Abstract
Introduction
The goal of this study was to examine patterns in the likelihood of consent to genetic research among participants in a prospective kidney disease cohort and biobank, and to determine demographic, clinical, and socioeconomic factors linked to consent for ongoing and future genetic research.
Methods
The Clinical Phenotyping Resource and Biobank Core (C-PROBE) enrolled 1628 adult and pediatric patients with chronic kidney disease from 2009 to 2017 across 7 sites in the United States. Participants were asked at annual study visits for consent to provide DNA samples for future genetic studies. We compared characteristics of participants by initial consent outcome and consent status at their most recent study visit.
Results
Of the C-PROBE participants, 96% consented to genetic studies at their initial study visit. Although African Americans were slightly less likely to consent at baseline (93% vs. 97%, odds ratio = 0.3, P < 0.02), there were no significant racial or ethnic differences with longitudinal participation. Also, pediatric and adult genetic consent rates were equivalent. The major persistent differences in the likelihood of consent were based on enrollment site, which ranged from 85% to 100% (P < 0.0001).
Conclusion
Overall, genetic consent rates for kidney research within the C-PROBE cohort were high. However, differences in consent rates over time and by recruitment site highlight the complexity of genetic consent for biobanking, and potential limitations for generalizability of observations.
Keywords: biorepository, CKD, DNA storage, genetic research, informed consent, minorities, translational research
See Commentary on Page 1245
Precision medicine research in nephrology offers significant potential to advance our knowledge of kidney disease pathogenesis, including insights into kidney health disparities. For example, the discovery of APOL1 genetic susceptibility among African Americans1, 2, 3 has the potential to guide systems biology research approaches to elucidate mechanisms of kidney injury and lead to targeted preventive and therapeutic strategies. These promising innovative research approaches rely on the availability of DNA samples among large, diverse, and well-phenotyped study cohorts. The C-PROBE, a longitudinal multisite cohort within the National Institute of Diabetes and Digestive and Kidney Diseases−funded George M. O’Brien Michigan Kidney Translational Core Center, is designed to streamline and facilitate kidney translational research toward precision medicine. C-PROBE provides a multidisciplinary network of investigators with longitudinal patient data and biological specimens obtained from consenting adult and pediatric patients with chronic kidney disease (CKD). In this cohort, the capacity to obtain genetic consent is fundamental to comprehensively explore novel inherited disease mechanisms that may contribute to kidney disease susceptibility, including racial/ethnic disparities.
Previous epidemiologic studies4, 5, 6, 7 and recent cardiovascular clinical trials8, 9 demonstrate some reluctance of ethnic and racial minority participants to consent to genetic studies. There is no information available on patterns of genetic consent frequency among kidney disease study adult or pediatric participants. Therefore, the purpose of this study was to examine consent outcomes with longitudinal study involvement of a diverse CKD study cohort and to determine whether demographic, clinical, and socio-economic characteristics are linked to genetic consent status.
Materials and Methods
The C-PROBE cohort study is an ongoing, prospective, observational cohort study of multi-ethnic patients with CKD, and functions as 1 of 3 complementary cores within the George M. O’Brien Michigan Kidney Translational Core Center (www.kidneycenter.med.umich.edu). Participants were enrolled at the following 7 sites after Institutional Review Board approval at each site: The University of Michigan (separate pediatric and adult sites), Ann Arbor, MI; Wayne State University, Detroit, MI; St. Clair Nephrology Research, with offices throughout southeast MI; John H. Stroger Hospital, Chicago, IL; Temple University, Philadelphia, PA; and Levine Children’s Hospital, Charlotte, NC. Enrollment for adults began in 2009; pediatric enrollment at the University of Michigan and Levine Children’s Hospital began in 2014 with the second wave of recruitment. Eligible candidates for C-PROBE are patients with CKD stages 1 to 4, followed up within the clinical site nephrology practices or undergoing kidney biopsy. Exclusion criteria include patients receiving chronic renal replacement therapy or who have had a kidney transplant, as well as patients unable or unwilling to consent, patients current participating in a blinded clinical trial, women who are pregnant or nursing, and adults with polycystic kidney disease. For the pediatric population, patient/guardian consent and, when appropriate, minor assent are obtained for enrollment. Data collection at annual study visits includes medical, social, and family histories, kidney-specific clinical information from chart review, and demographic information. In addition, blood and urine specimens are collected and stored in a biobank at each visit. For participants who undergo a clinically indicated kidney biopsy, extra tissue that is not required for diagnostic purposes is also collected and stored in the biobank.
