatically improved over the last several decades as slow culture-based techniques have given way to rapid multiplex high-throughput systems.
Against this backdrop, clinicians must decide if, when, and how to test for respiratory viral pathogens. Does testing improve antimicrobial stewardship and facilitate early initiation of antiviral therapy or merely identify pathogens of uncertain clinical significance and increase health-care costs? The goal of the present review was to answer these questions, focusing specifically on adults hospitalized with suspected LRTIs. The decision to pursue respiratory viral testing in children has been covered elsewhere.6, 7, 8
Rationale for Testing
The merits of respiratory viral testing must be considered in the context of recent changes in the epidemiology and management of severe LRTIs. Bacteria have traditionally been viewed as the dominant pathogens in severe LRTIs. Indeed, rates of Streptococcus pneumoniae infection in patients with community-acquired pneumonia (CAP) frequently approached 50% in historical studies.9, 10, 11 Several factors have altered this paradigm. Introduction of a seven-valent pneumococcal conjugate vaccine for children in 2000, followed by a 13-valent conjugate vaccine in 2010, markedly reduced rates of severe pneumococcal infection, even in adults.12, 13 Declining rates of cigarette smoking, one of the greatest risk factors for pneumococcal infection in immunocompetent adults, further reduced the incidence of invasive pneumococcal disease.14 Finally, an aging population has resulted in an ever-increasing number of individuals particularly susceptible to severe viral infection. The elderly are up to 10 times more likely to develop viral pneumonia than younger adults, and they experience substantially higher morbidity and mortality with viral infection.5, 15, 16 Illustrative of these trends, the Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study, a population-based surveillance study in which the etiology of CAP was rigorously investigated in 2,259 adult patients, detected a bacterial pathogen in < 15% of patients, and only 5% were infected with S pneumoniae.5
This change in LRTI epidemiology has been accompanied by marked improvements in the ability to detect respiratory viral pathogens in clinical practice. Slow and labor-intensive culture-based techniques have been largely replaced by increasingly sensitive diagnostic systems that pair nucleic acid amplification with multiplex technology, allowing multiple viruses to be tested on a single sample (discussed later in more detail). A number of studies have leveraged these advanced diagnostic techniques to better characterize the prevalence of viral infection in patients hospitalized with LRTI. In the CDC EPIC study, 23% of patients had evidence of a viral infection.5 Rhinovirus was the most frequently identified pathogen overall, found in 9% of patients. These results mirror findings of a meta-analysis that reported a combined incidence of respiratory viruses in hospitalized patients with CAP of 23%.17 Viruses seem to be just as common in patients admitted to the ICU (Fig 1). In a South Korean study of 198 patients with pneumonia admitted to the ICU, > 35% had a viral pathogen identified (of which 24% were rhinovirus).18 Other studies in ICU patients have reported viral infection rates between 18% and 41%.19, 20, 21
Figure 1.
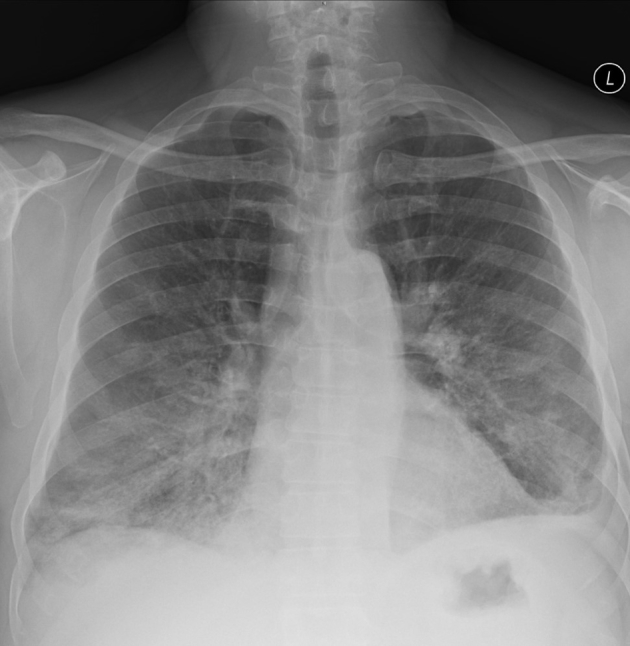
Posteroanterior chest radiograph in a middle-aged immunosuppressed male patient with acute hypoxemic respiratory failure secondary to respiratory syncytial virus demonstrating multifocal basilar predominant airspace opacities.
Recognition that viral pathogens are common causes of severe LRTI comes at a time when innovative approaches to curb antimicrobial use are urgently needed. Antibiotic overuse is a major driver of antimicrobial resistance.22 Rates of infection with drug-resistant pathogens frequently exceed 25% in severe hospital-acquired pneumonia, raising concern that whole classes of antibiotics may soon prove obsolete.23, 24 Respiratory viral testing offers a potential way forward. If severe LRTI is increasingly a viral illness, then perhaps leveraging improved diagnostic tools can rapidly identify a large number of patients in whom antibiotics are unnecessary.
How to Test
Before considering the clinical impact of respiratory viral testing, it is important to review the various ways in which viruses can be identified in clinical practice. Numerous techniques are currently available, including an ever-increasing number of multiplex molecular assays. The sheer number of available testing modalities can be disorienting for practicing clinicians, as each modality has its own strengths, limitations, and test characteristics. Although a comprehensive summary of each diagnostic technique is beyond the scope of the present review, this section provides a general overview of most commonly used testing platforms.
