Abstract
Objectives
Adolescent smoking has declined in New Zealand and in many other countries since the late 1990s, yet the reasons for the decline are not well understood. We investigated the extent to which established risk factors for adolescent smoking (parental, sibling and peer smoking, and exposure to smoking in the home) explained the downward trend.
Design
Trend analysis of repeat cross-sectional data from an annual nationally representative survey.
Setting
New Zealand.
Participants
Secondary school students aged 14–15 (n=398 221).
Outcome measure
Regular (at least monthly) smoking.
Methods
For each risk factor (parental smoking, best friend smoking, older sibling smoking and past week exposure to smoking in the home) we plotted prevalence of exposure, 2002–2015. Next, using multivariable logistic regression, we modelled the trend in regular smoking (expressed as an OR for year) adjusting for age, sex, ethnicity and socioeconomic position. The risk factors were added to the model—individually and collectively—to test whether they attenuated the OR for year.
Results
Exposure to all risk factors except ‘past week exposure to smoking in the home’ decreased between 2002 and 2015. We observed a strong downward trend in regular smoking among adolescents (OR=0.88 per year, 95% CI 0.88 to 0.88, p<0.001). ‘Best friend smoking’ was the only risk factor that significantly attenuated the trend. However, due to circularity, this factor provides an unsatisfactory explanation for population level smoking decline.
Conclusions
The established risk factors that we explored do not appear to have contributed to the remarkable decline in adolescent smoking in New Zealand between 2003 and 2015. Further research is needed to assess the possible contribution of factors outside our model, such as changes in the policy context, the social meaning of smoking and broader social and economic conditions.
Keywords: paediatrics, preventive medicine, public health, epidemiology
Strengths and limitations of this study.
The survey has a large sample size (n=20 443–31 833 per year), allowing precise population estimates based on individual-level data.
Due to data limitations, the study only includes a small number of risk factors, however the risk factors included have consistently been found to be among the strongest and most important predictors of adolescent smoking.
Our study design (using repeat cross sectional data) does not enable causal inferences to be drawn; rather our study draws on existing knowledge about the predictors of adolescent smoking initiation.
Introduction
Smoking is a leading cause of preventable illness and premature death,1 and a key driver of health disparities between ethnic and socioeconomic groups.2 3 Long-term tobacco use typically begins with experimental smoking in adolescence,4 and, internationally, considerable research and policy attention has focused on understanding and preventing smoking uptake in this age group.
The dramatic decline in adolescent smoking observed since the late 1990s in many high-income countries is good news from a public health perspective. In New Zealand (NZ), for example, regular smoking (defined as at least monthly) among adolescents aged 14–15 years declined from a peak of 29% in 1999 to 5% in 2015, with decreases across all main ethnic groups, and a convergence between boys and girls over the period.5 Over the same period, the proportion who had never smoked (ie, not even a few puffs) rose from 32% to 79%.5 However, as in other countries,6 ethnic and socioeconomic disparities remain pronounced. For example, among Māori (indigenous) smoking prevalence in this age group was 11% in 2015 compared with 4% among non-Māori.7
Other countries including the USA, England and Australia have also experienced a decline in adolescent smoking from the late 1990s, following a sharp rise in the early 1990s.8 It is important to understand the causes of this decline in order to help ensure it is sustained, and to enable replication in other countries. Yet little research has focused on explaining this phenomenon. Public health interventions such as increases in tobacco tax or smoke-free environment legislation may have played a role,9–11 but they do not fully explain the observed trends, since declines in adolescent smoking have occurred almost simultaneously in countries with widely differing regulatory contexts. This, and the fact that other adolescent risk behaviours (eg, alcohol use, teen pregnancy) have also declined over a similar time period,8 suggests that broader social or cultural changes rather than specific tobacco control policies may have contributed to this international trend.
Such shifts could be generated by new technologies, for example. When the use of cell phones rose and smoking fell among adolescents in the late 1990s, a causal association was hypothesised.12 More recently, attention has turned to other new technologies—smartphones and social media—and their potential role in driving generational change in attitudes and behaviour.13 There is face validity to the idea that these new technologies may have changed the way young people socialise or project their identity, displacing the role of smoking or providing less opportunity for it. However, this hypothesis is challenged by a consistent body of evidence showing a positive association between smoking and cell phone/internet/social media use at the individual level.14–23
Other major changes since the mid-90s that could potentially impact on youth behaviour include changes in parenting24–26; changes in the school environment and ethos27–29 and broad economic and labour market conditions resulting in young people leaving school and achieving independence later than previous cohorts.30 31 But before exploring these macro-level explanations for smoking decline, an initial step is to determine the extent to which the observed trends can be explained by changing exposure to established individual-level predictors of smoking initiation.
Proximal risk factors for adolescent smoking have been studied extensively. Parental, sibling and peer smoking have consistently been identified as key risk factors,4 32 with the Surgeon General’s 2012 evidence review concluding that the evidence is suggestive of a causal role for peer influences, and a potential causal role for parental smoking.4 The review found that smoking by older siblings influences smoking in adolescents more consistently than does smoking by parents.4 Exposure to smoking in the home, although a less studied factor, has also been shown to predict smoking in adolescents independent of parental smoking status in longitudinal and cross-sectional studies.33–37 Studies suggest secondhand smoke exposure may biologically predispose children to nicotine dependence38–42 in addition to providing pro-smoking socialisation.34 Could declining exposure to these proximal risk factors explain the dramatic decline in adolescent smoking since the turn of the century?
