Abstract
Introduction
The provision of healthcare for patients with inflammatory arthritis occurs in the context of somewhat conflicting targets, values and drivers. Therefore, there is a need for introducing ‘value-based healthcare’ defined as the value of patient relevant health outcomes in relation to costs. This term is a central part of tomorrow’s healthcare sector, especially for rheumatic diseases, yet the transition is a huge challenge, as it will impact the development, delivery and assessment of healthcare.
Aims
The aim of this study is to compare medical and patient evaluated impact of the traditional settlement and financing production (DAGS) controlled healthcare setting with a value-based and patient-centred adjunctive to standard care.
Methods and analysis
Patients with inflammatory arthritis receiving treatment in routine care at the outpatient clinics in the Capital Region of Denmark will prospectively and consecutively be enrolled in a Non-Intervention-Study framework providing a pragmatic value-based management model. A Danish reference cohort, used for comparison will be collected as part of routine clinical care. The enrolment period will be from 1 June 2018 until 31December 2023. Baseline and follow-up visits will be according to routine clinical care. Registry data will be obtained directly from patients and include personal, clinical and outcomes information. The study results will be reported in accordance with the STROBE statement.
Ethics and dissemination
The study has been notified to the Danish Data Protection Agency and granted authorisation for the period June 2018 to January 2025 (pending). Informed consent will be obtained from all patients before enrolment in the study. The study is approved by the ethics committee, Capital Region of Denmark (H-18013158). Results of the study will be disseminated through publication in international peer-reviewed journals.
Keywords: value-based health care, health care management model, patient centred value, inflammatory arthritis
Strengths and limitations of this study.
The protocol describes a novel pragmatic value-based management model used in real-life settings, that can evaluate and validate agreement of the patient identified values with patient perception of health, well-being and quality of life, and results are expected to delivery of healthcare at the right time and with optimal use of healthcare resources.
The study will hopefully identify patient characteristics that contribute to a poor medical prognosis (including loss of functioning) in patients with inflammatory arthritis that might guide future intervention matching (pharmacological and non-pharmacological) and delivery of stratified interventions based on a prognostic classification.
Although inflammatory arthritis care in outpatient clinics forms the basis of this protocol, the focus could be modified for application to other healthcare services, particularly for community-based treatments and/or treatment of other long-term conditions.
Including only inflammatory arthritis may hamper the external validity to other disease areas.
The open study design might introduce bias concerning selecting patient, performance and assessment of outcomes.
Introduction
Inflammatory arthritides are heterogeneous diseases with a wide clinical spectrum and diverse outcomes.1 Disease modifying antirheumatic drugs (DMARDs) including biological treatments have improved the management of inflammatory arthritis substantially during the last decades.2 Nevertheless, only around half of the patients experience remission and/or low disease activity to these drugs in routine care.3
A look into the future of healthcare shows several challenges ahead and also great opportunities. Healthcare systems around the world must accommodate and adjust to simultaneous developments in demographics, climatic changes, changing nutritional demands and increased nutritional understanding, citizens’ expectations, technological and scientific advancement and rises in chronic non-communicable musculoskeletal and associated diseases at pandemic proportions.
Increasing expectations, an ageing population and novel treatment options are driving healthcare related costs to increase at a faster rate than the expansion in the Gross Domestic Product in most high-income countries.4 As a result, healthcare systems across the world are challenged by a need to rationalise resources and to improve efficacy as well as effectiveness within the healthcare sector.5
The development of the healthcare sector occurs in the context of somewhat conflicting targets, values and drivers. Nevertheless, the principal focus for strategies describing this development denotes a need to introduce ‘value-based healthcare’ defined as the value of patient relevant health outcomes in relation to costs. This term is a central part of tomorrow’s healthcare sector, yet the transition is a huge challenge, as it will impact the development, delivery and assessment of healthcare.6
Another societal trend is a demand for a more individualised approach to healthcare. Advances within sciences and clinical healthcare are essential for the development of personalised medicine, but in order to fully capitalise on these improvements, there is a need to incorporate qualitative knowledge about the individual’s everyday life. People are distinguishable by their biological variability and in terms of how disease affects their lives and how individualities affect disease management.7 This is particularly important in the context of patients having to live with lifelong chronic musculoskeletal diseases, often associated to other comorbidities.