Serologic Testing
Serologic testing currently has a limited role in the diagnosis of viral infection in acutely ill patients. Pathogen-specific antibody titers can be checked in both the acute and convalescent phase of illness, with at least a fourfold increase in antibody levels viewed as diagnostic of recent infection. The requirement for testing weeks following an acute illness precludes use of this technique to guide real-time patient care. Additional serologic tests that are infrequently used include complement fixation and hemagglutination inhibition assays.25
Conventional Tube Culture
Tube culture was traditionally considered the “gold standard” for the diagnosis of viral infection.26 In this technique, a clinical specimen is added to a tube containing cells susceptible to a specific virus.27 Over the span of several weeks, microscopy is used to examine cells for characteristic cytopathic effects indicative of infection, which are then confirmed with immunofluorescence staining. This approach has multiple limitations. Viral culture is slow and labor-intensive, making it poorly suited to guide the management of acutely ill patients. Numerous clinically relevant respiratory viruses, such as respiratory syncytial virus (RSV) and human metapneumovirus, have proven uniquely difficult to isolate in culture.28, 29 Finally, conventional culture diagnosis may be particularly limited in older adults who can develop infection despite low viral burdens.30
Shell-vial Culture
Development of the shell-vial culture technique in the 1990s reduced the turnaround time for culture-based testing.27 Shell-vial culture is performed by inoculating a monolayer of susceptible cells grown on a cover slip with a clinical specimen. The shell-vial tube is then centrifuged to promote viral infection of the cellular monolayer. Depending on the virus being studied, confirmatory staining with fluorescent monoclonal antibodies can be performed following an incubation period of 1 to 3 days. Other than the improved processing time, shell-vial culture has similar limitations as traditional culture techniques.
Rapid Antigen Detection
Numerous rapid antigen detection kits are commercially available that produce test results in < 15 min. These kits are typically based on either immunofluorescent antibody testing or immunochromatographic assays. Although their quick turnaround time is appealing, widespread adoption of these kits in adults has been limited by their poor sensitivity.31, 32
Polymerase Chain Reaction
Nucleic acid amplification using polymerase chain reaction (PCR) has revolutionized the detection of respiratory viral pathogens. Developed in the 1980s, PCR allows for the exponential amplification of a target DNA sequence.33 Samples are placed in a special heating block called a thermocycler and cycled through three temperature-dependent steps: denaturing, annealing, and elongation. High heat (≈ 95°C) is initially used to separate the double-stranded DNA. The temperature is then lowered to facilitate annealing of single-stranded oligonucleotide primers to the complementary DNA template. In the final step, the temperature is raised to approximately 72°C, allowing a heat-resistant DNA polymerase to catalyze 5′→3′ synthesis of new DNA strands.
PCR-based testing offers appealing test characteristics, with sensitivity and specificity frequently approaching 100%.34 Results are available in a matter of hours, facilitating rapid pathogen detection for newly admitted patients. Current platforms obviate the need for more skilled technicians than basic laboratory personnel. However, PCR testing is typically more expensive than traditional techniques. In addition, results of PCR are largely qualitative and thus provide limited insight into the pathogenic load of a particular virus. Finally, given its exquisite sensitivity, PCR-based testing may frequently detect chronic colonization or low-level viral shedding following a previous infection, making it difficult to determine the clinical relevance of a positive test result.35
Reverse-transcriptase PCR
Reverse-transcriptase PCR (RT-PCR) allows RNA (rather than DNA) to serve as the starting material for PCR. This technology is particularly important for the study of LRTIs given the number of RNA viruses implicated in LRTIs (eg, rhinovirus, RSV, human metapneumovirus). In RT-PCR, a reverse transcriptase is used to synthesize complementary DNA from isolated RNA. Complementary DNA can then undergo PCR amplification as described earlier, either in the same tube or as part of a two-step process.
Quantitative Real-time PCR
Real-time PCR technology allows DNA amplification to be quantified following each PCR cycle.36 This goal is accomplished through the use of fluorescent DNA-binding dyes whose signal intensity when exposed to an energy source reflects the amount of amplified DNA. Many different probes are currently available, including ones that bind nonselectively to double-stranded DNA (eg, SYBR I) and sequence-specific hydrolysis probes (eg, TaqMan), which only fluoresce when they are cleaved by DNA polymerase I. Quantitative PCR technology can be applied to either traditional PCR or RT-PCR platforms. As with standard PCR, quantitative real-time PCR is highly sensitive and specific. In addition, the ability to provide quantitative information on pathogen burden may help distinguish viral carriage from true infection.35
High-throughput Multiplex Assays
Respiratory viral testing now commonly occurs on “multiplex” platforms, meaning that multiple analytes are tested on a single sample. As an example, the Respiratory Multi Well System r-gene assay (Argene/bioMerioux) can simultaneously test for 35 respiratory pathogens. Multiplex platforms are often “high-throughput,” signifying that multiple samples (up to 96 depending on the manufacturer) can be processed in a single run. These systems are appealing because multiple patient samples can be quickly tested for clinically relevant pathogens, facilitating both rapid diagnosis and the identification of polymicrobial infections.
Multiplex systems vary in their degree of automation, throughput, processing speed, cost, and ability to produce quantitative results (Table 1).37 Clinicians should in particular note that each platform tests for a slightly different group of viral pathogens. In addition, important viral causes of pulmonary disease, including varicella-zoster virus, herpes simplex virus, hantavirus, and Middle East respiratory syndrome coronavirus, are not covered by the listed PCR platforms and therefore require separate testing.
