Abstract
Introduction
Although sleep-disordered breathing has been found to be associated with higher urine albumin excretion, this association has not been evaluated in Hispanic/Latino populations, which experience a high burden of end-stage renal disease compared with non-Hispanics. We evaluated the association of sleep-disordered breathing with prevalent albuminuria among US Hispanics/Latinos.
Methods
This was a cross-sectional study of baseline data from participants in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a population-based study that enrolled 16,415 adults in 4 US communities. Sleep-disordered breathing was assessed using a home sleep apnea monitor for overnight recording and was defined using 3 thresholds of the apnea−hypopnea index (AHI; 3% desaturation): ≥5, ≥15, and ≥30. Albuminuria was defined as a urine albumin-to-creatinine ratio of ≥30 mg/g.
Results
There were 12,572 participants with complete data available for analysis. The age- and sex-adjusted prevalence of albuminuria was 9.1%. Mean age was 41 years, and 48% were men. Age- and sex-adjusted prevalence of sleep-disordered breathing was higher among individuals with albuminuria compared with those without albuminuria (36% vs. 25% had AHI ≥5, 18% vs. 9% had AHI ≥15, and 9% vs. 4% had AHI ≥30). In multivariable logistic regression analyses, AHIs ≥5, ≥15, and ≥30 were associated with greater odds of albuminuria compared with those with AHIs <5, <15, and <30 (odds ratio [OR] 1.42, 95% confidence interval [CI]: 1.14−1.76; OR: 1.71, 95% CI: 1.33−2.20; and OR 1.93, 95% CI 1.34−2.79), respectively. This association varied by Hispanic/Latino background group.
Conclusion
In US Hispanic/Latinos, sleep-disordered breathing was independently associated with higher odds of prevalent albuminuria.
Keywords: chronic kidney disease, Hispanics/Latinos, sleep apnea
Chronic kidney disease (CKD) is a global public health problem that affects 30 million people living in the United States.1 According to the US Renal Data System, the incident rate of end-stage renal disease is nearly 34% higher in Hispanics compared with non-Hispanics.2 Moreover, the burden of albuminuria is higher among Hispanics/Latinos.3, 4, 5, 6 Albuminuria is a well-established risk factor for CKD progression and cardiovascular events.7, 8, 9, 10 In a study from the Multiethnic Study of Atherosclerosis, Hispanics were found to have a nearly 2-fold higher prevalence of albuminuria compared with whites,5 which might be, in part, due to the higher prevalence of diabetes and obesity among Hispanics/Latinos compared with non-Hispanic whites.11, 12, 13, 14 These health-related disparities underscore the importance of identifying potentially modifiable risk factors for early CKD among Hispanics/Latinos.
Sleep-disordered breathing encompasses a spectrum of disorders characterized by breathing pauses during sleep, the most common of which is obstructive sleep apnea.15 Increasing evidence from epidemiologic and laboratory studies suggest that there is a significant association between sleep-disordered breathing and higher prevalence and severity of CKD risk factors, including hypertension, diabetes, and cardiovascular disease.16, 17, 18, 19
Observational studies have shown that sleep-disordered breathing is associated with higher urine albumin excretion.20, 21 However, the representation of Hispanic/Latinos individuals in these studies has been minimal. In a recent analysis from the HCHS/SOL, sleep-disordered breathing was found to be highly prevalent in US Hispanics/Latinos and was associated with diabetes and hypertension,22 which are major risk factors for kidney disease. We studied the cross-sectional association of sleep-disordered breathing with albuminuria in US Hispanics/Latinos.