Despite extensive risk factor research, few studies have explored how exposure to risk factors has changed over time, or how such changes may be contributing to changes in adolescent smoking at the population level. The current study explores trends in exposure to known risk factors for adolescent smoking (parental, sibling and peer smoking and exposure to smoking in the home) and investigates the extent to which these risk factors could explain the declining trend in adolescent smoking in NZ from 2003 to 2015.
Methods
Data
We used repeat cross-sectional data from the Action on Smoking and Health (ASH) NZ Year 10 Snapshot Survey series, an annual school-based survey of adolescents aged 14–15 years which is administered by ASH NZ and is part of the New Zealand Youth Tobacco Monitor. The questionnaire includes a set of ‘core’ questions that have remained consistent over time to enable trend monitoring, and additional questions which change from year to year. Years included in the current study were 2002–2015, since key variables of interest were unavailable prior to 2002. Furthermore, exposure to smoking in the home was not included in the questionnaire in 2002 or 2004–2005, and therefore multivariable trend analysis includes only data from 2003 and 2006–2015.
All public and private schools with year-10 students were invited to participate in the ASH NZ Year 10 Snapshot each year. Table 1 shows the sample size and student response rate (as a proportion of the total NZ year-10 population) by year. Non-response was almost entirely at the school level, with school response rates ranging from 44% to 67%.43 (The lower school response rate in 2015 was due to limited resources for liaising with schools that year.)
Table 1.
Sample size and student response rate by year
| Year | NZ Year 10 population |
Valid survey responses* | Valid survey responses that met all study inclusion criteria | Proportion of Year-10 population that met all study inclusion criteria (%) |
| 2002 | 58 812 | 29 173 | 28 088 | 50 |
| 2003 | 61 028 | 32 705 | 31 377 | 54 |
| 2004 | 62 852 | 31 630 | 30 807 | 46 |
| 2005 | 64 619 | 32 561 | 31 833 | 51 |
| 2006 | 63 086 | 32 844 | 31 690 | 52 |
| 2007 | 62 012 | 25 978 | 25 109 | 42 |
| 2008 | 61 485 | 30 903 | 29 682 | 50 |
| 2009 | 61 355 | 25 757 | 24 755 | 42 |
| 2010 | 61 210 | 32 832 | 31 696 | 54 |
| 2011 | 59 562 | 26 856 | 26 028 | 45 |
| 2012 | 59 627 | 31 983 | 30 396 | 43 |
| 2013 | 57 929 | 28 340 | 27 014 | 49 |
| 2014 | 59 612 | 31 125 | 29 303 | 47 |
| 2015 | 59 528 | 21 567 | 20 443 | 36 |
| Total | 852 717 | 414 254 | 398 221 | 47 |
*Valid survey responses=those with complete data for age (14 or 15), sex, ethnicity and smoking status.
NZ, New Zealand.
Following previously published ASH NZ analyses, our analysis was restricted to respondents aged 14 or 15 at the time of the survey. For consistency between descriptive and multivariable (ie, adjusted) analyses, only respondents with complete data for all variables (smoking status, parental smoking, sibling smoking, best friend smoking, age, gender, ethnicity, school decile and school ID, and for 2003 and 2006–2015 exposure to smoking in the home) were included in the analyses. In addition, only schools with at least 20 respondents were included so that results were based on stable estimates of smoking in each school. Table 1 shows the number of valid survey responses received based on the ASH NZ criteria for inclusion (ie, those with complete data for age (14/15 years), sex, ethnicity and smoking status), and the number included in our study (after exclusions above), by year. After application of our additional inclusion criteria 96% (398 221/414 254) of valid responses were included.
The final included sample (n=398, 221) comprised approximately half the year-10 population each year, and closely resembled the population in respect of demographic characteristics. A detailed comparison of the final included sample and population, by year, is provided in online supplementary table S1, showing that the sample is broadly representative although with modest but consistent under-representation of Māori and students from low decile schools. (School decile is a school-level measure of the socioeconomic position of a school’s student community, explained further below.)44
bmjopen-2017-020320supp001.pdf (887.6KB, pdf)
Fieldwork was undertaken earlier in the year in 2011 and subsequently, meaning respondents were 2–3 months younger on average in 2011 and subsequent years, than in 2010 and prior years. Excluding the timing of fieldwork and changes to non-core questions, there has been consistency in survey instruments, administration and data management across included years.
In participating schools, the one-page survey is completed in class time under the supervision of teaching staff. Individual students may choose not to participate. To protect the confidentiality of students’ responses, identifying information is not collected, and teachers are requested not to check the completed surveys. Completed surveys are returned to ASH NZ which oversees data entry, cleaning and coding.
Details on survey methodology are available elsewhere.43
Variables
The outcome variable, ‘regular smoking’ (Y/N) was defined as smoking at least monthly, based on the question ‘How often do you smoke now?’ The answer categories were: ‘I have never smoked/I am not a smoker now’, ‘At least once a day’, ‘At least once a week’, ‘At least once a month’ and ‘Less often than once a month’.