Table 1.
Characteristics of Commonly Used Multiplex Viral Testing Platforms
| Product | Manufacturer | Technology | Fully Automated | Throughput | Turnaround Time (h) | Viruses Detected |
|---|---|---|---|---|---|---|
| CLART PneumoVir | Genomica | Multiplex RT-PCR, low-density microarray | No | Moderate-high | > 6 | AdV, bocavirus, CoV (229E), Ev, hMPV A/B, Flu-A, Flu-A H1, H1 2009, Flu-A H3, Flu-B, Flu-C, PIV 1-4, RhV, RSV-A, RSV-B |
| eSensor Respiratory Viral Panela | GenMark Diagnostics | Multiplex RT-PCR, hybridization, electrochemical detection | Yes | Low | 1.5 | AdV-B/E, AdV-C, Flu-A, Flu-A H1N1, Flu-A H1 2009, Flu-A H3, Flu-B, hMPV, PIV 1-3, RhV, RSV-A, RSV-B, |
| FTD Respiratory Pathogens 33 | Fast Track Diagnostics | Multiplex qPCR | No | Moderate-high | > 6 | AdV, Bocavirus, CoV (4), Ev, Flu-A, Flu-A H1, Flu-B, hMPV A/B, parechovirus, PIV 1-4, RhV, RSV-A, RSV-B |
| FilmArray respiratory pathogen panela | BioFire Diagnostics | Nested multiplex RT-PCR, melting temperature analysis | Yes | Low | 1 | AdV, bocavirus, CoV (4), Flu-A, Flu-A H1, Flu-A H1-2009, Flu-A H3, Flu-B, Flu-C, hMPV, PIV 1-4, RhV/Ev, RSV |
| Infiniti respiratory pathogen panel | AutoGenomics | Multiplex PCR and RT-PCR, solid array analyzer | No | Moderate-high | > 6 | AdV, CoV, Ev, Flu-A, Flu-B, PIV 1-4, RhV-A, RhV-B, RSV-A, RSV-B |
| RespiFinder 22 | PathoFinder | Multiplex qPCR, melting temperature analysis | No | Moderate-high | > 6 | AdV, bocavirus, CoV (4), Flu-A, Flu-A H1 2009, Flu-B, hMPV, PIV 1-4, RhV/Ev, RSV-A, RSV-B |
| ResPlex II | Qiagen | Target-enriched multiplex PCR with Luminex suspension array | No | Moderate-high | 5-6 | AdV (B/E), bocavirus, CoV (4), CV/echovirus, Flu-A, Flu-B, hMPV-A, hMPV-B, RSV-A, PIV 1-4, RSV-B |
| xTAG Respiratory Viral Panela | Luminex Molecular Diagnostics | Multiplex PCR and RT-PCR with Luminex suspension array | No | Moderate | 8 | AdV, Flu-A, Flu-A H1, Flu-A H3, Flu-B, hMPV, PIV1-3, RhV/Ev, RSV-A, RSV-B |
| Verigene Respiratory Virus Plus Nucleic Acid Testa | Nanosphere | Multiplex RT-PCR, hybridization to gold nanoparticles | Yes | Low | 2 | AdV, Flu-A, Flu-A H1, Flu-A H3, Flu-B, PIV 1-4, RhV, RSV-A, RSV-B |
AdV = adenovirus; CoV = coronavirus; CV = coxsackievirus; Ev = enterovirus; Flu = influenza; hMPV = human metapneumovirus; PCR = polymerase chain reaction; PIV = parainfluenza virus; qPCR = quantitative real-time polymerase chain reaction; RhV = rhinovirus; RSV = respiratory syncytial virus; RT-PCR = reverse-transcriptase polymerase chain reaction.
Approved by the US Food and Drug Administration.
Many commercially available platforms pair multiplex PCR with hybridization of the amplified products onto a microarray that are often read with proprietary analyzers. Examples of this approach include the Infiniti (AutoGenomics) system in which sample processing is largely automated.38 Luminex platforms use liquid-phase bead-based arrays for high-throughput multiplex detection of up to 15 pathogens simultaneously. This technology has been incorporated into a number of commercially available kits, including the ResPlex assay (Qiagen) and the xTAG Respiratory Viral Panel (Luminex Molecular Diagnostics). Multiplex PCR has also been paired with mass spectrometry (PLEX-ID; Abbott Molecular), hybridization to gold nanoparticle-conjugated capture probes (Verigene; Nanosphere), and melt curve analysis (FilmArray; Idaho Technologies). One of the downsides of multiplex PCR testing is that reaction conditions may not be optimized for each specific analyte, potentially adversely affecting sensitivity. A number of advanced molecular assays, many of which occur in isothermal conditions, are currently being developed.39 Isothermal amplification obviates the need for a thermocycler and offers more rapid processing time.
For all testing methods described, lack of a consistent diagnostic gold standard confounds attempts to determine a platform’s true test characteristics. For example, studies describing the sensitivity and specificity of PCR-based platforms often define a true positive as a virus that is detected by at least two PCR assays.34 The performance of conventional detection techniques now frequently use PCR testing as the diagnostic standard.40 As such, when evaluating any study of respiratory viral testing, clinicians should be cognizant of how reference standards are defined.
Does Testing Work?