The C-PROBE informed consent document includes optional consents for obtaining archived kidney tissue, and DNA for future genetic studies. Although some sites include Health Insurance Portability and Accountability Act of 1996 (HIPAA) authorization within the informed consent document, some require a separate document. Initially, 1 site required a separate genetic consent document, but currently the genetic consent sections are embedded in the research project informed consent documents for all sites. To standardize the informed consent process among all sites, all coordinators receive formal education and training for C-PROBE consent consisting of review of the risks and benefits of all study procedures including the genetic consent. A “practice” consent is performed by all coordinators (observed and approved by the C-PROBE project manager) before engaging in actual participant enrollment. In addition, the consent process is reviewed with study teams on an annual basis. In the informed consent process, C-PROBE participants are asked to give their consent for genetic studies by checking “Yes” or “No” to the following question: “I give my permission to isolate DNA from my blood, urine and biopsy samples, and check my DNA for genes related to kidney disease” (Supplementary Appendix S1). Re-consent has been required for C-PROBE study amendments annually. Each participant’s genetic consent status is therefore re-addressed during subsequent study visits and can change over time. Results from genetic samples are not returned to participants. For the purposes of our analyses, we examined consent status in 2 ways: (i) initial consent at the baseline study visit and (ii) most recent consent status from last follow-up visit. First, we examined initial consent, obtained at the baseline study visit, for all C-PROBE participants. Subsequently, for participants with 2 or more study visits, we examined current consent status, defined as consent status at the most recent study visit.
For initial and current consent, we tested for associations between declining to consent and race, ethnicity, site, age, sex, education level, number of study visits, enrollment period, baseline estimated glomerular filtration rate (eGFR), diagnosis, and family history of kidney disease. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for adults aged 18 years and older, and the CKiD-Bedside formula for children and adolescents aged less than 18 years.10, 11 Diagnoses were coded as either glomerular or nonglomerular disease. Glomerular disease diagnoses included nephrotic syndrome, diabetic nephropathy, minimal change disease, focal segmental glomerulosclerosis, membranous nephropathy, IgA nephropathy, Henoch−Schoelein purpura, lupus nephritis, membranoproliferative glomerulonephritis, infection-related glomerulonephritis, immune-complex glomerulonephritis, vasculitis, thrombotic microangioapathy, Denys−Drash syndrome, brachio-oto-renal syndrome, C1Q nephropathy, Alport syndrome, thin basement membrane nephropathy, and amyloidosis. Nonglomerular diagnoses included sickle cell nephropathy, polycystic kidney disease (children only), acute or chronic interstitial nephritis, acute tubular necrosis, atheroembolic disease, sarcoidosis, myeloma kidney, hypertensive nephropathy, acquired obstructive uropathy, Wilm tumor, and congenital anomalies of the kidney and urinary tract.
Descriptive characteristics of the sample were described using frequencies and percentages for categorical variables and medians (25th and 75th percentiles) for continuous variables. Statistically significant differences of the distribution of variables of interest were tested across consent status using a χ2 test for categorical variables and a Kruskal-Wallis test for continuous variables. All variables significant at P < 0.25 were included for a series of multivariable backward selection logistic regressions modeling the outcome “declined consent.” Variables were removed in reverse order of P value until all variables were significant at the conventional level of P < 0.05. The same approach was used to model initial consent and consent at the most recent study visit. False discovery rate (FDR)−adjusted P values were calculated to correct for multiple comparisons. All analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, NC).
Finally, we conducted a brief internal and informal survey of all current C-PROBE principal investigators and study coordinators to better understand study team−specific factors that may play a role in genetic consent outcomes.
Results
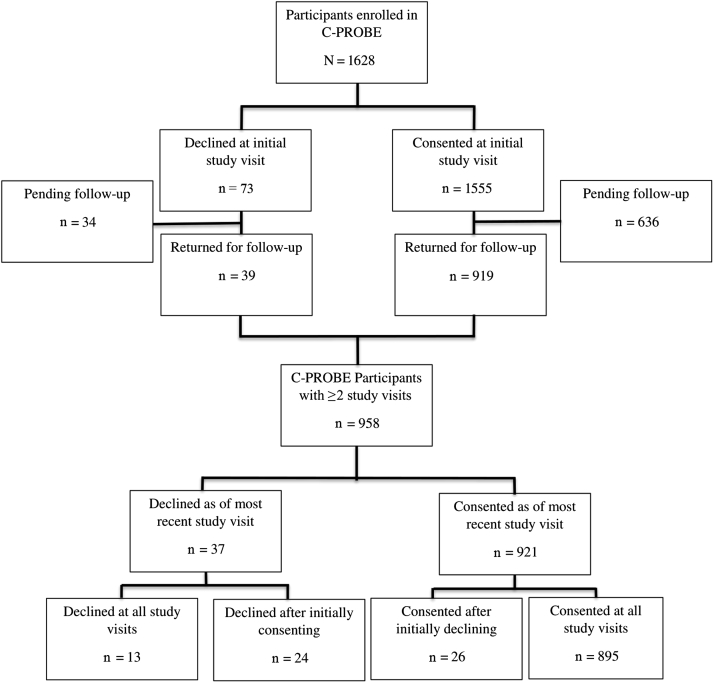
As of December 2018, a total of 1628 patients were enrolled into the C-PROBE cohort study with a completed baseline visit (Figure 1). At their initial study visit, 1555 patients (96%) agreed and 73 (4%) declined to consent for DNA storage and future research. Of the 1555 patients who consented, 919 (59%) have returned for a follow-up visit, compared to 39 (53%) of the 73 who initially declined. Notably, there was no statistical difference between initial consent status and likelihood of follow-up to date (59% vs. 53%, P = 0.34). Of the 958 patients with at least 1 follow-up visit, 921 (96%) consented to genetic studies as of their most recent study visit and 37 (4%) declined. There were 13 patients (1%) who declined at all study visits, 24 (3%) who declined after initially consenting, 26 (3%) who consented after initially declining, and 895 (93%) who consented at all study visits.