Smoking status of mother, father, older sibling(s) and best friend were based on the question ‘Which of the following people smoke?’ with a dichotomous variable (current smoker, yes/no) created for each. Previous research shows that maternal smoking is more strongly associated with adolescent smoking initiation than paternal smoking,45 therefore we examined exposure to maternal and paternal smoking separately. For the purposes of multivariable analysis, parental smoking was grouped into one variable, coded 0=neither parent smokes, 1=only mother smokes, 2=only father smokes, 3=both parents smoke.
Past week exposure to smoking in the home was based on the question ‘During the past 7 days, on how many days have people smoked around you in your home?’ Response categories were 0 days, 1–2 days, 3–4 days, 5–6 days and 7 days. For descriptive analysis only, we recoded the responses into three categories: ‘Daily exposure’ (7 days) ‘less than daily exposure’ (1–6 days) and ‘no exposure’ (0 days).
In previous research, using the same data set, we confirmed that all the above risk factors were significantly associated with adolescent smoking, and that these associations remained significant throughout the study period.46
Demographic variables were age (14 or 15 years old), sex (male or female), ethnicity (prioritised Māori, Pacific, Asian, NZ European/other) and school decile. School decile is calculated by the Ministry of Education for purposes of funding allocation, and is a school-level measure of the socioeconomic position (SEP) of a school’s student community. Details of how school decile is calculated are available from the Ministry of Education.44 For descriptive analysis only, we grouped school decile into low (deciles 1–3: most deprived), medium (4–7) and high (8–10: least deprived). Each school also had an identification number (school ID) which was assigned to all respondents from that school.
Analysis
To describe trends we used SPSS (IBM Released 2016. IBM SPSS Statistics for Windows, V.24.0) to tabulate prevalence of regular smoking and prevalence of exposure to risk factors (overall and by sex, ethnicity and school decile) for each year. We then quantified the mean annual absolute change in proportion of respondents exposed to each risk factor using weighted linear regression (to adjust for differing variance by year by giving more weight to more accurate estimates of prevalence) with year as the independent variable. The weights were 1/SD2 of the proportions.
Next, for the years 2003 and 2005–2015, we conducted trend analyses based on individual-level data using multivariable logistic regression. We used SAS/STAT software (V.9.4 of the SAS system for Windows). To test the extent to which the risk factors of interest accounted for the change over time in adolescent smoking, we modelled regular smoking as a function of survey year, adjusting for demographic factors (age, sex, ethnicity and school decile), and including school ID as a random effect to account for clustering at the school level (model 1). We then added the risk factors of interest to model 1, first individually then collectively. Attenuation of the OR for year, which was tested using Z tests to compare log odds, would indicate that the risk factor (partially) accounted for the trend over time.
Initially, we modelled the trend using year as a continuous variable, which provided a single OR describing average annual change in the odds of regular smoking compared with the reference year, 2003. This approach assumes a linear trend over time which may not be valid, so we also modelled the trend using year as a categorical variable. This provided an OR for regular smoking for each survey year 2006–2016, compared with the reference year.
To test whether the results were the same for Māori adolescents as for the sample as a whole, we recoded ethnicity into Māori (yes/no) and repeated the trend analysis above for Māori only.
Patient and public involvement
Patients were not involved in the design or conduct of this study, and nor were members of the general public.
Results
Prevalence of regular smoking
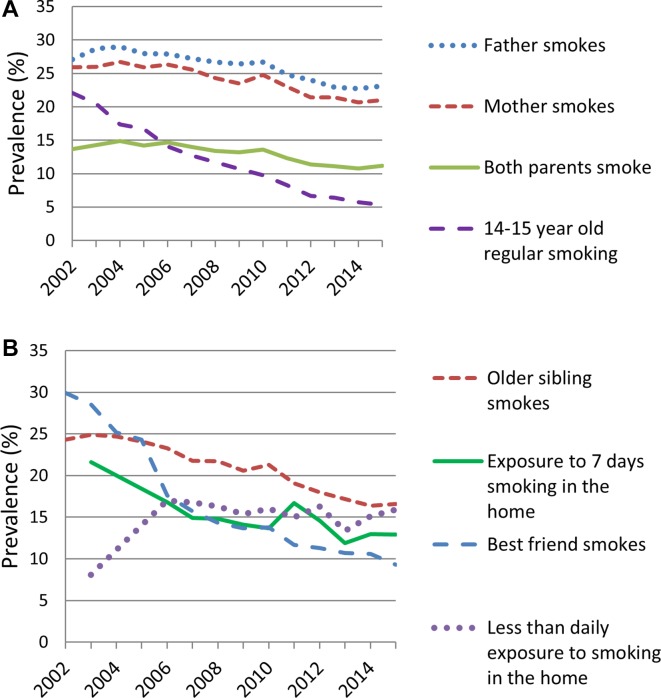
There was a long-term decline in prevalence of regular smoking among adolescents aged 14–15 years from 22% in 2002 to 5% in 2015 (figure 1). Based on weighted linear regression, the overall regular smoking rate reduced by an average of 1.2% per year (in absolute terms) from 2002 to 2015.