Methods
Study Participants
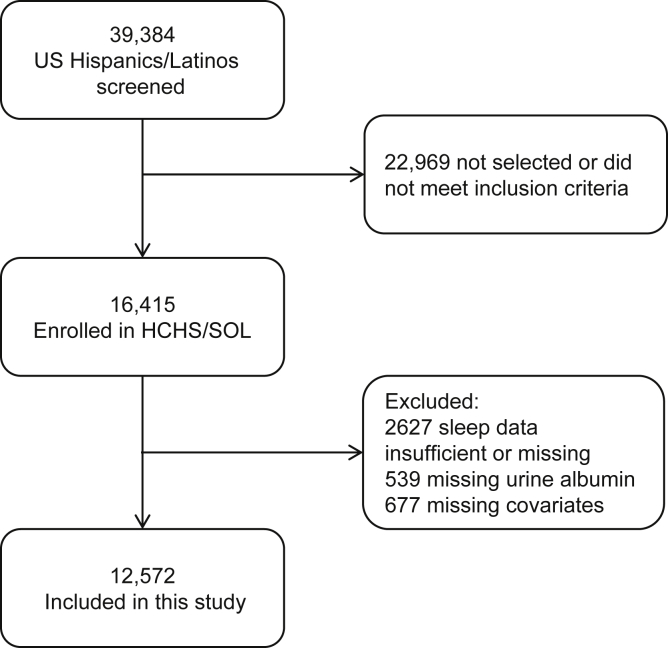
HCHS/SOL is a community-based cohort of Hispanics/Latinos from randomly selected households in 4 US field centers (Chicago, IL; Miami, FL; Bronx, NY; San Diego, CA) with baseline examinations (2008−2011) and yearly telephone follow-up assessment. Of 39,384 individuals who were screened and selected, and who met eligibility criteria, 41.7% were enrolled, representing 16,415 persons from 9872 households. Eligibility was defined as at least 1 Hispanic/Latino household member aged 18 to 74 years. The sample design and cohort selection were previously described.23, 24 Briefly, a stratified 2-stage area probability sample of household addresses was selected in each field center. The first sampling stage randomly selected census block groups with stratification based on Hispanic/Latino concentration and proportion of high and/or low socioeconomic status. The second sampling stage randomly selected households, with stratification, from US Postal Service registries that covered the randomly selected census block groups. Lastly, the study oversampled the 45- to 74-years age group (n = 9714; 59.2%) to facilitate examination of target outcomes. Sampling weights were generated to reflect the probabilities of selection at each stage. Participants self-reported their background as Cuban, Dominican, Mexican, Puerto Rican, Central American, or South American. The category “other” was used for participants belonging to a group not listed or to those who belonged to >1 group. All HCHS/SOL participants were eligible to be included in the present study. We used information from 12,572 participants with complete data (2627 individuals did not undergo the sleep study had insufficient sleep data for analysis, or had missing sleep questionnaire data; 539 had missing urine albumin measurements; and 677 had missing covariate data) (Figure 1). This study was approved by the institutional review boards for the reading centers, coordinating center, and at each field center where all participants gave written consent. The study adheres to the Declaration of Helsinki.
Figure 1.
Flowchart of participants included in the study. HCHS/SOL, Hispanic Community Health Study/Study of Latinos.
Data Collection and Variable Definition
The baseline study examination included clinical measurements, questionnaires, and fasting venous blood and urine specimens. Demographic factors, socioeconomic status, cigarette smoking, place of birth, language of interview, and medical history were obtained using standard questionnaires administered in Spanish or English depending on the participant’s language preference. Acculturation, which refers to the cultural modifications that occur as one takes on attitudes, customs, traditions, and behaviors of another culture,25 was measured using a modified version of the Short Acculturation Scale for Hispanics.26 Medication use, including sleeping aids, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs), was ascertained by conducting a scanned inventory of all currently used medications. Blood pressure (BP) was defined as the average of 3 repeat seated measurements obtained after a 5-minute rest. Hypertension was defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or use of antihypertensive medication. Diabetes mellitus was defined as fasting plasma glucose of ≥126 mg/dl, 2-hour postload glucose levels of ≥200 mg/dl, hemoglobin A1c level of ≥6.5%, or use of antidiabetic medication. The presence of cardiovascular disease was self-reported.
Sleep Variables
The main predictor for this study was the AHI, which is defined as the number of respiratory events per hour of sleep, with associated desaturations of ≥3%. Participants were instructed on the use of a sleep apnea monitor for overnight recording (Apnea Risk Evaluation System [ARES] Unicorder 5.2; B-Alert, Carlsbad, CA).27 This self-applied device measures airflow using the following: a nasal pressure cannula and transducer; hemoglobin oxygen saturation and pulse rate using finger pulse oximetry; head movement and position via accelerometers; and a microphone to record snoring levels. The ARES device does not include electroencephalography; therefore, the sleep time used to calculate AHI was estimated rather than measured. Sleep records were scored at a central sleep reading center. Respiratory events were identified as a ≥50% reduction in airflow lasting ≥10 seconds. Overall, 84.4% of sleep studies were graded as “excellent” quality (>3.8 hours of interpretable signals), whereas 5.8% of studies were of insufficient quality for analysis (<30 minutes of recording).22 For statistical analyses, we used AHI as a continuous variable and as a categorical variable. Three thresholds of AHI were used to define sleep apnea, AHI ≥5, ≥15, and ≥30, indicating minimal, moderate, and severe sleep-disordered breathing.28 In addition, we evaluated nocturnal hypoxemia, which was expressed as the percent of time during sleep in which arterial oxygen saturation (SpO2) was <90%. Daytime sleepiness was evaluated using the Epworth Sleepiness Scale.29 Data regarding sleep duration and symptoms were self-reported using the Sleep Heart Health Study Sleep Habits Questionnaire.30 Sleep duration was computed using self-reported bed and wake times on weekdays and weekends.