Figure 1.
Flow diagram of initial and longitudinal genetic consent status among Clinical Phenotyping and Resource Biobank Core (C-PROBE) participants (n = 1628).
This baseline cohort included 1381 adults and 247 children from diverse racial backgrounds reflective of CKD populations at C-PROBE recruiting sites (Table 1). Specifically, 39% self-identified as black/African American, 3% as Asian/Asian American, and 9% of Hispanic ethnicity. Participants with more than 1 study visit (n = 958) did not differ from the overall cohort by age, sex, race, ethnicity, baseline eGFR, diagnoses, family history of disease, history of hypertension, or weight.
Table 1.
Descriptive characteristics of the Clinical Phenotyping and Resource Biobank Core (C-PROBE) cohort
| Characteristic | All C-PROBE participants (n = 1628) n (%) |
C-PROBE participants with >1 study visit (n = 958) n (%) |
|---|---|---|
| Age, median (IQR) | 54 (30–64) | 54 (32–65) |
| Adults (≥18 yr) | 1381 (85) | 816 (85) |
| Children/adolescents (<18 yr) | 247 (15) | 142 (15) |
| Sex | ||
| Female | 837 (51) | 525 (55) |
| Male | 790 (49) | 433 (45) |
| Other | 1 (<1) | 0 (0) |
| Race | ||
| American Indian/Alaskan Native | 12 (1) | 7 (1) |
| Asian/Asian American | 48 (3) | 20 (2) |
| Black/African American | 636 (39) | 352 (37) |
| Native Hawaiian/other Pacific Islander | 1 (<1) | 0 (0) |
| White/Caucasian | 846 (52) | 534 (56) |
| Multiracial | 45 (3) | 29 (3) |
| Not reported | 40 (2) | 16 (2) |
| Ethnicity | ||
| Hispanic | 153 (9) | 73 (8) |
| Non-Hispanic | 1474 (91) | 884 (92) |
| Not reported | 1 (<1) | 1 (<1) |
| Site | ||
| 1 | 453 (28) | 294 (31) |
| 2 | 162 (10) | 98 (10) |
| 3 | 407 (25) | 262 (27) |
| 4 | 257 (16) | 113 (12) |
| 5 | 94 (6) | 45 (5) |
| 6 | 143 (9) | 81 (8) |
| 7 | 112 (7) | 65 (7) |
| Baseline eGFR,a median (IQR) | 49 (36–75) | 50 (38–76) |
| Diagnosis | ||
| Glomerular | 768 (47) | 462 (48) |
| Nonglomerular | 860 (53) | 496 (52) |
| Duration of disease at baseline | ||
| <6 mo | 151 (9) | 90 (9) |
| ≥6 mo | 1250 (77) | 742 (77) |
| Not reported | 227 (14) | 126 (13) |
| Family history of kidney disease | ||
| Family history | 381 (24) | 241 (25) |
| No family history | 1247 (76) | 717 (75) |
| History of hypertension | 1149 (71) | 682 (71) |
| Baseline weight statusb | ||
| Overweight | 417 (26) | 253 (26) |
| Obese | 765 (47) | 453 (47) |
There were no significant differences between all participants and those with 2 or more study visits. eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Estimated glomerular filtration rate calculated as ml/min per 1.73 m2.
Overweight defined as body mass index (BMI) 25 to 30 for adults or BMI percentile 85th to 95th for pediatric patients. Obese defined as BMI >30 for adults or BMI percentile >95th for pediatric patients.
Genetic consent status at the initial study visit was 96% among all participants (Table 2). Although there was a small but statistically significant difference in consent status by race for initial consent, racial differences became insignificant with longitudinal participation (Table 2). Notably, Hispanic ethnicity was not associated with a lower consent rate at either time point. Also, genetic consent among caregivers of pediatric participants was equivalent to consent among adult participants (initial consent rates 94% vs. 96%, P = 0.52). At baseline visit, individuals with recent diagnoses of CKD were more likely to consent than those with known disease duration of >6 months (99% vs. 96%, P = 0.002). Other clinical characteristics, including eGFR and family history of kidney disease, were not linked to consent status. Overall, site variation in consent status, which ranged from 85% to 100% (P < 0.0001), was highly significant and persists in the cohort. Also, likelihood of changing consent status was linked to study site (Table 3). This pattern persisted even after grouping of patients who initially declined and then consented or vice versa (data not shown).