Respiratory viral testing should ideally be incorporated into routine care only if it improves patient outcomes. This scenario might occur through a reduction in antibiotic exposure, more rapid initiation or discontinuation of antiviral therapy, avoidance of unnecessary diagnostic testing, or more effective use of respiratory isolation rooms. Clinical efficacy of respiratory viral testing has been best studied in children. In small pediatric studies, respiratory viral testing has been found to reduce exposure to antibiotics,41, 42, 43, 44, 45, 46, 47 increase administration of antiviral therapy,41, 42, 47 reduce diagnostic testing,41, 46, 48 and reduce time patients spend in isolation.43, 49
In adults, respiratory viral testing has been inconsistently associated with improved outcomes. Multiple observational studies of patients with influenza infection have found that respiratory viral testing infrequently alters management. In a single-center retrospective review of 166 adults admitted with a respiratory tract infection secondary to influenza, 35% were continued on antimicrobial therapy despite positive influenza test results and negative bacterial cultures.50 This group had a longer hospital length of stay and higher hospital costs compared with patients in whom antibiotics were stopped. Whether these adverse outcomes represent direct effects of continuing antibiotics or result from appropriate antibiotic use in patients at greater risk of bacterial superinfection is unclear. Similarly, in a recent retrospective review of 126 hospitalized patients with both CAP and hospital-acquired PCR-confirmed influenza, 75% received antibiotics.51 In addition, the diagnosis of influenza was not associated with a reduction in antibiotic duration. Falsey et al52 did find that patients who had a positive rapid influenza test result were less likely to receive antibiotics (86% vs 99%; P = .002) and more likely to receive antiviral therapy (73% vs 8%; P < .001) than patients whose rapid test result was negative. However, no difference in total days of antibiotic therapy between groups was found, and > 60% of low-risk patients with a positive rapid viral test result continued to receive antibiotics.
Further studies are needed to clearly determine the impact of influenza testing on antibiotic discontinuation. In addition, although influenza testing routinely increases the use of antiviral therapy, this effect likely depends on the relationship of testing to the start of influenza season and local management approaches. During peak influenza season at institutions where empirical antiviral therapy is routinely prescribed, a negative influenza test result may significantly decrease antiviral use. Conversely, viral testing may increase use of anti-influenza medications if empirical antiviral therapy is rarely used or if testing is being studied early in the influenza season when other viral illnesses are common.
Results have been similarly discouraging in studies of patients infected with any viral pathogen. In a large multiyear review of admissions to a single center in Canada, multiplex viral testing was not associated with a reduction in antibiotic therapy or time spent in isolation rooms.53 Similarly, in a review of 196 patients with a respiratory tract infection who had a viral pathogen identified by using quantitative real-time PCR, only 6% had antibiotic therapy stopped following viral testing, including 79 of 125 patients (63%) with normal chest imaging.54 Finally, PCR testing in a cohort of elderly patients admitted with a respiratory infection failed to reduce antibiotic duration or hospital length of stay.55
The utility of respiratory viral testing has been studied in several randomized trials. In a multicenter trial in the Netherlands, 107 newly admitted patients receiving antibiotics for an LRTI were randomized to receive standard care or viral testing according to multiplex PCR.56 Although the use of PCR doubled the number of patients with an identified pathogen, it increased hospital costs without reducing antibiotic use, diagnostic procedures, or duration of hospital stay. Branche et al57 randomized 300 patients admitted with an LRTI to receive either standard care (which included the use of an influenza/RSV duplex PCR) or measurement of procalcitonin (PCT) paired with testing of throat and nasal swabs for 14 viruses by using multiplex PCR. Initiation of antimicrobial therapy was discouraged for PCT values ≤ 0.24 ng/mL. Sixty-three of 151 patients (42%) in the intervention arm had a positive PCR, whereas 64 of 149 patients (40%) in the control arm had a positive viral test result. Although no difference in overall antibiotic use between the two arms was found, the subgroup of patients with a low PCT and a positive viral PCR were less likely to receive antibiotics at discharge (25% vs 45%; P = .002).
The recently published Routine Molecular Point-of-Care Testing for Respiratory Viruses in Adults Presenting to Hospital With Acute Respiratory Illness (ResPOC) study is the largest trial to date investigating the ability of respiratory viral testing to reduce antibiotic exposure.58 In this single-center study, 720 adults with an acute respiratory infection were recruited from both the ED and a short-stay acute medical unit. Patients in the control arm underwent standard diagnostic testing, including optional use of a laboratory-based PCR test for five respiratory viruses. Patients in the intervention arm had nasal and throat swabs tested on a multiplex PCR platform located in each unit. A total of 161 of 360 patients (45%) in the intervention arm had a virus detected, with influenza (17%) and rhinovirus/enterovirus (15%) being the most common pathogens identified. Only 158 of 354 patients (45%) in the control arm underwent PCR testing, with 52 (15%) of those patients found to have a viral infection. Turnaround time was significantly faster in the intervention arm (2 vs 37 h; P < .0001). Although no difference in the proportion of patients treated with antibiotics or duration of antibiotic therapy was found, patients in the intervention arm were more likely to receive only a single dose of antibiotics (10% vs 3%; P = .001) and receive antibiotics for < 48 h (17% vs 9%; P = .005). Patients with influenza infection in the intervention arm were more likely to receive neuraminidase inhibitors (82% vs 47%; P = .0001) and were placed in respiratory isolation more quickly (0.5 vs 1 day; P = .007). In a post hoc analysis focused only on patients who were randomized to treatment before the administration of antibiotics, significantly fewer patients in the intervention arm received antibiotic therapy (51% vs 64%; P = .029).