Technological advances and the data revolution are already transforming the healthcare landscape. First of all, it is already possible to apply home-based devices and apps to facilitate improvements in people’s health, and numerous wearable activity trackers and other gadgets connected to the cloud are widely used for health-related purposes. Second, health-related information is currently being captured via national quality databases, registries and electronic healthcare record systems, and this information is gradually becoming more accessible for citizens and healthcare professionals (HCPs) via online portals. Third, current evidence supports that online tools can in fact improve biomarkers, lifestyle characteristics including nutrition as well as patients’ competencies in coping with tackling their disease.8–10
Rationale and theoretical considerations
In inflammatory arthritis, we postulate that value can be assessed from a medical, patient-centred and/or a societal perspective.11–13 Medical value is the benefit obtained from controlling a disease and related conditions (associated conditions and comorbidities) by treating these to specified targets. Patient-centred value concerns patients’ contextual experiences arising from intrapersonal and social interactions (interpersonal) in relation to their health and well-being and will in the current study be addressed through individual goal identifying questionnaires. The International Classification of Functioning, Disability and Health (ICF) will be applied as the theoretical measurement framework and data structured within the body domain (body structures and functions), activity domain (execution of tasks), domain of participation (involvement in life situations) and contextual factors (personal and environmental factors)14 to support the patient-centred value approach. Societal values comprise both direct and indirect costs for society, in terms of usage of healthcare resources as well as economic issues related to loss of employment and/or early retirement in relation to inflammatory arthritis.12 The first two are pivotal for the well-being of the patients with inflammatory arthritis and the latter is critical for ensuring optimal usage of resources and costs in society. This study recognises that value can, and must, be defined by including and combining all three value perspectives as in ‘the value pyramid’ depicted below (figure 1). There is a potential positive and negative interaction between the two bottom layers. Together patient-centred value and medical value may create societal value in terms of increased work ability and diminished healthcare and allowance costs. Societal inequity in turn generates poor health and diminished personal value. Kinetics and impact of interventions (positive or negative) are presumably almost instant in the lower levels of the pyramid; however, the feedback between societal value and patient-centred and/or medical value is much more tardive and takes years to impact.
Figure 1.
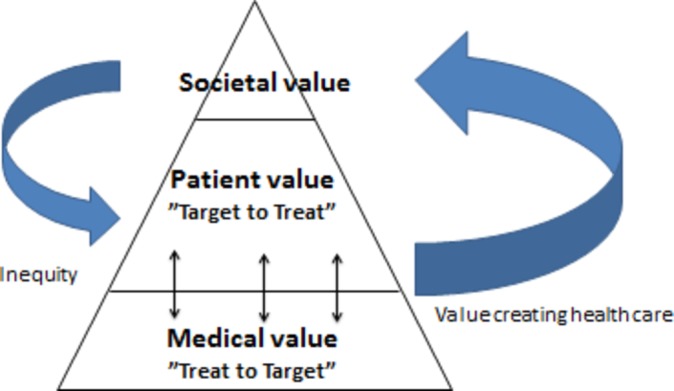
The value pyramid in inflammatory disease management. Treating patients with inflammatory arthritis to target is the backbone in creating value. However, individual patient-identified concerns, barriers or problems should be defined by patients and be regarded as adjunctive targets to treat to fully manage the impact of a chronic disease.
Incomplete awareness of underlying value creating mechanisms in inflammatory arthritis may influence the assessment and management of patients with arthritis in several ways. This may lead to misinterpretation of composite disease outcome measures, which could be driven by interpersonal and intrapersonal contextual factors that could increase the risk of overtreatment of conventional synthetic DMARD (csDMARD) and biological DMARD (bDMARD) treatments as well as insufficient non-pharmacological patient management and subsequent waste of resources.
Research on global patient assessments in inflammatory arthritis has been performed within several domains that are relevant to understand the impact of disease on patients’ lives, comprising, for example, disease activity, fatigue and work ability/productivity.15–17 In addition, overall functioning and health is a relevant outcome in studies and clinical care. However, limited research has been done in inflammatory arthritis on patient global assessment for overall functioning, health and value. The terminology used for such constructs varies from ‘general health’ and ‘well-being’ to’ quality of life’ (QoL) or ‘health-related quality of life’. Also, terms such as ‘happiness’ and ‘life satisfaction’ have been introduced.18 19
In its purest meaning, ‘health’ refers to the absence of disease, but in the broad definition of 1948 from the WHO, ‘health’ refers to overall physical, mental and social well-being.20 A tentative definition of the construct ‘well-being’ proposed that it is ‘an umbrella term for different valuations that people make regarding their lives, the events happening to them, their bodies and minds and the circumstances in which they live’.21 For QoL, several descriptions can be found in the literature, but usually they contain elements of a person’s physical, material, social and emotional status, while often emphasising the personal appraisal or satisfaction related to these aspects.22 23 More recently, the WHO concluded that the term ‘well-being’ in the 1948 definition was confusing and clarified that ‘functioning (and health)’ and ‘quality of life’ are interrelated but not interchangeable.24 While ‘functioning (and health)’ refers to the objective performance (either observed or self-reported), QoL refers to the subjects’ satisfaction about one’s performance.