Figure 1.
Prevalence of regular smoking and risk factors in adolescents aged 14-15 years, 2002–2015. (A) Trends in adolescent and parental smoking prevalence (B) Trends in exposure to other risk factors.
Online supplementary figures S1–S3 show trends in prevalence of regular smoking stratified by ethnicity, school decile and gender, and indicate that smoking has declined in all demographic groups. Furthermore, ethnic, SEP and gender disparities have narrowed over time in absolute terms.
Changes in exposure to risk factors over time
Trends in exposure to risk factors are shown in figure 1. Parental smoking (figure 1A) declined only modestly over the study period with maternal and paternal smoking both declining by an average rate of 0.5% per annum. As shown in figure 1B, smoking among older siblings declined slightly more, at an average rate of 0.7% per annum, and ‘best friend smokes’ had the highest rate of decline at 1.5% per annum. Past week exposure to smoking in the home did not change significantly over the study period. Prevalence of daily exposure to smoking in the home fell from 22% to 13% overall (an average decrease of 0.6% per annum), however less than daily exposure increased over the study period.
Online supplementary figures S4–S10 show exposure to risk factors over time by ethnicity and school decile. They show that trends in exposure to risk factors followed a similar pattern in all ethnic and SEP subgroups, but disparities in levels of exposure were marked at all years.
Trend analyses
Results of the trend analyses are shown in table 2. We observed a strong downward trend in regular smoking, with an OR of 0.88 per year (95% CI 0.88 to 0.88, p<0.001) based on the linear trend. When ‘best friend smokes’ was added to the model (model 1+best friend smokes) the size of the OR declined significantly (model 1), indicating that this risk factor partially (but not fully) accounted for the declining trend in adolescent smoking between 2003 and 2015. None of the other risk factors, when added to model 1, significantly attenuated the OR for year relative to the reference year, indicating that, individually, they did not contribute to the trend.
Table 2.
Results of multiple logistic regression analyses examining the impact of risk factors on the trend in regular smoking in adolescents
| Year | Model 1: OR for year partially adjusted (95% CI) |
Model 1+best friend smokes (95% CI) |
Model 1+ exposure to smoking in home (95% CI) |
Model 1+ parental smoking |
Model 1+ sibling smoking |
Model 1+all risk factors |
| 2003 | 1 | |||||
| 2006 | 0.62 (0.59 to 0.64) | 0.83* (0.78 to 0.87) | 0.58 (0.55 to 0.61) | 0.60 (0.57 to 0.63) | 0.61(0.58 to 0.64) | 0.75* (0.71 to 0.79) |
| 2007 | 0.56 (0.53 to 0.59) | 0.78* (0.74 to 0.83) | 0.54 (0.52 to 0.57) | 0.55 (0.52 to 0.58) | 0.56 (0.54 to 0.59) | 0.72* (0.68 to 0.76) |
| 2008 | 0.51 (0.48 to 0.53) | 0.74* (0.70 to 0.79) | 0.49 (0.47 to 0.52) | 0.50 (0.48 to 0.53) | 0.51 (0.48 to 0.53) | 0.68* (0.64 to 0.72) |
| 2009 | 0.47 (0.44 to 0.49) | 0.69*(0.65 to 0.73) | 0.46 (0.43 to 0.49) | 0.46 (0.44 to 0.49) | 0.47 (0.45 to 0.50) | 0.63* (0.59 to 0.67) |
| 2010 | 0.40 (0.38 to 0.42) | 0.58*(0.54 to 0.61) | 0.40 (0.38 to 0.42) | 0.39 (0.37 to 0.41) | 0.40 (0.38 to 0.42) | 0.53* (0.50 to 0.57) |
| 2011 | 0.36 (0.34 to 0.38) | 0.55* (0.51 to 0.58) | 0.33 (0.31 to 0.35) | 0.36 (0.34 to 0.38) | 0.37 (0.35 to 0.39) | 0.47* (0.44 to 0.51) |
| 2012 | 0.28 (0.27 to 0.30) | 0.42* (0.39 to 0.45) | 0.26 (0.25 to 0.28) | 0.29 (0.27 to 0.30) | 0.29 (0.27 to 0.31) | 0.37* (0.35 to 0.40) |
| 2013 | 0.27 (0.25 to 0.28) | 0.41* (0.39 to 0.44) | 0.28 (0.26 to 0.29) | 0.27 (0.26 to 0.29) | 0.28 (0.26 to 0.30) | 0.39* (0.37 to 0.42) |
| 2014 | 0.24 (0.22 to 0.25) | 0.36* (0.34 to 0.39) | 0.23 (0.22 to 0.25) | 0.24 (0.23 to 0.26) | 0.25 (0.24 to 0.27) | 0.34* (0.32 to 0.36) |
| 2015 | 0.21 (0.19 to 0.22) | 0.34* (0.32 to 0.37) | 0.20 (0.19 to 0.22) | 0.21 (0.20to 0.23) | 0.22 (0.21 to 0.24) | 0.31* (0.29 to 0.34) |
| All years combined, using year as a continuous variable | ||||||
| Linear trend (2003–2015) | 0.88 (0.88 to 0.88) | 0.91* (0.91 to 0.92) | 0.88 (0.88 to 0.88) | 0.88 (0.88 to 0.89) | 0.88(0.88 to 0.89) | 0.91* (0.90 to 0.91) |
Model 1 is adjusted for age, gender, ethnicity and school decile.