Lessons From Recent Trials
Why, despite remarkable advances in our ability to detect respiratory viral pathogens, does testing so inconsistently affect antibiotic use and other important patient outcomes? Several considerations deserve mention.
The clinical implications of a positive respiratory viral test remain a source of debate.35 A major current limitation of respiratory viral testing is the difficulty distinguishing viral carriage from true infection. Studies comparing the results of respiratory viral testing in patients with CAP and asymptomatic control subjects have found viral carriage rates of only 2% in the asymptomatic adult control subjects.5, 59 However, in a recent study of patients admitted to the ICU with acute respiratory failure (where the presence or absence of infection was determined according to admitting Acute Physiologic Assessment and Chronic Health Evaluation II diagnoses), 17% of patients undergoing mechanical ventilation for reasons other than a severe acute respiratory infection had a viral pathogen identified by using multiplex RT-PCR.21 Although these results may indicate that the admitting diagnoses misclassified a large number of truly infected patients, they may also show that viral carriage is common in acutely ill patients. Importantly, viral test results from the upper and lower airway were frequently discordant. Twenty percent of patients with a severe respiratory infection had a viral pathogen identified only in the upper airway, whereas 29% had a virus identified exclusively on tracheobronchial aspirate. These findings highlight the challenge of using a respiratory viral test result from the upper airway to rule in or out clinically significant lower airway infection. Finally, 25% of patients admitted with a severe respiratory tract infection had evidence of bacterial co-infection. Clinicians may understandably be reluctant to discontinue antibiotics even if viral test results are positive for fear of undertreating a mixed infection.
Second, because PCR-based testing is increasingly viewed as standard of care, it is often included in the control arm of trials. In a study by Branche et al,57 40% of patients in the control arm had either influenza or RSV detected by using duplex PCR. Similarly, in the ResPOC study, nearly one half of patients in the control arm underwent PCR testing for respiratory viruses.58 Although patients in the intervention arms of these trials may receive more expeditious or comprehensive testing, the concurrent use of PCR-based platforms for patients receiving “usual care” makes identifying significant differences in outcomes more challenging.
Third, the ResPOC study illustrates the challenge of enrolling patients with suspected LRTI sufficiently early in their course to prevent antibiotic administration. Despite targeting patients on presentation to the hospital, nearly one third of patients enrolled had already received antibiotics at the time of randomization. As such, a critical moment to reduce antibiotic administration was missed in a large number of patients. Although difficult to measure, the clinical momentum associated with antibiotic initiation in acutely ill patients likely contributes to ongoing antibiotic exposure even if viral test results are positive.
Fourth, heterogeneity in study populations makes generalizability challenging. Broad terms such as LRTI and acute respiratory infection capture different patient populations depending on the season and study center. Whether lessons learned from viral testing in patients presenting with a flu-like illness during peak influenza season are applicable to patients admitted with CAP is unclear.
Most importantly, the expectation that a single laboratory test in isolation will dramatically affect clinical care may be unrealistic. This concept is particularly true for multiplex viral detection when specific antiviral treatment is available for only a few detectable viral pathogens. The decision to administer antibiotics to patients with severe LRTI depends on numerous factors, including a clinician’s pretest suspicion for bacterial infection, the results of diagnostic testing (eg, studies have found an abnormal chest radiograph to be associated with continued antibiotic use54, 56, 60), and a patient’s clinical course. As noted earlier, the clinical significance of a positive or negative respiratory viral test result is often unclear, and thus it is understandable and perhaps advisable for clinicians not to use the results of testing as the sole determinant of antibiotic administration. Studies in other settings have shown that overreliance on antibiotics persists despite clear recommendations from consensus guidelines61 and focused interventions to reduce overuse.62 Given the pervasive and recalcitrant nature of antibiotic overuse, a multifaceted approach will surely be required to effectively solve this problem.
Our Approach
We agree with the recommendations of the Infectious Diseases Society of America that rapid respiratory viral testing should be used to help reduce unnecessary antibiotic administration to adults admitted with severe LRTIs.63 Clinician education regarding test characteristics of the diagnostic platforms available within their institution is important. In addition, testing should be implemented as part of a multidisciplinary and longitudinal antimicrobial stewardship program, as has been reported in small studies to be beneficial.64
We find it helpful to pair respiratory viral testing with measurement of PCT, a biomarker that has been shown to reduce antibiotic administration in patients presenting with respiratory infection.65 Even in patients requiring admission to the ICU, we will routinely discontinue antibiotic therapy if the PCT is low and a viral pathogen has been identified, especially if testing is performed on a sample collected from the lower respiratory tract. The pathogenicity of non-influenza viruses remains a source of debate. Rhinovirus is probably the most cited example. Rhinovirus clearly causes pneumonia in children with viremia, and detection in pleural fluid supports its pathogenicity. The data in adults are less established but, in our view, compelling evidence suggests that these viruses can indeed cause severe disease.5, 18, 66, 67, 68
Initiation of specific antiviral therapy for influenza or adenovirus in critically ill patients or discontinuation of empirical anti-influenza treatment during peak season can also be guided by using multiplex viral detection. Emerging specific treatment options for RSV and other viruses will further influence the clinical benefit of respiratory viral testing.