The aims of this study are to (1) compare medical and patient evaluated impact of the traditional settlement and financing production (DAGS) controlled healthcare setting with a value-based and patient-centred adjunctive to standard care; (2) identify patient characteristics that contribute to a poor medical prognosis (including loss of functioning) in patients with inflammatory arthritis that might guide future intervention matching (pharmacological and non-pharmacological) and delivery of stratified interventions based on a prognostic classification;25 (3) evaluate and validate agreement of the patient identified values with patient perception of health, well-being and QoL; (4) iteratively improve this pragmatic value-based management model (further elaborated below); (5) study societal value (health economics) as long-term outcomes of implementing the present pragmatic value-based management to patients with inflammatory arthritis, when delivered in real-world clinical practice to a heterogeneous patient population as compared with traditional DAGS-controlled healthcare.
In summary, there is a need to explore and to better understand solutions that can address the healthcare sectors tasks within the areas of value-based healthcare, specifically within the domains of medical, patient-centred and societal value. The ambitions are tightly coupled and to succeed with this challenging endeavour to exploit the full potential of each area, it will be necessary to address them in a coordinated manner.
Methods and analysis
Value-based management setting
Since 1 January 2018 and until end of 2020, the outpatient clinics at departments of Rheumatology involved in this study (Copenhagen University Hospitals, Glostrup and Gentofte) have been relieved of the DAGS-production reimbursement management. Instead, the sites have been given the opportunity to develop a value-based healthcare management system within fixed budgets, the latter defined as a lump-sum of the current budget given the previous 3 years and adjusted for inflation, without requiring certain amount of DAGS to earn the planned budget.
The management model will be designed and reiterated with assistance from all stakeholders including patient research partners and HCPs according to ‘The Parker Model’ a qualitative 3-step approach.26
Management team
In order to meet the objectives of this study, it is important for all partners to work successfully together. To fulfil this, it is important to provide this collaborative effort with an efficient management structure to make sure that the coherent nature of the project remains stable for the entire duration of the project and ensure timely and competent planning and conduction of the study.
The project management team consists of the Sponsor-Investigator, Dr Tanja Schjødt Jørgensen who is responsible for the execution of the project, the Principal Investigator Lars Erik Kristensen who is responsible for the overall scientific planning of the project and Chief Administrator Henrik Røgind who is responsible for administrative and financial tasks. The tasks of the management team in relation to the progress of the project are:
Monthly conferences with clinical and scientific staff members at the Parker Institute.
Review and management of project progress in relation to the objectives.
Coordinate the scientific publications of the project.
Head the dissemination process.
Head the data management team.
Manage financial tasks.
Facilitate audits.
Create awareness of the study in the non-scientific communities.
The data collected in the clinical study will on request be made available for further exploitation in the research community on publication of the main results of the studies. The Parker Institute guarantees to ensure future data storage, protection and availability.
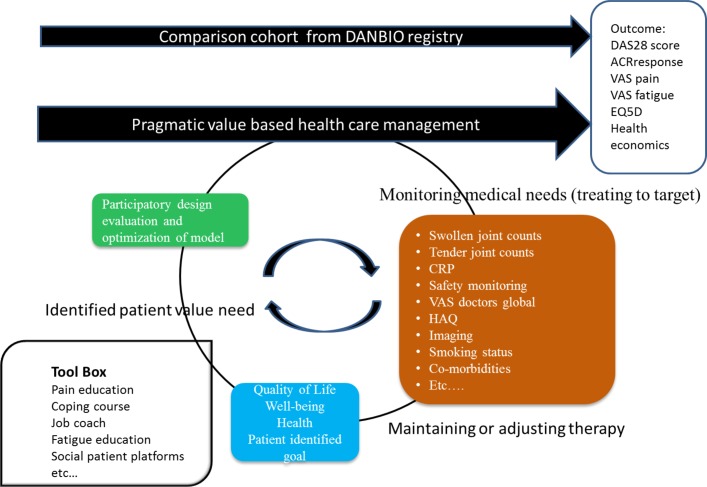
The value-based healthcare management model (figure 2) will integrate the traditional ‘treat to target’ approach with a patient identified goal and prioritised functional patient education, courses and/or solutions toolbox to be offered based on identified needs.
Figure 2.
Illustrates the principles of the pragmatic value-based healthcare model. ACR, American College of Rheumatology response criteria; CRP, C reactive protein; DAS28, Disease Activity Score 28; EQ-5D, based on the VAS scoring in the five domains; HAQ, Health Assessment Questionnaire; VAS, visual analogue scale.