Measures of Kidney Function
Albuminuria was defined as a urine albumin-to-creatinine ratio (UACR) of ≥30 mg/g. Urine creatinine was measured in serum and urine on a Roche Modular P Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN) using a creatinase enzymatic method. Urine albumin was measured using an immunoturbidometric method on the ProSpec nephelometric analyzer (Dade Behring GMBH, Marburg, Germany). The estimated glomerular filtration rate (eGFR) was assessed using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine-cystatin C equation.31 Serum creatinine measurements were traced by isotope dilution mass spectrometry. Serum cystatin C was measured using a turbidimetric method on the Roche Modular P Chemistry Analyzer (Gentian AS, Moss, Norway).
Statistical Analyses
Summary statistics, prevalence estimates, β coefficients, and ORs were weighted to adjust for sampling probability and nonresponse as previously described.16, 17 All analyses accounted for cluster sampling and the use of stratification in sample selection. Survey-specific procedures were used to compute 95% CIs to account for the 2-stage sampling design, stratification, and clustering. Age-adjusted estimates were calculated using the mean age of participants in HCHS/SOL (41 years). Multivariable survey logistic regression analyses were used to examine cross-sectional associations between sleep-disordered breathing and albuminuria (UACR ≥30 mg/g). ORs with 95% CIs were computed, adjusting for the following variables, which were chosen based on known clinical importance in previously published literature20, 21, 22: Hispanic/Latino background group, age, sex, income, education, acculturation, diabetes mellitus, cardiovascular disease, systolic BP, ACE inhibitor or ARB use, body mass index (BMI), low-density lipoprotein (LDL) cholesterol, C-reactive protein, and eGFR. Regression analyses were conducted in the overall study sample, and stratified by age (18−39, 40−59, and ≥60 years), sex (men and women), Hispanic/Latino background group (Mexican, Puerto Rican, Dominican, Central American, South American, Cuban, and other), and diabetes status (yes or no). Effect modification was tested separately by adding an interaction term between the potential effect modifier and each AHI threshold to the fully adjusted regression model. All statistical tests were 2-sided at a significance level of 0.05. No adjustments were made for multiple comparisons. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Overall, the mean ± SE age of participants was 41.1 ± 0.26 years, 47.7% were men, 14.9% had diabetes, and 21.5% had hypertension. Median (interquartile range [IQR]) of UACR was 6.5 mg/g (IQR: 4.5−12.0 mg/g), and mean ± SE eGFR was 107.2 ± 0.35 ml/min per 1.73 m2 (Table 1). Compared with individuals with AHI <5, those with AHI ≥5 were more likely to be older (51.2 years vs. 37.6 years), male (60.5% vs. 43.2%), had less than a high school education (38.1% vs. 30.4%), and had diabetes (29.3% vs. 9.9%) and hypertension (40.5% vs. 14.8%). Individuals with AHI ≥5 were more likely to prefer Spanish language (82.3% vs. 73.8%) and to have lived >10 years in the US (77.8% vs. 70.2%), but were less likely to be US born (13.7% vs. 25.1%). Individuals with AHI ≥ 5 were also more likely to have a higher BMI (32.3 vs. 28.3 kg/m2), higher LDL cholesterol (127.9 mg/dl vs. 117.3 mg/dl) and C-reactive protein (5.0 mg/l vs. 3.4 mg/l); and had lower eGFR levels (95.6 ml/min per 1.73 m2 vs. 110.9 ml/min per 1.73 m2). Similar associations were observed among those with AHIs of ≥15 versus <15 and those with AHIs of ≥30 versus <30 (Table 1). Compared with individuals included in the study (n = 12,572), those who were excluded due to insufficient or missing data (n = 3,843) had similar mean ages (41.0 years vs. 41.1 years), sex distribution (48.4% female vs. 47.7% male), educational attainment (32.1% more than high school vs. 32.4% less than high school), prevalent hypertension (22.8% vs. 21.5%), and diabetes (14.7% vs. 14.9%) (data not shown).
Table 1.