Robust cost-effectiveness data are needed to guide clinicians and health systems on when and how multiplex PCR testing should be implemented. Although small studies suggest that the use of multiplex PCR platforms can reduce costs by simplifying laboratory workflows, this analysis is highly dependent on the platform used and baseline operating expenses.69, 70 Competition and newer technology will likely result in some cost savings. Ultimately, unequivocal cost-effectiveness will depend on our ability to leverage multiplex PCR testing to reduce antibiotic exposure and hospital length of stay.71
Future Directions
A major challenge remains the large number of patients admitted with severe LRTIs in whom a pathogen is never identified. In the CDC EPIC study, a pathogen was not identified in 62% of patients despite diagnostic testing that exceeded usual practice.5 Advances in our ability to better phenotype these patients would have profound implications for antimicrobial stewardship efforts and clinical trial enrollment.
Conclusions
Viruses are a common cause of severe LRTI in adults. Multiplex high-throughput diagnostic platforms now allow clinicians to identify viral pathogens with unprecedented speed and accuracy. Although respiratory viral testing has not yet been shown to convincingly improve patient outcomes in randomized trials, it holds tremendous promise as a tool to aid rapid diagnosis and improve antimicrobial stewardship for patients with severe LRTIs.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. G. W. is a consultant to GenMark, bioMerieux, and Accelerate Diagnostics. None declared (J. M. W.).
Other contributions: The authors thank Chao Qi, PhD, for her review of Table 1.
Footnotes
FUNDING/SUPPORT: J. M. W. is supported by Northwestern University's Lung Sciences Training Program [Grant 5T32HL076139-13] and a Dixon Translational Research Grant. R. G. W. is supported by NIH/NIAID [Grant 1U19AI135964-01].
References
- 1.European Lung white book https://www.erswhitebook.org/chapters/acute-lower-respiratory-infections/ Accessed April 10, 2018.
- 2.Wunderink R.G., Waterer G.W. Clinical practice. Community-acquired pneumonia. N Engl J Med. 2014;370(6):543–551. doi: 10.1056/NEJMcp1214869. [DOI] [PubMed] [Google Scholar]
- 3.File T.M., Jr., Marrie T.J. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122(2):130–141. doi: 10.3810/pgm.2010.03.2130. [DOI] [PubMed] [Google Scholar]
- 4.Hayes B.H., Haberling D.L., Kennedy J.L., Varma J.K., Fry A.M., Vora N.M. Burden of pneumonia-associated hospitalizations: United States, 2001-2014. Chest. 2018;153(2):427–437. doi: 10.1016/j.chest.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain S., Self W.H., Wunderink R.G. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill P.J., Richardson S.E., Ostrow O., Friedman J.N. Testing for respiratory viruses in children: to swab or not to swab. JAMA Pediatr. 2017;171(8):798–804. doi: 10.1001/jamapediatrics.2017.0786. [DOI] [PubMed] [Google Scholar]
- 7.Zar H.J., Andronikou S., Nicol M.P. Advances in the diagnosis of pneumonia in children. BMJ. 2017;358:j2739. doi: 10.1136/bmj.j2739. [DOI] [PubMed] [Google Scholar]
- 8.Wishaupt J.O., Versteegh F.G., Hartwig N.G. PCR testing for paediatric acute respiratory tract infections. Paediatr Respir Rev. 2015;16(1):43–48. doi: 10.1016/j.prrv.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown P.D., Lerner S.A. Community-acquired pneumonia. Lancet. 1998;352(9136):1295–1302. doi: 10.1016/S0140-6736(98)02239-9. [DOI] [PubMed] [Google Scholar]
- 10.Boerner D.F., Zwadyk P. The value of the sputum Gram's stain in community-acquired pneumonia. JAMA. 1982;247(5):642–645. [PubMed] [Google Scholar]
- 11.Berntsson E., Blomberg J., Lagergard T., Trollfors B. Etiology of community-acquired pneumonia in patients requiring hospitalization. Eur J Clin Microbiol. 1985;4(3):268–272. doi: 10.1007/BF02013650. [DOI] [PubMed] [Google Scholar]
- 12.Griffin M.R., Zhu Y., Moore M.R., Whitney C.G., Grijalva C.G. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369(2):155–163. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore M.R., Link-Gelles R., Schaffner W. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–309. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuorti J.P., Butler J.C., Farley M.M. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342(10):681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 15.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 16.Iuliano A.D., Roguski K.M., Chang H.H. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X., Wang Q., Wang M. Incidence of respiratory viral infections detected by PCR and real-time PCR in adult patients with community-acquired pneumonia: a meta-analysis. Respiration. 2015;89(4):343–352. doi: 10.1159/000369561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi S.H., Hong S.B., Ko G.B. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012;186(4):325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 19.Shorr A.F., Fisher K., Micek S.T., Kollef M.H. The burden of viruses in pneumonia associated with acute respiratory failure: an underappreciated issue. Chest. 2018;154(1):84–90. doi: 10.1016/j.chest.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Tramuto F., Maida C.M., Napoli G. Burden and viral aetiology of influenza-like illness and acute respiratory infection in intensive care units. Microbes Infect. 2016;18(4):270–276. doi: 10.1016/j.micinf.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Someren Greve F., Juffermans N.P., Bos L.D. Respiratory viruses in invasively ventilated critically ill patients—a prospective multicenter observational study. Crit Care Med. 2018;46(1):29–36. doi: 10.1097/CCM.0000000000002752. [DOI] [PubMed] [Google Scholar]