The first part of defining patient value measures (through individual patient goal identification) will be derived from current patient preference studies.11 12 These measures will be used to form a core set of outcome measures, which will be assessed continuously by all patients and to form a dynamic and individualised sets of outcomes measures by asking patients to tailor their reporting by appraising available outcome measures that are of key relevance for them as individuals. The process of outcome identification and testing will be started in March 2018. Subsequent iterations and calibration of the instrument and value-based healthcare plan will be performed yearly throughout the following 5 years. Furthermore, as part of the routine clinical care, participants will undergo an examination programme to assess the variables of interest (table 1). During the implementation process of this pragmatic value-based healthcare model, functional tailored generic educational courses and/or solutions will be developed; these will among other themes consist of interdisciplinary educational courses, work coaches, social patient platforms and referral to other health professionals, for example, occupational and physical therapy. The interventions programmed (educational courses and/or solutions) will be paid for by redistributing resources (the lump-sum) as the project is not accountable for certain amount of DAGS. Thus, the budget will equal the ‘normal’ budget and be compared with a standard of care setting both in terms of quantity and quality of patient care.
Table 1.
Examination and interview at baseline and follow-up (follow-up will be every 6 months)
| Demographics and disease-related characteristics (interview) | Baseline | Follow-ups |
| Sex (M/F), no. (%) | X | |
| Age (years) | X | |
| Diagnosis (eg, RA, PsA, AS) | X | |
| Disease duration (years) | X | |
| Smoking (current (average per week)/previous (average per week)/never) | X | |
| Alcohol consumption (no. per week) | X | |
| Diabetes (y/n) | X | |
| Cardiovascular disease (y/n) | X | |
| Dyslipidaemia (or treatment for this) (y/n) | X | |
| Mental disorder (depression, anxiety) (y/n) | X | |
| Medication | ||
| Use of mild analgesics including NSAIDs (days per month) | X | X |
| Cumulated dose of oral prednisolone during the last month (mg) | X | X |
| Medication history (current and previous csDMARDs and bDMARDs) | X | X |
| Interval (days) between study baseline visit and initiation of new treatment | X | X |
| Date for treatment termination of new drug | X | X |
| Reason for withdrawal of treatment during the study period (lack of effect, adverse events, other) | X | X |
| Clinical examination | ||
| VAS physician (0–100 mm) | X | X |
| Height (cm) | X | |
| Weight (kg) | X | |
| Swollen joint count (0–28) | X | X |
| Tender joint count (0–28) | X | X |
| Manual tender point examination (no), only scores≥2 are interpreted as a tender point | X | X |
| Spondyloarthritis Research Consortium of Canada enthesitis score (SPARCC) (xx-xx) | X | X |
| Patient-reported outcomes (PROs) | ||
| EQ-5D; VAS pain (0–100 mm) | X | X |
| VAS Health (0–100 mm) | X | X |
| VAS Well-being (0–100 mm) | X | X |
| Multi-Dimensional Health Assessment Questionnaire disability index (MD-HAQ, including visual analogue scale for pain and global) (0–3) | X | X |
| PainDETECT Questionnaire (PDQ) | X | X |
| Personal factors and coping | X | X |
| VAS pain (0–100 mm) | X | X |
| VAS global (0–100 mm) | X | X |
| VAS fatigue (0–100 mm) | X | X |
Patient Prioritised Problem Identification (PPPI) Questionnaire
|
X | X |
| Transition Questionnaire score (Trans-Q) | X | X |
| Imaging and blood sampling as per routine care |
ADL, activities of daily living; AS, ankylosing spondylitis; bDMARD, biological DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease modifying anti rheumatic drugs; EQ-5D, based on the VAS scoring in the five domains; NSAIDs, Nonsteroidal anti-inflammatory drugs; PsA, psoriatic arthritis; RA, rheumatoid arthritis; VAS, visual analogue scale.
However, the exact nature and extent of these solutions are subject to change and will be developed depending on demand and results of the current study.
Study design
A cohort of patients with inflammatory arthritis receiving treatment in routine care at the outpatient clinics at Center for Rheumatology and Spine Diseases (VRR), Glostrup and Gentofte Hospitals (Capital Region of Denmark) will prospectively be enrolled in a Non-Intervention-Study framework providing the pragmatic value-based management model. A Danish reference cohort, used for comparison will be collected as part of routine clinical care through the nationwide Danish registry of biological therapies (DANBIO).27 All participants will be assessed at baseline. The enrolment period will be from 1 June 2018 until 31December 2023. Follow-up will be assessed every 6th month in accordance with routine clinical care.
Participants
Patients with a diagnosis of inflammatory arthritis who may be considered for inclusion will be identified by doctors and nurses during routine care, according to expert clinical opinion of treating specialist, at departments of Rheumatology in the Capital Region of Denmark (Gentofte and Glostrup Hospital locations).
Data collection
Registry data will be obtained directly from patients and include personal, clinical and outcomes information. Data collection at baseline and follow-up visits will be based on questionnaires placed in the clinic and data exported to a designated research database. Clinical data collection at follow-ups will be based on electronic questionnaires accessed via DANBIO; a locked online it-platform hosted by the Capital Region of Denmark. Questionnaires covering patient value identified key evaluation and outcome domains will be implemented in paper form and subsequently submitted to a research database.