Table 2.
Characteristics of participants in the Clinical Phenotyping and Resource Biobank Core (C-PROBE) by genetic consent status
| Characteristic | Genetic consent status at baseline (n = 1628) |
Current genetic consent status among participants with >1 study visit (n = 958) |
||||
|---|---|---|---|---|---|---|
| Declined consent (n = 73) n (%) |
Consented (n = 1555) n (%) |
P valuea | Declined consent (n = 37) n (%) |
Consented (n = 921) n (%) |
P valuea | |
| Age | 0.52 | 0.93 | ||||
| Adults (≥18 yr) | 59 (4) | 1322 (96) | 31 (4) | 785 (96) | ||
| Children/adolescents (<18 yr) | 14 (6) | 233 (94) | 6 (3) | 136 (97) | ||
| Race | 0.001 | 0.08 | ||||
| American Indian/Alaskan Native | 0 (0) | 12 (100) | 0 (0) | 7 (100) | ||
| Asian/Asian American | 4 (8) | 44 (92) | 0 (0) | 20 (100) | ||
| Black/African American | 47 (7) | 589 (93) | 23 (7) | 329 (93) | ||
| Native Hawaiian/other Pacific Islander | 0 (0) | 1 (100) | 0 (0) | 0 (0) | ||
| White/Caucasian | 18 (2) | 821 (98) | 13 (2) | 521 (98) | ||
| Multiracial | 3 (7) | 42 (93) | 0 (0) | 29 (100) | ||
| Not reported | 1 (2) | 39 (98) | 1 (7) | 15 (93) | ||
| Ethnicity | 0.98 | 0.98 | ||||
| Hispanic | 7 (5) | 146 (95) | 3 (4) | 70 (96) | ||
| Non-Hispanic | 66 (5) | 1408 (95) | 34 (4) | 850 (96) | ||
| Not reported | 0 (0) | 1 (100) | 0 (0) | 1 (100) | ||
| Site | <0.0001 | <0.0001 | ||||
| 1 | 8 (2) | 445 (98) | 5 (2) | 289 (98) | ||
| 2 | 9 (6) | 153 (94) | 12 (12) | 86 (88) | ||
| 3 | 3 (1) | 404 (99) | 2 (1) | 260 (99) | ||
| 4 | 38 (15) | 219 (85) | 12 (11) | 101 (89) | ||
| 5 | 0 (0) | 94 (100) | 0 (0) | 45 (100) | ||
| 6 | 10 (7) | 133 (93) | 2 (3) | 79 (97) | ||
| 7 | 5 (4) | 107 (96) | 4 (6) | 61 (94) | ||
| Education (among adults >22 yr old) (n = 768) | 0.08 | 0.10 | ||||
| Some College/college degree | 21 (3) | 677 (97) | 11 (3) | 421 (97) | ||
| No college | 32 (5) | 561 (95) | 18 (5) | 318 (95) | ||
| Baseline eGFR,b median (IQR) | 53 (38–86) | 49 (36–74) | 0.51 | 54 (37–80) | 50 (38–75) | 0.52 |
| Diagnosis | 0.76 | 0.98 | ||||
| Glomerular | 32 (4) | 736 (96) | 18 (4) | 444 (96) | ||
| Nonglomerular | 41 (5) | 819 (95) | 19 (4) | 477 (96) | ||
| Duration of disease at baseline | 0.002 | 0.96 | ||||
| <6 mo | 1 (1) | 150 (99) | 3 (3) | 87 (97) | ||
| ≥6 mo | 52 (4) | 1198 (96) | 30 (4) | 712 (96) | ||
| Not reported | 20 (9) | 207 (91) | 4 (3) | 122 (97) | ||
| Family history of kidney disease | 0.93 | 0.73 | ||||
| Family history | 18 (5) | 363 (95) | 11 (4) | 230 (95) | ||
| No family history | 55 (4) | 1192 (96) | 26 (4) | 691 (96) | ||
eGFR, estimated glomerular filtration rate; IQR, interquartile range.
False discovery rate−adjusted P value.
Estimated glomerular filtration rate calculated as ml/min/1.73 m2.
Table 3.