- 22.Marston H.D., Dixon D.M., Knisely J.M., Palmore T.N., Fauci A.S. Antimicrobial resistance. JAMA. 2016;316(11):1193–1204. doi: 10.1001/jama.2016.11764. [DOI] [PubMed] [Google Scholar]
- 23.Bassetti M., Welte T., Wunderink R.G. Treatment of gram-negative pneumonia in the critical care setting: is the beta-lactam antibiotic backbone broken beyond repair? Crit Care. 2016;20:19. doi: 10.1186/s13054-016-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner L.M., Webb A.K., Limbago B. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37(11):1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahony J.B. Nucleic acid amplification-based diagnosis of respiratory virus infections. Expert Rev Anti Infect Ther. 2010;8(11):1273–1292. doi: 10.1586/eri.10.121. [DOI] [PubMed] [Google Scholar]
- 26.Talbot H.K., Falsey A.R. The diagnosis of viral respiratory disease in older adults. Clin Infect Dis. 2010;50(5):747–751. doi: 10.1086/650486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAdam A.J., Riley A.M. Developments in tissue culture detection of respiratory viruses. Clin Lab Med. 2009;29(4):623–634. doi: 10.1016/j.cll.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths C., Drews S.J., Marchant D.J. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30(1):277–319. doi: 10.1128/CMR.00010-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen S.C., Williams J.V. New approaches for immunization and therapy against human metapneumovirus. Clin Vaccine Immunol. 2015;22(8):858–866. doi: 10.1128/CVI.00230-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.She R.C., Polage C.R., Caram L.B. Performance of diagnostic tests to detect respiratory viruses in older adults. Diagn Microbiol Infect Dis. 2010;67(3):246–250. doi: 10.1016/j.diagmicrobio.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chartrand C., Tremblay N., Renaud C., Papenburg J. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta-analysis. J Clin Microbiol. 2015;53(12):3738–3749. doi: 10.1128/JCM.01816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chartrand C., Leeflang M.M., Minion J., Brewer T., Pai M. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med. 2012;156(7):500–511. doi: 10.7326/0003-4819-156-7-201204030-00403. [DOI] [PubMed] [Google Scholar]
- 33.Ishmael F.T., Stellato C. Principles and applications of polymerase chain reaction: basic science for the practicing physician. Ann Allergy Asthma Immunol. 2008;101(4):437–443. doi: 10.1016/S1081-1206(10)60323-7. [DOI] [PubMed] [Google Scholar]
- 34.Popowitch E.B., O'Neill S.S., Miller M.B. Comparison of the Biofire FilmArray RP, Genmark eSensor RVP, Luminex xTAG RVPv1, and Luminex xTAG RVP fast multiplex assays for detection of respiratory viruses. J Clin Microbiol. 2013;51(5):1528–1533. doi: 10.1128/JCM.03368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zautner A.E., Gross U., Emele M.F., Hagen R.M., Frickmann H. More pathogenicity or just more pathogens? On the interpretation problem of multiple pathogen detections with diagnostic multiplex assays. Front Microbiol. 2017;8:1210. doi: 10.3389/fmicb.2017.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubista M., Andrade J.M., Bengtsson M. The real-time polymerase chain reaction. Mol Aspects Med. 2006;27(2-3):95–125. doi: 10.1016/j.mam.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Reddington K., Tuite N., Barry T., O'Grady J., Zumla A. Advances in multiparametric molecular diagnostics technologies for respiratory tract infections. Curr Opin Pulm Med. 2013;19(3):298–304. doi: 10.1097/MCP.0b013e32835f1b32. [DOI] [PubMed] [Google Scholar]
- 38.Caliendo A.M. Multiplex PCR and emerging technologies for the detection of respiratory pathogens. Clin Infect Dis. 2011;52(suppl 4):S326–S330. doi: 10.1093/cid/cir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu W., Tang Y.W. Emerging molecular assays for detection and characterization of respiratory viruses. Clin Lab Med. 2009;29(4):673–693. doi: 10.1016/j.cll.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Templeton K.E., Scheltinga S.A., van den Eeden W.C., Graffelman A.W., van den Broek P.J., Claas E.C. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41(3):345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonner A.B., Monroe K.W., Talley L.I., Klasner A.E., Kimberlin D.W. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112(2):363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- 42.Noyola D.E., Demmler G.J. Effect of rapid diagnosis on management of influenza A infections. Pediatr Infect Dis J. 2000;19(4):303–307. doi: 10.1097/00006454-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Rogers B.B., Shankar P., Jerris R.C. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139(5):636–641. doi: 10.5858/arpa.2014-0257-OA. [DOI] [PubMed] [Google Scholar]
- 44.Schulert G.S., Lu Z., Wingo T., Tang Y.W., Saville B.R., Hain P.D. Role of a respiratory viral panel in the clinical management of pediatric inpatients. Pediatr Infect Dis J. 2013;32(5):467–472. doi: 10.1097/INF.0b013e318284b146. [DOI] [PubMed] [Google Scholar]
- 45.Byington C.L., Castillo H., Gerber K. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children's hospital. Arch Pediatr Adolesc Med. 2002;156(12):1230–1234. doi: 10.1001/archpedi.156.12.1230. [DOI] [PubMed] [Google Scholar]