Focus group interviews, in which the subjects can reflect what constructs (Patient centred Value, Health, Well-being and QoL) represent to them, will be categorised into themes by going back and forward between answers and the (expanding) list of themes. Themes will further be linked to ICF components (body functions, activities and participation, environmental factor or personal factor) and ICF chapters.14 For each construct, the average number of themes identified as well as the proportion of themes representing a specific ICF category will be calculated for patients and controls separately. An ICF category will be considered relevant, when 10% or more of all themes within the construct is related to this ICF category.
The focus group interviews use the methodology of Concept mapping (CM), a formal group process with a structured approach used to identify and organise ideas on a topic of interest.28 CM is highly effective for the development of outcome measures, such as key patient considerations.29 In this study, CM will be conducted through three to four, full-day, focus groups including patients with inflammatory arthritis or until qualitative data saturation (defined as the presence of redundancy in emerging concepts) is achieved. If data saturation is not achieved additional sessions will be run. At the start of each focus group, the CM process will be introduced. Clustering analysis will be performed on the participant statements generated during the focus groups using multidimensional scaling (MDS) analysis (CS Global MAX; Concept Systems).30 The x and y values from the MDS will be used to perform a hierarchical cluster analysis dividing the statements into non-overlapping clusters;31 32 any duplicate statements within the concept maps will be removed.29 33 Independent and thematic analysis of the reduced statement pool will be performed separately by two healthcare specialists, to identify common clusters while preserving both the exact wording of the statements and the cluster labels assigned to them by patients during the workshops. To identify which issues are of most importance participants will be asked to rate the importance of each statement on a 5-point scale, from 1 (‘not important’ for people with inflammatory arthritis) to 5 (‘very important’ for people with inflammatory arthritis).
Patient and public involvement
Collaboration between patients and professionals in developing and disseminating research is relatively new. Nevertheless, this study follows the EULAR recommendations34 for the inclusion of patient representatives in the contemporary scientific process by adhering to eight important aspects. Representative healthcare professionals and patients were involved in all aspects of the protocol development to ensure a systematic representation of the real care processes and identification of real issues.
Results from the current study will be disseminated to participants through newsletter(s) in layman terms and the Parker Institute website (http://parkerinst.dk/). In order to maximise impact, we will, with the input of patient research partners, also communicate our results through a number of other scientific and non-scientific channels including, but not limited to: (1) presentations at relevant congresses; (2) presentations in relevant fora and (3) press briefings.
We would like to thank our patient research partners, Søren Herlev Jørgensen and Lilian Dalsgaard for taking part in the whole process of preparing the current study.
Variables and outcome measures
Clinical examination
HCPs will perform the interview and clinical examination, consisting of variables shown in table 1.
Patient demographics and patient-reported outcomes
Patient demographics and medication profile will be collected from the participant by interview and from the patient files. Patient-reported outcomes (PROs) will be obtained from electronic questionnaires (table 1) accessible from computer touch screens at the study site.35 Furthermore, Patient Prioritised Problem Identification Questionnaires will be recorded in paper formats and serve as a tool for patient empowerment. These outcomes will be assessed via a prioritised questionnaire (0–10 scale) including assessments of pain, fatigue, work/leisure activities, function, discomfort, coping and/or anxiety/depression/stress. The results from these outcomes will be used to define the personalised target to treat and will then be allocated if necessary to predefined educational tools.
Multi-Dimensional Health Assessment Questionnaire (MD-HAQ) derived from the HAQ Health Assessment Questionnaire, which includes an index of the three rheumatoid arthritis (RA) core data set measures (physical function, pain and global estimate). It consists of 10 questions addressing eight different areas of functional ability and yields a total score between 0 and 3, with a higher score representing increasing disability.36 The MD-HAQ is useful in all rheumatic diseases by saving time, documenting changes in status over long periods and by improving rheumatology care and outcomes.
Health and well-being during the last week on a horizontal VAS from 0 (worst) to 100 (best health, well-being, respectively). To avoid an influence of task description across global, instructions are similarly formulated with a single sentence item. No reference to a specific disease will be made. Second, a subgroup of participants will be asked to indicate whether they consider dissimilarities between the constructs to be present (yes/no). Next, they will be invited to think about each of the two constructs and to write down what they are taking into account when scoring themselves (five lines available per construct). Finally, a subgroup of subjects will be asked to score the three globals a second time after this forced reflection.