Characteristics of participants in the Clinical Phenotyping and Resource Biobank Core (C-PROBE) by consent status over time among participants with more than 1 study visit (n = 958)
| Characteristic | Changed consent status over time (n = 50) n (%) |
Consent remained the same over time (n = 908) n (%) |
P valuea |
|---|---|---|---|
| Age | 0.14 | ||
| Adults (≥18 yr) | 38 (5) | 778 (95) | |
| Children/adolescents (<18 yr) | 12 (8) | 130 (92) | |
| Race | 0.18 | ||
| American Indian/Alaskan Native | 0 (0) | 7 (100) | |
| Asian/Asian American | 2 (10) | 18 (90) | |
| Black/African American | 27 (8) | 325 (92) | |
| Native Hawaiian/other Pacific Islander | 0 (0) | 0 (0) | |
| White/Caucasian | 18 (3) | 516 (97) | |
| Multiracial | 2 (7) | 27 (93) | |
| Not reported | 1 (6) | 15 (94) | |
| Ethnicity | 0.93 | ||
| Hispanic | 5 (7) | 68 (93) | |
| Non-Hispanic | 45 (5) | 839 (95) | |
| Not reported | 0 (0) | 1 (100) | |
| Site | <0.0001 | ||
| 1 | 9 (3) | 285 (97) | |
| 2 | 14 (14) | 84 (86) | |
| 3 | 3 (1) | 259 (99) | |
| 4 | 11 (10) | 102 (90) | |
| 5 | 0 (0) | 45 (100) | |
| 6 | 7 (9) | 74 (91) | |
| 7 | 6 (9) | 59 (91) | |
| Education (among adults >22 yr old) (n = 768) | 0.15 | ||
| Some college/college degree | 14 (3) | 418 (97) | |
| No college | 20 (6) | 316 (94) | |
| Baseline eGFR,b median (IQR) | 62 (46, 90) | 50 (37, 75) | 0.08 |
| Diagnosis | 0.49 | ||
| Glomerular | 28 (6) | 434 (94) | |
| Nonglomerular | 22 (4) | 474 (96) | |
| Duration of disease at baseline | 0.52 | ||
| <6 mo | 4 (4) | 86 (96) | |
| ≥6 mo | 36 (5) | 706 (95) | |
| Not reported | 10 (8) | 116 (92) | |
| Family history of kidney disease | 0.63 | ||
| Family history | 15 (6) | 226 (94) | |
| No family history | 35 (5) | 682 (95) | |
eGFR, estimated glomerular filtration rate; IQR, interquartile range.
False discovery rate−adjusted P value.
Estimated glomerular filtration rate calculated as ml/min per 1.73 m2.
Final multivariable logistic regression models of predictors of consent status are shown in Table 4. In these models, site 4 was used as the reference category for the site variable to stabilize estimates for other sites, given that site 4 had the highest decline rate. For initial consent, race and site were significant independent predictors of consent. Independent of site variation, black/African American participants, but not Asian American or other racial/ethnic groups, were less likely to consent at baseline visit compared to white/Caucasian participants (odds ratio = 0.3, 95% confidence interval = 0.2–0.6). However, among participants with 2 or more annual study visits, race was not a statistically significant predictor of consent status after accounting for differences by site. Enrollment site remained the only significant independent predictor of genetic consent status (P < 0.001). Reclassifying race as “black/African American versus all other races” did not change these results. In additional sensitivity analyses, we tested whether the effect of race on consent status varied by site using a race × site interaction term in our models. These interactions were not statistically significant, and there was no subgroup variation in the effect of race by site on initial or most recent consent status.
Table 4.
Multivariable logistic regression analysis on predictors of declining to consent to genetic studies
| Characteristic | Initial consent among all participants (n = 1628): 73 declined consent, 1555 consented |
Current consent status among participants with ≥2 study visits (n = 958): 37 declined consent, 921 consented |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P valuea | Odds ratio (95% CI) | P valuea | |
| Race | 0.02 | |||
| Asian/Asian American | 0.4 (0.1–1.4) | |||
| Black/African American | 0.3 (0.2–0.6) | |||
| Other | 0.6 (0.2–1.9) | |||
| White/Caucasian | Reference | |||
| Site | <0.0001 | <0.0001 | ||
| 1 | 6.3 (2.8–14.3) | 6.8 (2.3–19.8) | ||
| 2 | 3.8 (1.9–8.2) | 0.9 (0.4–2.0) | ||
| 3 | 18.7 (5.7–61.8) | 15.4 (3.4–70.0) | ||
| 4 | Reference | Reference | ||
| 5 | Undefinedb | Undefinedb | ||
| 6 | 1.7 (0.8–3.6) | 5.9 (0.7–46.5) | ||
| 7 | 2.2 (0.8–6.1) | 2.2 (0.5–10.1) | ||
CI, confidence interval.
False discovery rate adjusted P value.
Undefined denotes that odds ratio is undefined because no participants at this site had declined at their last visit.