*OR >model 1 OR (p<0.05).
When all four risk factors were entered into the model together (model 1+all risk factors), the attenuation of the OR was significant (p<0.05) but the magnitude of the change was no greater than for ‘model 1+best friend smokes’.
The pattern of results described above was observed regardless of whether year was used as a categorical variable (modelling change relative to 2003 for each year), or a continuous variable (modelling the linear trend, to give an annual average change over the study period, as shown in the final row of table 2). The same patterns were also seen in Māori respondents (see online supplementary table S2).
There was a residual effect of year (ie, unexplained change over time indicated by an OR for year that was significantly less than 1) in all the models, including the fully adjusted model. This suggests that there were factors outside our fully adjusted model that were influencing the change over time in smoking prevalence.
Discussion
Exposure to best friend smoking declined strongly during the 2002–2015 period, while exposure to other established risk factors for smoking decreased more modestly, if at all. There was no change in past week exposure to smoking in the home overall (ie, 1–7 days), but daily exposure fell significantly, while less than daily exposure increased. The primary aim of this study was to determine whether these known risk factors explained (in statistical terms) the dramatic decline in adolescent smoking seen recently in NZ. Despite declining exposure to many of the included risk factors, modelling showed that most of the factors we explored (parental and sibling smoking, and exposure to smoking in the home) did not account for the trend in any measurable way, either individually or collectively.
Only ‘best friend smokes’ appeared to contribute to the declining trend in adolescent smoking. This was unsurprising, given that exposure to this risk factor declined markedly over the study period, and a previous study using the same data set has shown that the smoking status of respondents’ best friend was by far the strongest risk factor for regular smoking in NZ adolescents aged 14–15 years of the factors we explored.46 However, research on peer influence suggests that causality is likely to be bidirectional, and the association is due, in part, to smokers seeking out other smokers as friends.4 Furthermore, at the population level, it would be a circular to suggest that declining best friend smoking explained the decline in adolescent smoking, since survey respondents and their best friends belong to the same cohort of adolescents in which smoking is declining. The question remains: if decreases in best friend smoking are resulting in reduced risk of adolescent smoking at the individual level, what is driving the decline in best friend smoking?
One possibility is that, since younger adolescents are strongly influenced by peers and adolescents slightly older than themselves, a virtuous cycle may have developed whereby a decline in adolescent smoking at time 1 has led to a subsequent decline in adolescent smoking at time 2 and so on. Further research, perhaps drawing on communicable disease methodology, could test this hypothesis and explore how the ‘social transmission’ of smoking (and other health risk behaviours) influences population prevalence over time. Should this hypothesis prove to be correct, the trigger for the sudden change from rapidly rising to rapidly falling adolescent tobacco use from the late 1990s to the early 2000s still remains to be identified.
Our findings suggest that there are other factors influencing the decline in adolescent smoking that this study did not address. For example, it is possible that changes in the social meaning of smoking47 48 and the policy context11 49—factors that were not included in our analyses—may have played a role in triggering adolescent smoking decline. For example, policy responses to rising adolescent smoking in the 1990s may have influenced teen smoking in NZ, as they appear to have done in Australia,10 11 the UK6 and the USA.9 In NZ, such policy responses included raising the legal age of tobacco purchase from 16 to 18 years of age in 1997, a tax increase which raised the price of a packet of 20 cigarettes by 13% in 199850 and the ‘Why start?’ mass media campaign which ran from 1996 to 1998. It is plausible that, collectively, these measures contributed to the denormalisation of smoking which, together with any specific intervention effects, may have been the trigger for adolescent smoking decline both in NZ and other jurisdictions. Mass media campaigns from 2000 focusing on secondhand smoke and a 2004 ban on smoking in pubs and all other indoor workplaces likely contributed to the ongoing denormalisation of smoking (in particular indoor smoking) in NZ,51 and may underpin the observed decline in daily exposure to smoking in the home. As Simon Chapman has pointed out, denormalisation involves an ‘interplay of continuous, uncontrolled, unmeasured, and sometimes unmeasurable variables intended to influence [tobacco] consumption’, and cannot be reduced to the sum of its parts.52
However, if tobacco denormalisation, along with the other factors discussed above, explains the decline in adolescent smoking, is it simply a coincidence that adolescent alcohol use, teen pregnancy and juvenile crime have also declined over the same period? Or does this suggest there are additional overarching influences that are impacting on a range of adolescent risk-taking behaviours?
As far as we are aware, this is the first study to explore trends in exposure to known risk factors with the purpose of better understanding the drivers of the decline in adolescent smoking prevalence. Definitively establishing the reason(s) for the decline in adolescent smoking is not possible using repeat cross-sectional data (or indeed via any single study). However, trend analysis using statistical modelling allowed us to explore the relationships between survey year, risk factors and outcomes, and thereby (potentially) account for changes over time in statistical terms. This approach has allowed us to rule out hypothesised explanations for population level change over time, and adds to the evidence base about the most likely explanations for the decline of smoking in young people.