The answers to the open question, in which the subjects could reflect what the constructs represent to them, will be categorised into themes by going back and forward between answers and the (expanding) list of themes. Themes will be further linked to ICF components (body functions, activities and participation, environmental factor or personal factor) and ICF chapters.14 For each construct, the average number of themes identified as well as the proportion of themes representing a specific ICF category will be calculated for patients and controls separately. An ICF category will be considered relevant, when 10% or more of all themes within the construct related to this ICF category.
The Transition Questionnaire (Trans-Q) consists of three main questions addressing whether there has been an improvement, deterioration or no change regarding pain, function and overall condition between the two visits.37 38
Exploratory outcomes and response criteria
Response to treatment and care during the study period will be assessed by various outcome variables covering composite, clinician and individual patient prioritised outcome measures and the composite patient value measure (table 2). The latter is a weighted average of the individual patient prioritised outcome measures based on the patient’s own evaluation or relative importance (each scale rated 0–10). The composite measures are chosen based on their extensive use in inflammatory arthritis trials and routine care, respectively. These are described in the following section. Clinician and PROs are shown in table 2.
Table 2.
Outcome measures assessed at follow-up baseline
| Composite outcomes | ACR20/50/70 (at least 20%/50%/70% improvement in ACR response criteria) MDA (% achieving minimal disease activity) Δ CDAI Δ DAS28 |
| Clinical outcomes | Δ No. of tender and swollen joints Δ CRP level Δ Physician’s global assessment (VAS global) |
| Patient-reported outcomes | Δ VAS-fatigue, Δ VAS-pain, pain detect score, Δ Patient VAS-global Δ Patient Value (composite score 0–100 mm of patient prioritised outcome measures questionnaire) Δ EQ-5D (VAS) Δ Well-being Δ Health Δ HAQ-MD Trans-Q |
ACR, American College of Rheumatology response criteria; CDAI, Clinical Disease Activity Index; CRP, C reactive protein; DAS28, Disease Activity Score 28; EQ-5D, based on the VAS scoring in the five domains; HAQ-MD Multi-Dimensional Health Assessment Questionnaire disability index (including visual analogue scale for pain and global); Trans-Q, Transition score; VAS, visual analogue scale.
Analysis and statistics
Sample size considerations
Due to the exploratory design, no statistical power calculation has been performed. The study will enrol consecutively for 17 months, which presumably allows us to recruit 2.500 patients based on the flow in the out-patient clinics at Glostrup and Gentofte. A subsequent 5-year follow-up period is planned.
Descriptive statistics and main analyses
The study results will be reported in accordance with the STROBE statement.39 Missing data at follow-up will be imputed by a non-responder assumption (applying baseline observation carried forward technique for continuous data).
Baseline variables will be described for all participants and in relevant subgroups. The cohort of patients enrolled in the management model will be compared with patients being part of routine clinical care and collected in DANBIO on means and SD or medians and IQRs will be calculated depending on data distribution and comparisons will be performed by χ² test for categorical data and Mann-Whitney or Kruskal Wallis test for continuous data. P<0.05 are considered to be statistically significant. The total number of participants with recorded values will be reported. Correlations will be explored by Spearman’s rank-order correlation.
Achievement of patient, clinician and composite response measures will be described for all patients and in subgroups (according to, eg, treatment, PRO profile). Regression models will be applied to study if pain measurements and/or ultrasonic activity have an impact on the treatment response measures (table 2). Crude and adjusted estimates will be reported.
Long-term (5 and 10 year) income, allowance and healthcare cost estimates (both direct and indirect) will be calculated and presented for the cohort of patients enrolled in the management model and compared with patients being part of routine clinical care (DANBIO) (see online supplementary file 1).
bmjopen-2018-023915supp001.pdf (186.5KB, pdf)
Discussion
The vision of the current study is closely aligned with the healthcare sectors commitment to help patient’s live healthier and to ensure high-quality lives.
The described approach will advance the current setup, function, performance and evaluation of the healthcare sector in a value-based perspective (what’s of importance for the patient). Finally, the study’s combined efforts within clinical care and science will create an in-depth understanding of the implications of a personalised value-based healthcare approach.
In the short term, a successful development and evaluation of the study is expected to result in delivery of a pragmatic value-based management model used in real-life settings that provides patients with needed and relevant healthcare at the right time and without wasting healthcare resources. As such, the study will put forward a model that empowers patients, facilitates healthcare-promoting interactions between patients, caregivers and HCPs and encourages a more patient-focused partnership aspiring to optimise the disease management in a holistic and cooperative manner. This model will offer the possibility for management plans to be tailored according to the individual patient’s consent, need, aspirations and competencies and ensure that the patient, the HCPs and other key stakeholders are all engaged in the advancement of our healthcare sector.