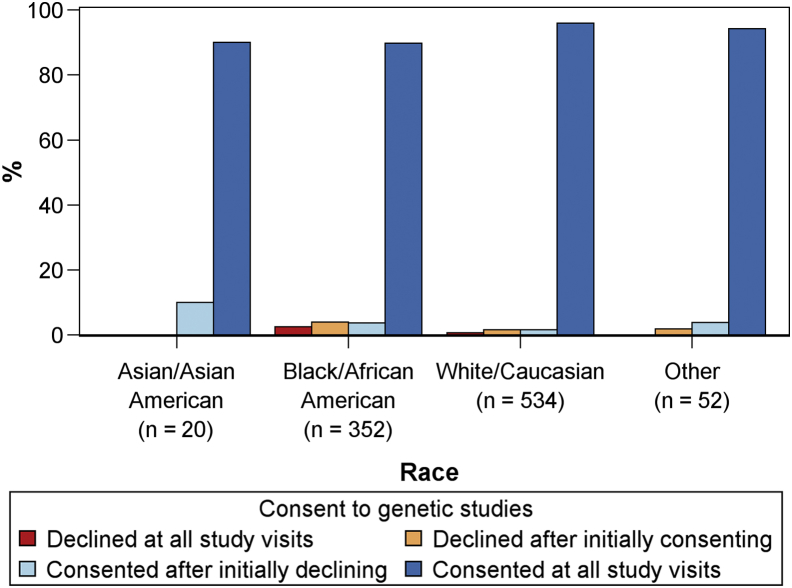
Consent status by race among patients with more than 1 study visit is displayed in Figure 2. The vast majority of participants in each group consented at all study visits.
Figure 2.
Genetic consent status over time in the Clinical Phenotyping and Resource Biobank Core (C-PROBE) by race among participants with more than 1 study visit (n = 958).
The informal survey found that all study coordinators felt knowledgeable and adequately trained in conducting genetic consent, and the majority of coordinators reported that they found the consent process easy to discuss with eligible candidates. Notably, all sites that had a >90% consent rate had either 1 or 2 coordinators during the observation period. However, site 4, with relatively lower genetic consent (85% baseline and 89% current), had more staff turnover, with 4 study coordinator transitions. Research coordinators at all sites reported that relatively few patients raised questions about the genetic component of the study, and that questions centered around privacy concerns, availability of data to insurance companies, and duration of storage.
Discussion
A major finding of this study is that the overwhelming majority of C-PROBE participants of all racial backgrounds and all ages were consistently willing to contribute DNA samples for future research in kidney disease. Although patterns of genetic consent have been described in the general population and in patients with other diseases, this study uniquely describes genetic consent rates among adult and pediatric participants with CKD. As in other study groups, African Americans show slightly more reluctance for genetic consent initially in the C-PROBE cohort compared to white Americans. However, with re-consent at subsequent study visits, this racial difference disappears, resulting in equivalent consent rates after accounting for study site variation. The evolution of consent with longitudinal participation has important implications for study design strategies, specifically, for health disparities research involving banking of genetic material. In contrast, site variations in genetic consent were persistent and remind us that in multisite studies, despite significant efforts to standardize procedures, local factors influence the extent of engagement of study populations. Finally, we demonstrate that a small but significant group of participants (5%) were inconsistent in their decision regarding genetic consent when asked annually. These results, along with variations by study site, highlight the complexity and sensitivity of the genetic consent process and issues surrounding biobank management.
As genetic-based research provides hope for personalized medicine in kidney disease and other life-threatening illnesses, there is increasing enthusiasm to collect, store, and collaboratively use genetic materials to advance therapies. The well-established opt-in consent process for use of DNA among participants engaged in research is a fundamental step. Insight into current patterns can guide best practices aimed at adequately informing eligible patients with kidney disease and ensuring their comfort with the process and their decision about genetic consent.
In the general population, the American Healthstyles Survey conducted in 1998 estimated that 1 in 5 Americans were unwilling to contribute DNA samples under any circumstances.12 More recent estimates suggest that participants were more agreeable to consent to genetic research. The Baltimore Epidemiologic Catchment Area survey, conducted from 2000 to 2004, found that 86% of participants consented to genetic studies and storage of their DNA for future research.7 The National Health and Nutritional Examination Survey, another general population survey, found 85% to 90% of participants willing to donate blood samples and consent to future genetic studies in 3 recruitment periods spanning 1999 to 2008.4, 5, 6 Growing support and level of comfort with genetic research may have occurred because of increasing public awareness of the potential value of genetics information in health and disease from the Human Genome Project, the use of gene-based cancer therapies, the United States Supreme Court decision to strike down gene patents,13 and, more recently, the commercialization of DNA testing kits.
Previous studies in various study populations have shown lower genetic consent rates in minority participants despite their willingness to participant in nongenetic components of the study.4, 5, 6, 7, 8, 9, 14 Our consent results in the C-PROBE cohort are consistent with 2 recent cardiovascular clinical studies, the Action to Control Cardiovascular Risk in Diabetes trial (ACCORD) and the Multi-Ethnic Study of Atherosclerosis study (MESA), in which overall consent rates were 89% and 95%, respectively.8 As observed in MESA and ACCORD, significantly more African American and Asian participants refused to consent at their initial C-PROBE visit independent of other factors such as diagnosis, education level, and study site. In contrast to MESA, we did not observe differences in consent rates by Hispanic ethnicity.