Baseline characteristics by three thresholds of apnea-hypopnea index
| All (N = 12,572) | AHI <5 (n = 8731) | AHI ≥5 (n = 3841) | AHI <15 (n = 11,144) | AHI ≥15 (n = 1428) | AHI <30 (n = 12,023) | AHI ≥30 (n = 549) | |
|---|---|---|---|---|---|---|---|
| Age, yr | 41.1 ± 0.26 | 37.6 ± 0.26 | 51.2a ± 0.35 | 39.8 ± 0.26 | 52.7b ± 0.61 | 40.7 ± 0.26 | 51.6c ± 0.98 |
| Male | 47.7 | 43.2 | 60.5a | 45.7 | 65.6b | 46.7 | 71.9c |
| Hispanic/Latino | |||||||
| Mexican | 41.1 | 42.7 | 36.4a | 41.8 | 34.9b | 41.2 | 38.3 |
| Puerto Rican | 15.3 | 14.7 | 16.9a | 15.1 | 16.5 | 15.3 | 14.4 |
| Dominican | 9.7 | 10.1 | 8.5 | 9.7 | 9.1 | 9.8 | 7.3 |
| Central American | 7.2 | 7.4 | 6.6 | 7.3 | 6.2 | 7.2 | 6.5 |
| South American | 4.8 | 4.8 | 4.51 | 4.9 | 3.9 | 4.8 | 4.2 |
| Cuban | 18.5 | 16.5 | 24.1a | 17.6 | 26.7b | 18.2 | 26.8c |
| Other | 3.6 | 3.75 | 3.1 | 3.7 | 2.7 | 3.6 | 2.7 |
| Education <high school | 32.4 | 30.4 | 38.1a | 31.8 | 37.9b | 32.3 | 35.6 |
| Income ≤$20,000 | 44.9 | 44.4 | 46.4 | 45.0 | 44.5 | 45.0 | 42.5 |
| Diabetes mellitus | 14.9 | 9.9 | 29.3a | 12.8 | 34.5b | 14.0 | 39.1c |
| Hypertension | 21.5 | 14.8 | 40.5a | 18.4 | 49.2b | 20.4 | 48.1c |
| Never smoker | 62.9 | 65.8 | 54.7a | 64.0 | 52.7b | 63.5 | 49.2 |
| SASH Score | 2.2 ± 0.02 | 2.2 ± 0.02 | 2.0a ± 0.03 | 2.2 ± 0.02 | 2.0b± 0.04 | 2.2 ± 0.02 | 2.0 ± 0.05 |
| Spanish language | 76.0 | 73.8 | 82.3a | 75.2 | 82.6b | 75.8 | 80.4 |
| US born | 22.2 | 25.1 | 13.7a | 23.1 | 13.2b | 22.4 | 15.8c |
| >10 yr in US | 72.2 | 70.2 | 77.8a | 71.3 | 80.4b | 71.8 | 80.8c |
| Sleep duration, h | 8.0 ± 0.02 | 8.0 ± 0.03 | 7.9a ± 0.04 | 8.0 ± 0.02 | 7.9b ± 0.07 | 8.0 ± 0.02 | 7.9 ± 0.06 |
| Sleep duration | |||||||
| <7 | 19.7 | 19.1 | 21.6a | 19.4 | 22.3 | 19.6 | 21.5 |
| 7−8 | 33.9 | 33.0 | 36.7 | 33.6 | 36.6 | 33.8 | 37.1 |
| > 8 | 46.4 | 48.0 | 41.7 | 46.9 | 41.0 | 46.5 | 41.4 |
| ESS >10 | 14.3 | 13.2 | 17.7a | 13.8 | 19.0b | 13.9 | 25.3c |
| Sleeping medication use | 9.2 | 8.4 | 11.5a | 9.0 | 11.1 | 9.1 | 10.6 |
| ACE inhibitor or ARB se | 11.5 | 7.5 | 23.0a | 9.8 | 27.1a | 10.7 | 31.1 |
| Systolic BP, mm Hg | 120 ± 0.26 | 117 ± 0.24 | 128a ± 0.41 | 119 ± 0.25 | 130b ± 0.70 | 119 ± 0.26 | 129c ± 1.20 |
| Diastolic BP, mm Hg | 72 ± 0.19 | 70 ± 0.19 | 77a ± 0.27 | 71 ± 0.18 | 79b ± 0.42 | 72 ± 0.19 | 79c ± 0.66 |
| BMI, kg/m2 | 29.3 ± 0.09 | 28.3 ± 0.10 | 32.3a ± 0.14 | 28.8 ± 0.09 | 33.7b ± 0.25 | 29.1 ± 0.09 | 34.9c ± 0.33 |
| LDL, mg/dl | 120.0 ± 0.51 | 117.3 ± 0.60 | 127.9a ± 0.88 | 119.2 ± 0.53 | 127.8b ± 1.26 | 119.8 ± 0.52 | 126.6c ± 2.27 |
| C-reactive protein mg/l | 3.8 ± 0.10 | 3.4 ± 0.12 | 5.0a ± 0.19 | 3.6 ± 0.10 | 5.7b ± 0.41 | 3.7 ± 0.10 | 6.3c ± 0.88 |
| eGFR, ml/min per 1.73 m2 | 107.0 ± 0.35 | 110.9 ± 0.36 | 95.6a ± 0.51 | 108.5 ± 0.36 | 93.3b ± 0.87 | 107.5 ± 0.36 | 93.8c ± 1.29 |
| UACR median (IQR) | 6.5 (4.5−12.0) | 6.3 (4.4−10.7) | 7.6 (4.8−15.6) | 6.4 (4.4−11.3) | 8.3 (5.1−17.9) | 6.5 (4.46−11.7) | 9.0 (5.23−21.4) |
ACE, angiotensin-converting enzyme; AHI, apnea−hypopnea index; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESS, Epworth Sleepiness Scale; LDL, low-density lipoprotein cholesterol; SASH, Short Acculturation Scale for Hispanics; SpO2, peripheral capillary oxygen saturation; UACR, urine albumin to creatinine ratio.