Ethics and dissemination
The study has been notified to the Danish Data Protection Agency and granted authorisation for the period June 2018 to January 2025 (pending). Sensitive personal data will be pseudonymised and encrypted according to regulations stipulated by the Danish Data Protection Agency and informed consent will be obtained from all patients before enrolment in the study. Patient research partners have been involved in the preparation of the study protocol. From our point of view, this observational study withholds only minimal or no risk of harm, since no change of treatment strategies or any invasive examinations is applied. Results of the study will be disseminated through publication in international peer-reviewed journals. With the input of patient research partners, public outreach will be performed by layman articles and reports at the Parker Institutes’ website.
Supplementary Material
Acknowledgments
Thank you to patient research partners, Søren Herlev Jørgensen and Lilian Dalsgaard for taking part in the whole process of preparing the current study.
Footnotes
Contributors: TSJ and LEK have made substantial contributions to the conception and design of the protocol and been responsible for drafting the protocol manuscript. They are also taking responsibility to the integrity of the protocol and approve the final version for publication. JJøL, AH, HMøSø, BS, A-MS, BAE, BB, KC, EF-M, HRø, TL, PCT, IFP, EEW, JK and HG have all made substantial contribution to the conception and design of the protocol, revised the protocol for important intellectual content and approved the final version to be published.
Funding: The study is supported by The Parker Institute (Oak Foundation) and The Capital Region of Denmark.
Competing interests: TSJ has received fees for speaking by Abbvie, Roche, UCB, Novartis, Biogen, Eli Lilly and Pfizer. HG has received fees for speaking by MSD and Pfizer, LEK has received fees for speaking and consultancy by Pfizer, AbbVie, Amgen, UCB, Celgene, BMS, MSD, Novartis, Eli Lilly and Janssen Pharmaceuticals.
Patient consent: Not required.
Ethics approval: The ethics committee of the Capital Region of Denmark (H-18013158).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Helliwell P, Coates L, Chandran V, et al. Qualifying unmet needs and improving standards of care in psoriatic arthritis. Arthritis Care Res 2014;66:1759–66. 10.1002/acr.22404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ash Z, Gaujoux-Viala C, Gossec L, et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71:319–26. 10.1136/ard.2011.150995 [DOI] [PubMed] [Google Scholar]
- 3. Combe B, Landewe R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:34–45. 10.1136/ard.2005.044354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. OECD, 2015. Health Statistics. http://www.oecd.org/els/health-systems/health-data.htm
- 5. Andersen TM. Sundhedsområdet åd halvdelen af velfærdsløft Altinget. 2014.
- 6. Porter ME. A strategy for health care reform--toward a value-based system. N Engl J Med 2009;361:109–12. 10.1056/NEJMp0904131 [DOI] [PubMed] [Google Scholar]
- 7. Ziegelstein RC. Personomics. JAMA Intern Med 2015;175:888–9. 10.1001/jamainternmed.2015.0861 [DOI] [PubMed] [Google Scholar]
- 8. Klinisk Integreret Hjemmemonitorering (KIH). Slutrapportering til fonden for velfærdsteknologi. 2015.
- 9. Chow CK, Redfern J, Hillis GS, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: A randomized clinical trial. JAMA 2015;314:1255–63. 10.1001/jama.2015.10945 [DOI] [PubMed] [Google Scholar]
- 10. Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev 2015;16:376–92. 10.1111/obr.12268 [DOI] [PubMed] [Google Scholar]
- 11. Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 2014;73:1012–9. 10.1136/annrheumdis-2014-205207 [DOI] [PubMed] [Google Scholar]
- 12. Kristensen LE, Jørgensen TS, Christensen R, et al. Societal costs and patients’ experience of health inequities before and after diagnosis of psoriatic arthritis: a Danish cohort study. Ann Rheum Dis 2017;76:1495–501. 10.1136/annrheumdis-2016-210579 [DOI] [PubMed] [Google Scholar]
- 13. Zangi HA, Ndosi M, Adams J, et al. EULAR recommendations for patient education for people with inflammatory arthritis. Ann Rheum Diss 2015;74:954–62. 10.1136/annrheumdis-2014-206807 [DOI] [PubMed] [Google Scholar]
- 14. World Health Organisation. International classification of functioning, disability and health. 2001.