In longitudinal assessment, 5% of C-PROBE participants with 2 or more study visits have changed their genetic consent status during the study thus far. Re-consent rates, 18 months after initial consent, were also reported in MESA.9 Approximately 5% of the MESA cohort changed their status, with a significantly larger proportion of African Americans changing to refusal of genetic consent.9 In contrast, our annual consent results demonstrated no racial differences in current consent after adjusting for site variation. Overall, the group in longitudinal follow-up tended to be more agreeable to genetic studies (96%). Reasons for the change in consent status were not investigated here and are therefore unclear. Potentially, greater familiarity with the study and study team could play a role in increasing consent. Conversely, study team turnover leading to participant unfamiliarity may increase instability of consent status.
Interestingly, family history of CKD, reported in 24% of C-PROBE participants, did not influence genetic consent rates. In contrast, family history of cancer was a significant predictor of consent in cancer genetics research.14 The discrepancy in results among participants with cancer and kidney disease, along with the observations of evolving consent, may represent an opportunity to improve education of the potential benefits (and risks) of genetic kidney research.
Clearly, APOL1-related nephropathy is a research topic for which the contributions of people of African descent will be needed to advance our knowledge on genetic susceptibility. Although it is encouraging to see that initial racial differences in consent rates are minimized with longitudinal data collection, these findings may suggest that the nephrology community has an opportunity to more effectively engage patient populations and the public by conveying the potential impact for genetic-based research on kidney health, especially among minority populations.
Although we did not explore the reasons for the initial racial differences in C-PROBE consent rates, mistrust, racially based discrimination, lack of benefit, religion, risk perceptions, and the complexity of the informed consent process have been noted previously and could have affected consent rates in our study.15, 16, 17 For all populations with CKD, further insight into perceptions of risks, benefits, and uncertainty related to genetic research may help to guide effective approaches for genetic consent.
We did not observe differences in genetic consent rates between pediatric and adult participants. Genetic studies in pediatric research engender additional challenges.18, 19 Some studies have noted equivalent consent rates among adults and children,20, 21 whereas other studies have suggested that parents/guardians tend to be more restrictive when considering participation for minors.22 With 2 pediatric sites enrolling 15% of the study population, our study was not powered to detect small differences in consent outcomes between children and adults. Further investigation is needed to understand consent frequency in pediatric populations with kidney disease, and studies such as Chronic Kidney Disease in Children (CKiD) should be well positioned to provide additional information.23
As in other multisite studies,8, 9 we observed substantial variation in consent rates (85%–100%) among the 7 enrollment sites. Site variation in genetic consent may be due to the experience of the local study teams in obtaining consent, exposure to studies with genetic consent, consent procedures dictated by local institutional review boards, state policy, or local public opinion. It is noteworthy that the site that experienced the most staff turnover had relatively lower consent. Potentially, the comfort level of the participant in discussing genetic consent may be negatively affected by staff turnover. Unfortunately, there is little guidance on the most effective strategies for consenting to genetic research that would lead to standardization of techniques to assist study teams.24 Harmonization of policies across institutions and states around genetic consent may facilitate a national standard. Further insight into approaches that properly inform patients of the risks and benefits of genetic research may limit potential selection biases introduced by site differences in consent processes.
There are several limitations of this study. First, we did not directly determine reasons for willingness or refusal to consent among C-PROBE participants. We also did not link involvement of the primary nephrologist in recruitment with consent status. An increased engagement of the treating physician may have increased the participant’s level of trust with the study team. In addition, the pediatric population, added to the cohort more recently, was significantly smaller than the adult population and limited our ability to fully assess differences by age of participants. Also, the pediatric population were enrolled from 2 sites, whereas the adults were enrolled from 5 sites. Finally, when interpreting these results, it is critical to consider selection bias. The baseline consent analysis is limited to patients who consented to participate in C-PROBE; the most recent consent analysis is also limited to those who returned for a follow-up visit.
These results, however, indicate that further investigation of attitudes and insights regarding participants and consent processes are required to disentangle these influences and to define the most effective procedures for informed genetic consent in CKD research. Because C-PROBE does not return results of genetic research to participants, it is not known to what degree genetic research results might influence future consent rates.
In summary, we found that the majority of C-PROBE participants were willing to donate DNA samples for future CKD-focused genetic studies. Gaining a better understand of factors associated with changing consent status over time and how consent rates may differ by study site will help in creating unbiased population samples for genetic research.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was supported, in part, by The George M. O’Brien Michigan Kidney Translational Core Center (NIH/NIDDK: P30-DK081943-01), and the University of Michigan School of Medicine, Department of Internal Medicine, and Department of Pediatrics & Communicable Diseases. We would like to thank the patients and families who graciously participated in this research.
Footnotes
Appendix S1. Example C-PROBE consent form.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Example C-PROBE consent form.