Data are expressed as weighted mean ± SE, median (IQR), or %.
P < 0.05 for comparison of AHI ≥5 versus <5.
P < 0.05 for comparison of AHI ≥15 versus <15.
P < 0.05 for comparison of AHI ≥30 versus <30.
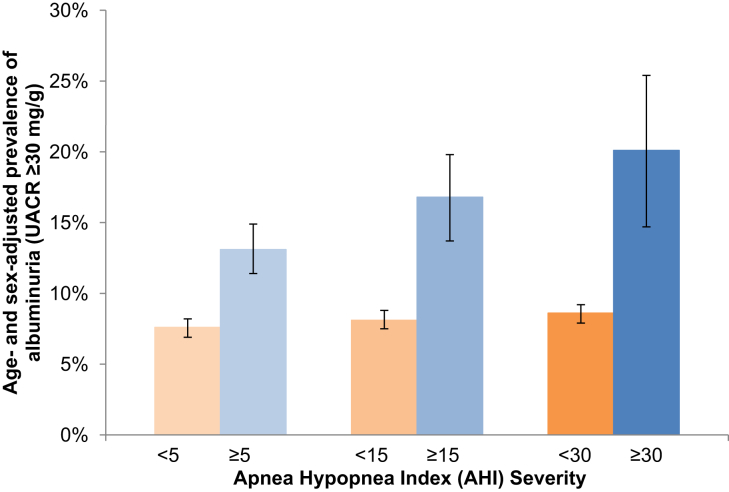
The overall age- and sex-adjusted prevalence of albuminuria (UACR ≥30 mg/g) was 9.1%, and it was highest among individuals with more severe sleep-disordered breathing (Figure 2).
Figure 2.
Age- and sex-adjusted prevalence of urine albumin-to-creatinine ratio (UACR) ≥30 mg/g by apnea−hypopnea index severity.
In multivariable analyses, the odds of albuminuria were higher for Hispanics/Latinos with a high AHI. Compared with individuals with AHIs <5, individuals with AHIs ≥5 were 42% more likely to have albuminuria (OR: 1.40; 95% CI: 1.12−1.74); the corresponding albuminuria ORs for participants with AHIs ≥15 versus <15 and AHIs ≥ 30 versus <30 were 1.69 (95% CI: 1.32−2.18) and 1.87 (1.29−2.72), respectively. In addition, every percentage point increase in nocturnal hypoxemia (percent of sleep time with SpO2 <90%) was associated with a 6% increased odds of albuminuria (OR: 1.06; 95% CI: 1.04−1.08) (Table 2).
Table 2.
Association (adjusted odds ratio) between sleep measures and albuminuria (UACR ≥ 30 mg/g)
| OR (95% CI) | P value | |
|---|---|---|
| AHI, per 1 unit increase | 1.02 (1.01−1.02) | <0.001 |
| AHI ≥ 5 (yes vs. no) | 1.40 (1.12−1.74) | 0.003 |
| AHI ≥ 15 (yes vs. no) | 1.69 (1.32−2.18) | <0.001 |
| AHI ≥ 30 (yes vs. no) | 1.87 (1.29−2.72) | <0.001 |
| Percent of sleep time with SpO2 <90%, per 1% increase | 1.06 (1.04−1.08) | <0.001 |
AHI, apnea−hypopnea index; CI, confidence interval; OR, odds ratio; SpO2, hemoglobin oxygen saturation; UACR, urine albumin-to-creatinine ratio.
Adjusted for Hispanic/Latino background group, age, sex, income, education, acculturation, diabetes, cardiovascular disease, systolic blood pressure, body mass index, angiotensin-converting inhibitor or angiotensin receptor use, low-density lipoprotein cholesterol, C-reactive protein, and estimated glomerular filtration rate.