Strengths of the study include the large sample size, and demographic similarity between the sample and the year-10 population, suggesting response bias was not a substantial issue. Systematic under-representation and over-representation were found to be relatively consistent over time and therefore unlikely to affect trend analysis which was the focus of our study. The methods for the ASH NZ survey were broadly consistent between years, with minor changes (eg, a change in fieldwork timing from 2011) unlikely to contribute significantly to the trends observed. Since there is a strong similarity between NZ and other countries at a late stage in the tobacco epidemic in terms of trends in adolescent smoking and known risk factors, it is likely that our conclusions may be generalisable to similar countries, but this remains to be confirmed through further research.
Given the complex array of factors at various levels that are known to influence smoking uptake, one of the limitations of our study was the limited number of risk factors for which consistent data were available. Clearly, there are other contributing factors, and our study was unable to explore these. The study was based on self-report questionnaire data, with its inherent limitations (eg, potential for social desirability bias, and misinterpretation of questions resulting in misclassification); however, recent biomarker testing of a subsample of ASH NZ year-10 participants indicated that the survey provides an accurate population estimate of smoking prevalence.53 We used school decile as a proxy for SEP, since more direct measures were unavailable. Because school communities are heterogeneous, it is an imperfect measure at the individual level, and residual confounding by SEP is possible in our adjusted analyses.
Conclusions
In summary, our findings suggest that the remarkable decline in adolescent smoking in NZ cannot be explained by declining exposure to parental smoking, sibling smoking or past week exposure to smoking in the home. These factors have not contributed measurably to the trend, either individually or collectively. Declining ‘best friend smoking’ partially accounts for declining adolescent smoking in our statistical model, but this finding contributes little to our understanding of the drivers of population-level decline since respondents and their best friends largely come from the same population. It is clear that factors other than those in our model are at play, with changes in the social meaning of smoking, the policy context and broader sociocultural changes all potential contributors. Further research is needed to identify other contributing factors and determine their relative importance.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the data owners, ASH New Zealand, for access to the ASH NZ Year 10 Snapshot Survey data. The ASH Year 10 Snapshot Survey is part of the New Zealand Youth Tobacco Monitor, a national, school-based survey of young people’s smoking behaviours, attitudes and associated risk factors. We also thank the advisory group who provided project oversight, feedback and/or technical advice. In particular we thank Rob McGee (University of Otago), Sicily Sunseri (Health Promotion Agency), and Andrew Waa (University of Otago) for feedback on earlier drafts.
Footnotes
Contributors: All authors contributed to the conception and design of the study. The analysis was executed by DS and JB, with oversight by RE. JB drafted the manuscript with input from all authors.
Funding: This work was supported by a University of Otago Research Grant 507415.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The survey was approved, as a component of the NZ Youth Tobacco Monitor, by the Ministry of Health Multiregional Health and Disability Ethics Committee in 2007.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data are owned by ASH New Zealand, and cannot be shared by the authors.
References
- 1. Ministry of Health. Health loss in New Zealand 1990-2013: a report from the New Zealand burden of diseases, injuries and risk factors study. Wellington: Ministry of Health, 2016. [Google Scholar]
- 2. Blakely T, Fawcett J, Hunt D, et al. What is the contribution of smoking and socioeconomic position to ethnic inequalities in mortality in New Zealand? Lancet 2006;368:44–52. 10.1016/S0140-6736(06)68813-2 [DOI] [PubMed] [Google Scholar]
- 3. David A, Esson K, Perucic A-M, et al. Tobacco use: equity and social determinants : B E, Sivasankara Kurup A, Equity, social determinants and public health programmes. Geneva: World Health Organization, 2010. [Google Scholar]
- 4. Centers for Disease Control. Preventing tobacco use among youth and young adults: a report of the Surgeon General. Atlanta, USA: Centers for Disease Control and Prevention, 2012. [PubMed] [Google Scholar]
- 5. Action on Smoking and Health. ASH year 10 snapshot survey 2015: factsheet 1 topline results. Auckland: ASH New Zealand, 2018. [Google Scholar]
- 6. Green MJ, Leyland AH, Sweeting H, et al. Socioeconomic position and early adolescent smoking development: evidence from the British Youth Panel Survey (1994-2008). Tob Control 2016;25:203–10. 10.1136/tobaccocontrol-2014-051630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Action on Smoking and Health. ASH year 10 snapshot survey 2015: factsheet 4 Maori smoking. Auckland, New Zealand: ASH New Zealand, 2018. [Google Scholar]