References
- 1.Kopp J.B., Winkler C.A., Zhao X. Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol. 2015;26:1443–1448. doi: 10.1681/ASN.2013111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzur S., Rosset S., Shemer R. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sampson M.G., Robertson C.C., Martini S. Integrative genomics identifies novel associations with APOL1 risk genotypes in black NEPTUNE subjects. J Am Soc Nephrol. 2016;27:814–823. doi: 10.1681/ASN.2014111131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQuillan G.M., Porter K.S., Agelli M., Kington R. Consent for genetic research in a general population: the NHANES experience. Genet Med. 2003;5:35–42. doi: 10.1097/00125817-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 5.McQuillan G.M., Pan Q., Porter K.S. Consent for genetic research in a general population: an update on the National Health and Nutrition Examination Survey experience. Gen Med. 2006;8:354–360. doi: 10.1097/01.gim.0000223552.70393.08. [DOI] [PubMed] [Google Scholar]
- 6.McQuillan G.M., Porter K.S. Consent for future genetic research: the NHANES experience in 2007-2008. IRB. 2011;33:9–14. [PubMed] [Google Scholar]
- 7.Mezuk B., Eaton W.W., Zandi P. Participant characteristics that influence consent for genetic research in a population-based survey: the Baltimore Epidemiologic Catchment Area follow-up. Community Genet. 2008;11:171–178. doi: 10.1159/000113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simons-Morton D.G., Chan J.C., Kimel A.R. Characteristics associated with informed consent for genetic studies in the ACCORD trial. Contemp Clin Trials. 2014;37:155–164. doi: 10.1016/j.cct.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green D., Cushman M., Dermond N. Obtaining informed consent for genetic studies: the Multiethnic Study of Atherosclerosis. Am J Epidemiol. 2006;164:845–851. doi: 10.1093/aje/kwj286. [DOI] [PubMed] [Google Scholar]
- 10.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz G.J., Munoz A., Schneider M.F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S.S., Fridinger F., Sheedy K.M., Khoury M.J. Public attitudes regarding the donation and storage of blood specimens for genetic research. Commun Genet. 2001;4:18–26. doi: 10.1159/000051152. [DOI] [PubMed] [Google Scholar]
- 13.Ravicher D.B., Hassan S.Y., Cardozo B.N. The Association for Molecular Pathology, et al., Petitioners, v. Myriad Genetics, Inc., et al., Respondents (Supreme Court of the United States) On petition for a writ of certiorari to the United States Court of Appeals for the Federal Circuit. Biotechnol Law Rep. 2012;31:607–618. [Google Scholar]
- 14.Ford B.M., Evans J.S., Stoffel E.M. Factors associated with enrollment in cancer genetics research. Cancer Epidemiol. 2006;15:1355–1359. doi: 10.1158/1055-9965.EPI-05-0816. [DOI] [PubMed] [Google Scholar]
- 15.Mittman I.S., Secundy M.G. A national dialogue on genetics and minority issues. Community Genet. 1998;1:190–200. doi: 10.1159/000016162. [DOI] [PubMed] [Google Scholar]
- 16.Schulz A., Caldwell C., Foster S. “What are they going to do with the information?” Latino/Latina and African American perspectives on the Human Genome Project. Health Educ Behav. 2003;30:151–169. doi: 10.1177/1090198102251026. [DOI] [PubMed] [Google Scholar]
- 17.Halverson C.M., Ross L.F. Engaging African-Americans about biobanks and the return of research results. J Community Genet. 2012;3:275–283. doi: 10.1007/s12687-012-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klima J., Fitzgerald-Butt S.M., Kelleher K.J. Understanding of informed consent by parents of children enrolled in a genetic biobank. Genet Med. 2014;16:141–148. doi: 10.1038/gim.2013.86. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman D., Geller G., Leroy L. Ethical implications of including children in a large biobank for genetic-epidemiologic research: a qualitative study of public opinion. Am J Med Genet Part C Semin Med Genet. 2008;148C:31–39. doi: 10.1002/ajmg.c.30159. [DOI] [PubMed] [Google Scholar]
- 20.Papaz T., Safi M., Manickaraj A.K. Factors influencing participation in a population-based biorepository for childhood heart disease. Pediatrics. 2012;130:e1198–e1205. doi: 10.1542/peds.2012-0687. [DOI] [PubMed] [Google Scholar]
- 21.Wu A.C., Davis R., Tantisira K. Acceptance of asthma pharmacogenetic study by children and adults. J Pharmacogenom Pharmacoproteom. 2011;2:103. doi: 10.4172/2153-0645.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burstein M.D., Robinson J.O., Hilsenbeck S.G. Pediatric data sharing in genomic research: attitudes and preferences of parents. Pediatrics. 2014;133:690–697. doi: 10.1542/peds.2013-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furth S.L., Cole S.R., Moxey-Mims M. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jallo N., Lyon D.E., Kinser P.A. Recruiting for epigenetic research: facilitating the informed consent process. Nurs Res Pract. 2013;2013:935740. doi: 10.1155/2013/935740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Example C-PROBE consent form.