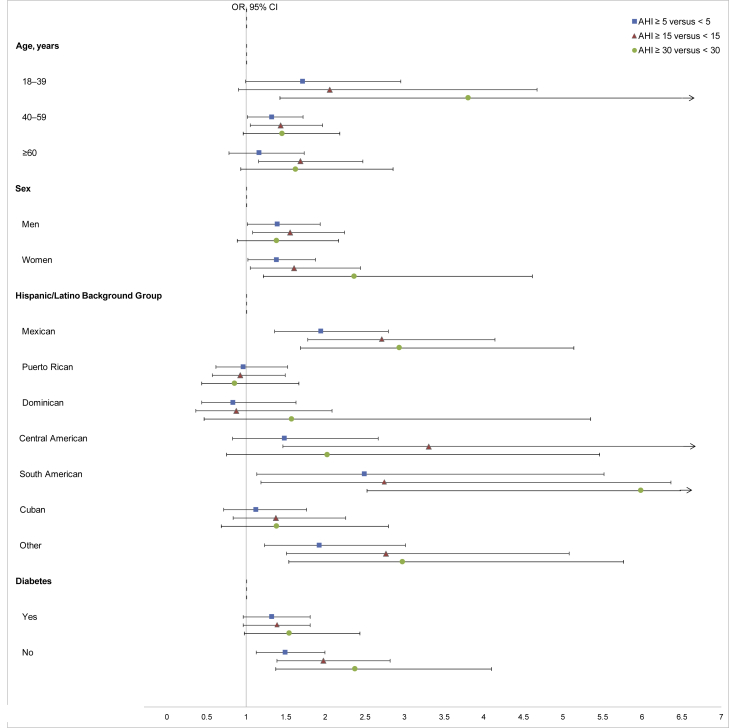
The results of analyses stratified by age, sex, Hispanic/Latino background group, and diabetes mellitus for the association of AHI (≥5 vs. <5, ≥15 vs. <15, and ≥30 vs. <30) with albuminuria are depicted in Figure 3 and summarized in the Supplementary Table S1. These subgroup analyses resulted in similar findings as in the overall cohort. However, there was evidence of effect modification by Hispanic/Latino background group. Particularly, the association between sleep AHI ≥15 versus <15 and albuminuria was statistically significant in certain Hispanic/Latino background groups (Mexican, Central American, South American, and other Hispanic/Latino background) but not others (Puerto Rican, Dominican, and Cuban) (p value for interaction < 0.001).
Figure 3.
Association (adjusted odds ratio [OR] with 95% confidence interval [CI]) between thresholds of apnea−hypopnea index (AHI) severity (AHI ≥ 5 vs. < 5, ≥ 15 vs. < 15, and ≥ 30 vs. < 30) and albuminuria (urine albumin-to-creatinine ratio ≥30 mg/g), stratified by age, sex, Hispanic/Latino background group, and diabetes status. The upper limit of wide CIs was truncated at 6.5 (indicated with an arrow); actual values are provided in Supplementary Table S1.
Discussion
In this large cohort of US Hispanic/Latino adults, the prevalence of sleep-disordered breathing was higher in persons with albuminuria compared with those without albuminuria. In addition, sleep-disordered breathing was independently associated with higher odds of albuminuria. Furthermore, this association varied by Hispanic/Latino background group. To the best of our knowledge, this was the largest population-based study to evaluate the association of sleep-disordered breathing with prevalent albuminuria and the first study to assess this issue in US Hispanics/Latinos.
There is ample evidence that indicates a greater prevalence of sleep-disordered breathing in patients with end-stage renal disease than in people with normal kidney function.32, 33, 34 In contrast, research on sleep-disordered breathing in earlier stages of kidney disease is limited, particularly with regard to albuminuria with a preserved eGFR. To date, these studies have been conducted at single centers and have not included a significant proportion of Hispanics/Latinos.20, 21, 35 Faulx et al. reported that among 496 participants in the Cleveland Family Study, obstructive sleep apnea was associated with a prevalence of albuminuria (UACR 25−249 mg/d) of 20.3% in those with AHIs ≥30 compared with 4.7% among participants with AHIs <5.20 The only previous population-based study of sleep-disordered breathing in early stages of CKD (normal eGFR with albuminuria) was conducted in Switzerland in 1726 adults who underwent polysomnography at home.36 These investigators reported a higher prevalence of severe sleep-disordered breathing among individuals with CKD stages 1 to 2. Our results reinforced and extended these findings to the US Hispanic/Latino population.
In our multivariable analyses, we found that an elevated AHI was associated with prevalent albuminuria, independent of hypertension, diabetes, and obesity. This finding suggested that sleep-disordered breathing might contribute to the excess burden of albuminuria in Hispanics/Latinos. Similar to our findings, in the Cleveland Family Study, severe obstructive sleep apnea was significantly associated with increased urine albumin excretion, even after adjusting for obesity, diabetes, and hypertension.20 Several biological mechanisms might be responsible for the association between sleep-disordered breathing and albuminuria.37 Specific effects of sleep-disordered breathing that might lead to albuminuria include nocturnal sympathetic activation with loss of BP dipping, vascular stiffness, and insulin resistance.38, 39, 40 In addition, cycles of hypoxia and re-oxygenation stimulate the formation of reactive oxygen species that promote inflammation and systemic endothelial dysfunction,41, 42, 43 all of which could have adverse effects on kidney function.
The impact of continuous positive airway pressure (CPAP) on albuminuria has not been thoroughly investigated. However, there is evidence that sleep-disordered breathing influences inflammation and endothelial function, and that treatment with CPAP may ameliorate these mediating effects.34, 44, 45 Chen et al.46 evaluated 60 individuals with AHIs >15 with albuminuria who were treated with CPAP for 6 months. A significant decrease in UACR was noted from baseline to the end of follow-up among participants with good compliance to CPAP. If future longitudinal studies find sleep-disordered breathing to be a risk factor for CKD progression, then clinical trials that assess the impact of CPAP treatment on CKD progression should be considered.