- 8. Ball J, Edwards R, Cook H. Why are today’s adolescents less likely to smoke, drink, take drugs or have sex than those in the 1990s? 15th World Congress on Public Health. Melbourne, Australia, 2017. [Google Scholar]
- 9. Pampel FC, Aguilar J. Changes in youth smoking, 1976-2002: a time-series analysis. Youth Soc 2008;39:453–79. 10.1177/0044118X07308070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White VM, Durkin SJ, Coomber K, et al. What is the role of tobacco control advertising intensity and duration in reducing adolescent smoking prevalence? Findings from 16 years of tobacco control mass media advertising in Australia. Tob Control 2015;24:198–204. 10.1136/tobaccocontrol-2012-050945 [DOI] [PubMed] [Google Scholar]
- 11. White VM, Warne CD, Spittal MJ, et al. What impact have tobacco control policies, cigarette price and tobacco control programme funding had on Australian adolescents’ smoking? Findings over a 15-year period. Addiction 2011;106:1493–502. 10.1111/j.1360-0443.2011.03429.x [DOI] [PubMed] [Google Scholar]
- 12. Charlton A, Bates C. Decline in teenage smoking with rise in mobile phone ownership: hypothesis. BMJ 2000;321:1155 10.1136/bmj.321.7269.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Twenge JM. IGen: Why Today’s Super-Connected Kids Are Growing Up Less Rebellious, More Tolerant, Less Happy-and Completely Unprepared for Adulthood. New York: Simon and Schuster, 2017. [Google Scholar]
- 14. Durkee T, Carli V, Floderus B, et al. Pathological internet use and risk-behaviors among European Adolescents. Int J Environ Res Public Health 2016;13:294 10.3390/ijerph13030294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gommans R, Stevens GW, Finne E, et al. Frequent electronic media communication with friends is associated with higher adolescent substance use. Int J Public Health 2015;60:167–77. 10.1007/s00038-014-0624-0 [DOI] [PubMed] [Google Scholar]
- 16. Huang GC, Okamoto J, Valente TW, et al. Effects of media and social standing on smoking behaviors among adolescents in China. J Child Media 2012;6:100–18. 10.1080/17482798.2011.633411 [DOI] [Google Scholar]
- 17. Iannotti RJ, Kogan MD, Janssen I, et al. Patterns of adolescent physical activity, screen-based media use, and positive and negative health indicators in the U.S. and Canada. J Adolesc Health 2009;44:493–9. 10.1016/j.jadohealth.2008.10.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koivusilta L, Lintonen T, Rimpelä A. Mobile phone use has not replaced smoking in adolescence. BMJ 2003;326:161 10.1136/bmj.326.7381.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leena K, Tomi L, Arja RR. Intensity of mobile phone use and health compromising behaviours–how is information and communication technology connected to health-related lifestyle in adolescence? J Adolesc 2005;28:35–47. 10.1016/j.adolescence.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 20. Meldrum RC, Clark J. Adolescent virtual time spent socializing with peers, substance use, and delinquency. Crime & Delinquency 2015;61:1104–26. 10.1177/0011128713492499 [DOI] [Google Scholar]
- 21. Morioka H, Itani O, Osaki Y, et al. Association between smoking and problematic internet use among Japanese adolescents: large-scale nationwide epidemiological study. Cyberpsychol Behav Soc Netw 2016;19:557–61. 10.1089/cyber.2016.0182 [DOI] [PubMed] [Google Scholar]
- 22. Osaki Y, Ohida T, Kanda H, et al. Mobile phone use does not discourage adolescent smoking in Japan. Asian Pac J Cancer Prev 2012;13:1011–4. 10.7314/APJCP.2012.13.3.1011 [DOI] [PubMed] [Google Scholar]
- 23. Steggles N, Jarvis MJ. Do mobile phones replace cigarette smoking among teenagers? Tob Control 2003;12:339-a–40. 10.1136/tc.12.3.339-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gauthier AH, Smeeding TM, Furstenberg FF. Are parents investing less time in children? Trends in selected industrialized countries. Popul Dev Rev 2004;30:647–72. 10.1111/j.1728-4457.2004.00036.x [DOI] [Google Scholar]
- 25. Guryan J, Hurst E, Kearney M. Parental education and parental time with children. Journal of Economic Perspectives 2008;22:23–46. 10.1257/jep.22.3.23 [DOI] [Google Scholar]
- 26. Morman MT, Floyd K. A “changing culture of fatherhood”: effects on affectionate communication, closeness, and satisfaction in men’s relationships with their fathers and their sons. West J Commun 2002;66:395–411. 10.1080/10570310209374746 [DOI] [Google Scholar]
- 27. Thapa A, Cohen J, Guffey S, et al. A review of school climate research. Rev Educ Res 2013;83:357–85. 10.3102/0034654313483907 [DOI] [Google Scholar]
- 28. Cornell D, Huang F. Authoritative school climate and high school student risk behavior: a cross-sectional multi-level analysis of student self-reports. J Youth Adolesc 2016;45:2246–59. 10.1007/s10964-016-0424-3 [DOI] [PubMed] [Google Scholar]
- 29. Jamal F, Fletcher A, Harden A, et al. The school environment and student health: a systematic review and meta-ethnography of qualitative research. BMC Public Health 2013;13:798 10.1186/1471-2458-13-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Settersten RA, Ray B. What’s going on with young people today? The long and twisting path to adulthood. Future Child 2010;20:19–41. 10.1353/foc.0.0044 [DOI] [PubMed] [Google Scholar]