- 15. Spoorenberg A, van Tubergen A, Landewé R, et al. Measuring disease activity in ankylosing spondylitis: patient and physician have different perspectives. Rheumatology 2005;44:789–95. 10.1093/rheumatology/keh595 [DOI] [PubMed] [Google Scholar]
- 16. van Tubergen A, Coenen J, Landewé R, et al. Assessment of fatigue in patients with ankylosing spondylitis: a psychometric analysis. Arthritis Rheum 2002;47:8–16. 10.1002/art1.10179 [DOI] [PubMed] [Google Scholar]
- 17. Escorpizo R, Bombardier C, Boonen A, et al. Worker productivity outcome measures in arthritis. J Rheumatol 2007;34:1372. [PubMed] [Google Scholar]
- 18. Fowler JH, Christakis NA. Dynamic spread of happiness in a large social network: longitudinal analysis over 20 years in the Framingham Heart Study. BMJ 2008;337:a2338 10.1136/bmj.a2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naci H, Ioannidis JP. Evaluation of wellness determinants and interventions by citizen scientists. JAMA 2015;314:121 10.1001/jama.2015.6160 [DOI] [PubMed] [Google Scholar]
- 20. World Health Organisation, 1948. Constitution of the World Health Organization: Principles. http://apps.who.int/gb/bd/PDF/bd47/EN/constitution-en.pdf?ua=1
- 21. Diener E. Guidelines for National Indicators of Subjective Well-Being and Ill-Being. J Happiness Stud 2006;7:397–404. 10.1007/s10902-006-9000-y [DOI] [Google Scholar]
- 22. Felce D, Perry J. Quality of life: its definition and measurement. Res Dev Disabil 1995;16:51–74. 10.1016/0891-4222(94)00028-8 [DOI] [PubMed] [Google Scholar]
- 23. Eurostat. Quality of life indicators - measuring quality of life 2015 (updated 05-11-2015). http://ec.europa.eu/eurostat/statistics-explained/index.php/Quality_of_life_indicators_-_measuring_quality_of_life.
- 24. World Health Organisation. WHOQOL: Measuring Quality of Life. 2017.
- 25. Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med 2000;19:453–73. [DOI] [PubMed] [Google Scholar]
- 26. Jørgensen TS, Skougaard M, Taylor PC, et al. The parker model: applying a qualitative three-step approach to optimally utilize input from stakeholders when introducing new device technologies in the management of chronic rheumatic diseases. Patient 2018;11:515–26. 10.1007/s40271-018-0306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hetland ML. DANBIO--powerful research database and electronic patient record. Rheumatology 2011;50:69–77. 10.1093/rheumatology/keq309 [DOI] [PubMed] [Google Scholar]
- 28. Busija L, Buchbinder R, Osborne RH. A grounded patient-centered approach generated the personal and societal burden of osteoarthritis model. J Clin Epidemiol 2013;66:994–1005. 10.1016/j.jclinepi.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 29. Trochim W, Kane M. Concept mapping: an introduction to structured conceptualization in health care. Int J Qual Health Care 2005;17:187–91. 10.1093/intqhc/mzi038 [DOI] [PubMed] [Google Scholar]
- 30. MAXTM, 2016. Ithaca The Concept System Global MAXTM (Build 2016.046.12) [Web-based Platform] http://www.conceptsystemsglobal.com [Google Scholar]
- 31. Trochim WM, Cook JA, Setze RJ. Using concept mapping to develop a conceptual framework of staff’s views of a supported employment program for individuals with severe mental illness. J Consult Clin Psychol 1994;62:766–75. 10.1037/0022-006X.62.4.766 [DOI] [PubMed] [Google Scholar]
- 32. Trochim WM, Linton R. Conceptualization for planning and evaluation. Eval Program Plann 1986;9:289–308. 10.1016/0149-7189(86)90044-3 [DOI] [PubMed] [Google Scholar]
- 33. Crabtree BF, Miller WL. Using codes and code manuals—a template organizing style of interpretation : Crabtree BF, Miller WL, Doing qualitative research. 2nd ed Thousand Oaks: SAGE Publications Inc, 1999:163–77. [Google Scholar]
- 34. de Wit MP, Berlo SE, Aanerud GJ, et al. European league against rheumatism recommendations for the inclusion of patient representatives in scientific projects. Ann Rheum Dis 2011;70:722–6. 10.1136/ard.2010.135129 [DOI] [PubMed] [Google Scholar]
- 35. Gudbergsen H, Bartels EM, Krusager P, et al. Test-retest of computerized health status questionnaires frequently used in the monitoring of knee osteoarthritis: a randomized crossover trial. BMC Musculoskelet Disord 2011;12:190 10.1186/1471-2474-12-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pincus T. A multidimensional health assessment questionnaire (MDHAQ) for all patients with rheumatic diseases to complete at all visits in standard clinical care. Bull NYU Hosp Jt Dis 2007;65:150–60. [PubMed] [Google Scholar]
- 37. van der Roer N, Ostelo RW, Bekkering GE, et al. Minimal clinically important change for pain intensity, functional status, and general health status in patients with nonspecific low back pain. Spine 2006;31:578–82. 10.1097/01.brs.0000201293.57439.47 [DOI] [PubMed] [Google Scholar]
- 38. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–15. [DOI] [PubMed] [Google Scholar]
- 39. Vandenbroucke JP, Von Elm E, Altman DG, et al. [Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration]. Gac Sanit 2009;23:158 10.1016/j.gaceta.2008.12.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023915supp001.pdf (186.5KB, pdf)