We found evidence of effect modification by Hispanic/Latino background with a statistically significant association between moderate-to-severe sleep-disordered breathing and albuminuria among mainland Hispanics/Latinos (Mexican, Central, and South American) but not among Caribbean background groups (Puerto Rican, Dominican, and Cuban). Reasons for these findings are unknown. Compared with mainland Hispanics/Latinos, Caribbeans have a higher prevalence of APOL1 risk variant alleles, which is a known risk factor for albuminuria.47 It is possible that those with the APOL1 risk variant may be at such high risk for albuminuria that the association with sleep-disordered breathing is obscured. Nevertheless, our findings underscored the heterogeneity of this population, which needs to be taken into account in designing future studies.
Our study had several strengths, including the large sample size of Hispanics/Latinos with diverse backgrounds recruited from across the US, and standardized objective measures of sleep-disordered breathing with strict quality control. Our findings should be considered with the following limitations. First, sleep was not recorded using electroencephalography; therefore, the sleep time used to calculate AHI was estimated rather than measured. This might have led to AHI underestimation. Second, the definition of albuminuria was based on single measurements of urine albumin. Third, the cross-sectional study design limited the interpretation of the results presented.
In conclusion, this study provided evidence of a strong association between sleep-disordered breathing and albuminuria, independent of hypertension, diabetes, and obesity. The direction of this association needs to be evaluated in future studies. Nevertheless, clinicians should be aware of the high prevalence and potential implications of sleep-disordered breathing in Hispanics/Latinos with kidney disease.
Disclosure
The authors declared no competing interests.
Acknowledgments
The authors thank the staff and participants of HCHS/SOL for their important contributions. A complete list of staff and investigators has been provided by Sorlie et al.24 and is also available on the study website http://www.cscc.unc.edu/hchs. The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following institutes, centers, or offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, the National Institute on Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. ACR and JPL are funded by the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK094829 and K24 DK092290, respectively).
Footnotes
Table S1. Association between apnea-hypopnea index (AHI) thresholds and albuminuria (UACR ≥30 mg/g) stratified by age, sex, Hispanic/Latino background, and diabetes.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Association between apnea-hypopnea index (AHI) thresholds and albuminuria (UACR ≥30 mg/g) stratified by age, sex, Hispanic/Latino background, and diabetes.
References
- 1.Center for Disease Control and Prevention. Chronic kdney disease initiative. 2018. Available at: https://www.cdc.gov/kidneydisease/index.html. Accessed March 20, 2018.
- 2.United States Renal Data System. 2017 annual data report. Available at: https://www.usrds.org/adr.aspx. Accessed February 1, 2018.
- 3.Afkarian M., Zelnick L.R., Hall Y.N. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryson C.L., Ross H.J., Boyko E.J., Young B.A. Racial and ethnic variations in albuminuria in the US Third National Health and Nutrition Examination Survey (NHANES III) population: associations with diabetes and level of CKD. Am J Kidney Dis. 2006;48:720–726. doi: 10.1053/j.ajkd.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Peralta C.A., Li Y., Wassel C. Differences in albuminuria between Hispanics and whites: an evaluation by genetic ancestry and country of origin: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Genet. 2010;3:240–247. doi: 10.1161/CIRCGENETICS.109.914499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricardo A.C., Flessner M.F., Eckfeldt J.H. Prevalence and correlates of CKD in Hispanics/Latinos in the United States. Clin J Am Soc Nephrol. 2015;10:1757–1766. doi: 10.2215/CJN.02020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemmelgarn B.R., Manns B.J., Lloyd A. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 8.Hallan S.I., Ritz E., Lydersen S., Romundstad S. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peralta C.A., Shlipak M.G., Judd S. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klausen K., Borch-Johnsen K., Feldt-Rasmussen B. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2017. Available at: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 29, 2018.