- 31. Rea D, Callister P. The changing nature of young people’s transitions in New Zealand. Wellington: Institute of Policy Studies, Victoria University, 2009. [Google Scholar]
- 32. Tyas SL, Pederson LL. Psychosocial factors related to adolescent smoking: a critical review of the literature. Tob Control 1998;7:409–20. 10.1136/tc.7.4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Darling H, Reeder A. Is exposure to secondhand tobacco smoke in the home related to daily smoking among youth? Aust N Z J Public Health 2003;27:655–6. 10.1111/j.1467-842X.2003.tb00617.x [DOI] [PubMed] [Google Scholar]
- 34. Waa A, Edwards R, Newcombe R, et al. Parental behaviours, but not parental smoking, influence current smoking and smoking susceptibility among 14 and 15 year-old children. Aust N Z J Public Health 2011;35:530–6. 10.1111/j.1753-6405.2011.00772.x [DOI] [PubMed] [Google Scholar]
- 35. Becklake MR, Ghezzo H, Ernst P. Childhood predictors of smoking in adolescence: a follow-up study of Montreal schoolchildren. CMAJ 2005;173:377–9. 10.1503/cmaj.1041428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang MP, Ho SY, Lam TH. Parental smoking, exposure to secondhand smoke at home, and smoking initiation among young children. Nicotine Tob Res 2011;13:827–32. 10.1093/ntr/ntr083 [DOI] [PubMed] [Google Scholar]
- 37. Voorhees CC, Ye C, Carter-Pokras O, et al. Peers, tobacco advertising, and secondhand smoke exposure influences smoking initiation in diverse adolescents. Am J Health Promot 2011;25:e1–11. 10.4278/ajhp.090604-QUAN-180 [DOI] [PubMed] [Google Scholar]
- 38. Selya AS, Dierker LC, Rose JS, et al. Risk factors for adolescent smoking: parental smoking and the mediating role of nicotine dependence. Drug Alcohol Depend 2012;124:311–8. 10.1016/j.drugalcdep.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bélanger M, O’Loughlin J, Okoli CT, et al. Nicotine dependence symptoms among young never-smokers exposed to secondhand tobacco smoke. Addict Behav 2008;33:1557–63. 10.1016/j.addbeh.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schuck K, Kleinjan M, Otten R, et al. Responses to environmental smoking in never-smoking children: can symptoms of nicotine addiction develop in response to environmental tobacco smoke exposure? J Psychopharmacol 2013;27:533–40. 10.1177/0269881112466184 [DOI] [PubMed] [Google Scholar]
- 41. Okoli CT, Kelly T, Hahn EJ. Secondhand smoke and nicotine exposure: a brief review. Addict Behav 2007;32:1977–88. 10.1016/j.addbeh.2006.12.024 [DOI] [PubMed] [Google Scholar]
- 42. Brody AL, Mandelkern MA, London ED, et al. Effect of secondhand smoke on occupancy of nicotinic acetylcholine receptors in brain. Arch Gen Psychiatry 2011;68:953–60. 10.1001/archgenpsychiatry.2011.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Action on Smoking and Health. 2014 ASH year 10 snapshot survey: information and methodology. Auckland: Action on Smoking and Health, 2014. [Google Scholar]
- 44. Ministry of Education, 2017. School deciles: Ministry of Education https://education.govt.nz/school/running-a-school/resourcing/operational-funding/school-decile-ratings/ (accessed 29 Jun 2017).
- 45. Leonardi-Bee J, Jere ML, Britton J. Exposure to parental and sibling smoking and the risk of smoking uptake in childhood and adolescence: a systematic review and meta-analysis. Thorax 2011;66:847–55. 10.1136/thx.2010.153379 [DOI] [PubMed] [Google Scholar]
- 46. Ball J, Sim D, Edwards R. Addressing ethnic disparities in adolescent smoking: Is reducing exposure to smoking in the home a key? Nicotine Tob Res 2018. [Epub ahead of print 15 Mar 2018]. 10.1093/ntr/nty053 [DOI] [PubMed] [Google Scholar]
- 47. Plumridge EW, Fitzgerald LJ, Abel GM. Performing coolness: smoking refusal and adolescent identities. Health Educ Res 2002;17:167–79. 10.1093/her/17.2.167 [DOI] [PubMed] [Google Scholar]
- 48. Nichter M. Smoking: what does culture have to do with it? Addiction 2003;98(Suppl 1):139–45. 10.1046/j.1360-0443.98.s1.9.x [DOI] [PubMed] [Google Scholar]
- 49. Dessaix A, Maag A, McKenzie J, et al. Factors influencing reductions in smoking among Australian adolescents. Public Health Res Pract 2016;26:e2611605 doi:10.17061/phrp2611605 [DOI] [PubMed] [Google Scholar]
- 50. O’Dea D, Thomson G. Report on tobacco taxation in New Zealand Volume I. Wellington, New Zealand, 2007. [Google Scholar]
- 51. Edwards R, Thomson G, Wilson N, et al. After the smoke has cleared: evaluation of the impact of a new national smoke-free law in New Zealand. Tob Control 2008;17:e2 10.1136/tc.2007.020347 [DOI] [PubMed] [Google Scholar]
- 52. Chapman S. Unravelling gossamer with boxing gloves: problems in explaining the decline in smoking. BMJ 1993;307:429–32. 10.1136/bmj.307.6901.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sunseri S, White J. Class, please spit: using a biochemical validation of self-reported smoking in a national sample of young people. 13th Behavioural Research in Cancer Control Conference. Melbourne, Australia: Cancer Council Victoria, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-020320supp001.pdf (887.6KB, pdf)