- 46.Jennings L.C., Skopnik H., Burckhardt I., Hribar I., Del Piero L., Deichmann K.A. Effect of rapid influenza testing on the clinical management of paediatric influenza. Influenza Other Respir Viruses. 2009;3(3):91–98. doi: 10.1111/j.1750-2659.2009.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadmon G., Levy I., Mandelboim M. Polymerase-chain-reaction-based diagnosis of viral pulmonary infections in immunocompromised children. Acta Paediatr. 2013;102(6):e263–e268. doi: 10.1111/apa.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iyer S.B., Gerber M.A., Pomerantz W.J., Mortensen J.E., Ruddy R.M. Effect of point-of-care influenza testing on management of febrile children. Acad Emerg Med. 2006;13(12):1259–1268. doi: 10.1197/j.aem.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 49.Mills J.M., Harper J., Broomfield D., Templeton K.E. Rapid testing for respiratory syncytial virus in a paediatric emergency department: benefits for infection control and bed management. J Hosp Infect. 2011;77(3):248–251. doi: 10.1016/j.jhin.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Ghazi I.M., Nicolau D.P., Nailor M.D., Aslanzadeh J., Ross J.W., Kuti J.L. Antibiotic utilization and opportunities for stewardship among hospitalized patients with influenza respiratory tract infection. Infect Control Hosp Epidemiol. 2016;37(5):583–589. doi: 10.1017/ice.2016.17. [DOI] [PubMed] [Google Scholar]
- 51.Akers I.E., Weber R., Sax H., Boni J., Trkola A., Kuster S.P. Influence of time to diagnosis of severe influenza on antibiotic use, length of stay, isolation precautions, and mortality: a retrospective study. Influenza Other Respir Viruses. 2017;11(4):337–344. doi: 10.1111/irv.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falsey A.R., Murata Y., Walsh E.E. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med. 2007;167(4):354–360. doi: 10.1001/archinte.167.4.ioi60207. [DOI] [PubMed] [Google Scholar]
- 53.Mulpuru S., Aaron S.D., Ronksley P.E., Lawrence N., Forster A.J. Hospital resource utilization and patient outcomes associated with respiratory viral testing in hospitalized patients. Emerg Infect Dis. 2015;21(8):1366–1371. doi: 10.3201/eid2108.140978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiley K.T., Lautenbach E., Lee I. The use of antimicrobial agents after diagnosis of viral respiratory tract infections in hospitalized adults: antibiotics or anxiolytics? Infect Control Hosp Epidemiol. 2010;31(11):1177–1183. doi: 10.1086/656596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernes S.S., Hagen E., Quarsten H., Bjorvatn B., Bakke P.S. No impact of early real-time PCR screening for respiratory viruses on length of stay and use of antibiotics in elderly patients hospitalized with symptoms of a respiratory tract infection in a single center in Norway. Eur J Clin Microbiol Infect Dis. 2014;33(3):359–364. doi: 10.1007/s10096-013-1963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oosterheert J.J., van Loon A.M., Schuurman R. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41(10):1438–1444. doi: 10.1086/497134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Branche A.R., Walsh E.E., Vargas R. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. J Infect Dis. 2015;212(11):1692–1700. doi: 10.1093/infdis/jiv252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brendish N.J., Malachira A.K., Armstrong L. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5(5):401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Self W.H., Williams D.J., Zhu Y. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis. 2016;213(4):584–591. doi: 10.1093/infdis/jiv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semret M., Schiller I., Jardin B.A. Multiplex respiratory virus testing for antimicrobial stewardship: a prospective assessment of antimicrobial use and clinical outcomes among hospitalized adults. J Infect Dis. 2017;216(8):936–944. doi: 10.1093/infdis/jix288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston S.L., Szigeti M., Cross M. Azithromycin for acute exacerbations of asthma: the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176(11):1630–1637. doi: 10.1001/jamainternmed.2016.5664. [DOI] [PubMed] [Google Scholar]
- 62.Meeker D., Linder J.A., Fox C.R. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562–570. doi: 10.1001/jama.2016.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barlam T.F., Cosgrove S.E., Abbo L.M. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lowe C.F., Payne M., Puddicombe D. Antimicrobial stewardship for hospitalized patients with viral respiratory tract infections. Am J Infect Control. 2017;45(8):872–875. doi: 10.1016/j.ajic.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 65.Schuetz P., Wirz Y., Sager R. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498. doi: 10.1002/14651858.CD007498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang K., Xi W., Yang D. Rhinovirus is associated with severe adult community-acquired pneumonia in China. J Thorac Dis. 2017;9(11):4502–4511. doi: 10.21037/jtd.2017.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan F., Xiao Y., Li M. Metagenomic analysis identified human rhinovirus B91 infection in an adult suffering from severe pneumonia. Am J Respir Crit Care Med. 2017;195(11):1535–1536. doi: 10.1164/rccm.201609-1908LE. [DOI] [PubMed] [Google Scholar]
- 68.Choi S.H., Huh J.W., Hong S.B. Clinical characteristics and outcomes of severe rhinovirus-associated pneumonia identified by bronchoscopic bronchoalveolar lavage in adults: comparison with severe influenza virus-associated pneumonia. J Clin Virol. 2015;62:41–47. doi: 10.1016/j.jcv.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahony J.B., Blackhouse G., Babwah J. Cost analysis of multiplex PCR testing for diagnosing respiratory virus infections. J Clin Microbiol. 2009;47(9):2812–2817. doi: 10.1128/JCM.00556-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dundas N.E., Ziadie M.S., Revell P.A. A lean laboratory: operational simplicity and cost effectiveness of the Luminex xTAG respiratory viral panel. J Mol Diagn. 2011;13(2):175–179. doi: 10.1016/j.jmoldx.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vallieres E., Renaud C. Clinical and economical impact of multiplex respiratory virus assays. Diagn Microbiol Infect Dis. 2013;76(3):255–261. doi: 10.1016/j.diagmicrobio.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