- 12.Burke J.P., Williams K., Gaskill S.P. Rapid rise in the incidence of type 2 diabetes from 1987 to 1996: results from the San Antonio Heart Study. Arch Intern Med. 1999;159:1450–1456. doi: 10.1001/archinte.159.13.1450. [DOI] [PubMed] [Google Scholar]
- 13.Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 14.Daviglus M.L., Talavera G.A., Avilés-Santa M.L. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colten H.R., Altevogt B.M., editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. National Academies Press; Washington, DC: 2006. [PubMed] [Google Scholar]
- 16.Peppard P.E., Young T., Palta M., Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor G.T., Caffo B., Newman A.B. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179:1159–1164. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marin J.M., Carrizo S.J., Vicente E., Agusti A.G.N. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 19.Anothaisintawee T., Reutrakul S., Van Cauter E., Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 2015;30:11–24. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Faulx M.D., Storfer-Isser A., Kirchner H.L. Obstructive sleep apnea is associated with increased urinary albumin excretion. Sleep. 2007;30:923–929. doi: 10.1093/sleep/30.7.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canales M.T., Paudel M.L., Taylor B.C. Sleep-disordered breathing and urinary albumin excretion in older men. Sleep Breath Schlaf Atm. 2011;15:137–144. doi: 10.1007/s11325-010-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redline S., Sotres-Alvarez D., Loredo J. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189:335–344. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavange L.M., Kalsbeek W.D., Sorlie P.D. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorlie P.D., Avilés-Santa L.M., Wassertheil-Smoller S. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaFromboise T., Coleman H.L.K., Gerton J. Psychological impact of biculturalism: evidence and theory. Psychol Bull. 1993;114:395–412. doi: 10.1037/0033-2909.114.3.395. [DOI] [PubMed] [Google Scholar]
- 26.Marin G., Sabogal F., Marin B.V. Development of a short acculturation scale for Hispanics. Hispanic J Behav Sci. 1987;9:183–205. [Google Scholar]
- 27.Westbrook P.R., Levendowski D.J., Cvetinovic M. Description and validation of the apnea risk evaluation system: a novel method to diagnose sleep apnea-hypopnea in the home. Chest. 2005;128:2166–2175. doi: 10.1378/chest.128.4.2166. [DOI] [PubMed] [Google Scholar]
- 28.Kapur V.K., Auckley D.H., Chowdhuri S. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johns M.W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Lind B.K., Goodwin J.L., Hill J.G. Recruitment of healthy adults into a study of overnight sleep monitoring in the home: experience of the Sleep Heart Health Study. Sleep Breath Schlaf Atm. 2003;7:13–24. doi: 10.1007/s11325-003-0013-z. [DOI] [PubMed] [Google Scholar]
- 31.Inker L.A., Schmid C.H., Tighiouart H. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unruh M.L., Sanders M.H., Redline S. Sleep apnea in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol. 2006;17:3503–3509. doi: 10.1681/ASN.2006060659. [DOI] [PubMed] [Google Scholar]
- 33.Hanly P. Sleep disorders and end-stage renal disease. Curr Opin Pulm Med. 2008;14:543–550. doi: 10.1097/MCP.0b013e3283130f96. [DOI] [PubMed] [Google Scholar]
- 34.Turek N.F., Ricardo A.C., Lash J.P. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis. 2012;60:823–833. doi: 10.1053/j.ajkd.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou Y.-T., Lee P.-H., Yang C.-T. Obstructive sleep apnea: a stand-alone risk factor for chronic kidney disease. Nephrol Dial Transplant. 2011;26:2244–2250. doi: 10.1093/ndt/gfq821. [DOI] [PubMed] [Google Scholar]
- 36.Ogna A., Forni Ogna V., Haba Rubio J. Sleep characteristics in early stages of chronic kidney disease in the HypnoLaus Cohort. Sleep. 2016;39:945–953. doi: 10.5665/sleep.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adeseun G.A., Rosas S.E. The impact of obstructive sleep apnea on chronic kidney disease. Curr Hypertens Rep. 2010;12:378–383. doi: 10.1007/s11906-010-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato M., Roberts-Thomson P., Phillips B.G. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 39.Clausen P., Jensen J.S., Jensen G. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001;103:1869–1874. doi: 10.1161/01.cir.103.14.1869. [DOI] [PubMed] [Google Scholar]
- 40.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 41.Lavie L. Oxidative stress–a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51:303–312. doi: 10.1016/j.pcad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Shamsuzzaman A.S.M., Winnicki M., Lanfranchi P. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 43.Doonan R.J., Scheffler P., Lalli M. Increased arterial stiffness in obstructive sleep apnea: a systematic review. Hypertens Res. 2011;34:23–32. doi: 10.1038/hr.2010.200. [DOI] [PubMed] [Google Scholar]
- 44.Baessler A., Nadeem R., Harvey M. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers - a meta-analysis. J Inflamm (Lond) 2013;10:13. doi: 10.1186/1476-9255-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu H., Wang Y., Guan J., Yi H., Yin S. Effect of CPAP on endothelial function in subjects with obstructive sleep apnea: a meta-analysis. Respir Care. 2015;60:749–755. doi: 10.4187/respcare.03739. [DOI] [PubMed] [Google Scholar]
- 46.Chen N.-H., Chou Y.-T., Lee P.-H. Reversibility of albuminuria and continuous positive airway pressure compliance in patients of obstructive sleep apnea syndrome. Medicine (Baltimore) 2016;95:e4045. doi: 10.1097/MD.0000000000004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kramer H.J., Stilp A.M., Laurie C.C. African ancestry-specific alleles and kidney disease risk in Hispanics/Latinos. J Am Soc Nephrol. 2017;28:915–922. doi: 10.1681/ASN.2016030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association between apnea-hypopnea index (AHI) thresholds and albuminuria (UACR ≥30 mg/g) stratified by age, sex, Hispanic/Latino background, and diabetes.