Abstract
Introduction
Chronic kidney disease (CKD) and acute kidney injury (AKI) are strongly associated with excess morbidity and mortality and frequently co-occur in critically ill septic patients, but how their interplay affects clinical outcomes is not well elucidated.
Methods
We conducted a single-center, retrospective cohort study of 2632 adult patients admitted to the intensive care unit (ICU) with severe sepsis or septic shock. Subjects were classified into 6 groups according to baseline CKD (no-CKD: estimated glomerular filtration rate [eGFR] ≥60; CKD: eGFR 15−59 ml/min per 1.73 m2) and incident AKI by the Kidney Disease: Improving Global Outcomes (KDIGO) serum creatinine criteria (no-AKI, AKI stage 1, AKI stages ≥2) during ICU stay. Study outcomes were 90-day mortality (in hospital or within 90 days of discharge) and incident/progressive CKD.
Results
Prevalent CKD was 46% and incident AKI was 57%. Adjusted hazard ratios (95% confidence intervals) for 90-day mortality relative to the reference group of no-CKD/no-AKI were 1.5 (1.1−2.0) in no-CKD/AKI stage 1, 2.4 (1.9−3.1) in no-CKD/AKI stages≥2, 1.1 (0.8−1.4) in CKD/no-AKI, 1.2 (0.9−1.6) in CKD/AKI stage 1, and 2.2 (1.7−2.9) in CKD/AKI stages ≥2. A similar trend was observed for incident/progressive CKD during a median follow-up of 15.3 months.
Conclusion
Stage 1 AKI on CKD was not associated with an independent increased risk of adverse outcomes in critically ill septic patients. AKI stages ≥2 on CKD and any level of AKI in no-CKD patients were strongly and independently associated with adverse outcomes. Sepsis-associated stage 1 AKI on CKD may represent distinct underlying pathophysiology, with more prerenal cases and less severe de novo intrinsic damage, which needs further investigation.
Keywords: AKI, CKD, mortality, outcomes, sepsis
See Commentary on Page 1258
AKI is a serious condition that affects at least 1 out of 2 critically ill patients and is associated with adverse short- and long-term outcomes.1, 2 About 50% of patients with AKI requiring renal replacement therapy in the ICU die in the hospital, and AKI survivors have an excess risk of mortality over the following years.3, 4, 5, 6 Other dire sequelae of AKI include the development and progression of CKD or end-stage renal disease.7, 8, 9, 10, 11, 12, 13, 14, 15 Conversely, CKD patients carry an elevated risk of AKI, so that AKI and CKD predispose to each other in a vicious circle and may exert negative impacts independently or synergistically.16, 17, 18, 19, 20, 21, 22 Approximately 30% of critically ill AKI patients have underlying CKD.23, 24 CKD by itself portends worse outcomes in hospitalized patients and is associated with a marked increase in the long-term risk of death and cardiovascular events.25, 26, 27
Both AKI and CKD confer adverse prognosis; however, whether and how their coexistence affects outcomes has not been well elucidated. In several observational studies, AKI on CKD patients were found unexpectedly to have better in-hospital outcomes than their counterparts with AKI but without CKD, including shorter length of hospital stay and lower mortality rates.28, 29, 30, 31, 32 As for long-term outcomes in AKI survivors, pre-existing CKD has been associated with higher risk of progression to end-stage renal disease and death.32, 33, 34, 35, 36 Nevertheless, the great majority of these studies adopted heterogeneous, nonconsensus definitions of AKI, and did not take into account the severity of AKI episodes, thus making it difficult to fully appreciate the prognostic impact of AKI on CKD for clinical practice and research purposes.
The objective of our study was to examine how the combination of prevalent CKD and incident AKI, defined by the KDIGO criteria and stratified by AKI severity,37 are related to short- and long-term outcomes in critically ill septic patients, a setting in which both CKD and AKI are common and overall prognosis is dismal.38, 39, 40, 41
Methods
Population and Variables
We conducted a single-center, retrospective cohort study of patients admitted to the ICU in an urban tertiary care hospital. The observation period was from May 2007 to April 2012. Clinical variables were extracted from electronic health records (EHRs) and validated through comprehensive individual review of 10% of EHRs by data management personnel blinded to the study.
The analysis included adult (≥18 years old) patients who were admitted to the ICU with a diagnosis of severe sepsis or septic shock, defined on the basis of International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes, as per the criteria of Angus et al.,42 and had at least 1 serum creatinine (sCr) measurement during the 3 months prior to the index hospitalization. The closest sCr >1 day and <3 months before the index hospitalization was used to calculate the baseline estimated glomerular filtration rate (eGFR) through the 4-variable Modification of Diet in Renal Disease (MDRD) study equation.43 A cutoff of 60 ml/min per 1.73 m2 eGFR was used to define CKD at baseline (eGFR ≥60 ml/min per 1.73 m2 = no-CKD; eGFR <60 ml/min per 1.73 m2 = CKD). Patients with end-stage renal disease or baseline eGFR <15 ml/min per 1.73 m2 were excluded. AKI was defined as ≥26.4 μmol/l (0.3 mg/dl) or 50% increase in sCr from baseline to the peak value during the ICU stay and was graded according to KDIGO SCr criteria.37 Patients were then classified as no-AKI, AKI 1 (AKI stage 1), or AKI ≥2 (AKI stage 2 or 3).
Comorbidities were identified through ICD-9-CM codes, except anemia, which was defined as admission hematocrit <39% for men and <36% for women. Data regarding drug exposure (within 72 hours of ICU stay), red blood cell transfusion (within 72 hours of ICU stay), and mechanical ventilation during ICU stay were derived from hospital billing codes. Scores of critical illness severity (Sequential Organ Failure Assessment [SOFA]44 and Acute Physiology and Chronic Health Evaluation II [APACHE II]45) scores were calculated on the basis of clinical and laboratory data within the first 24 hours of ICU admission. The SOFA score was expressed as “nonrenal SOFA,” equivalent to the SOFA score minus the points attributed for renal function.
Study Outcomes
Two outcomes were evaluated: (i) 90-day mortality, defined as all-cause mortality during the index hospitalization or up to 90 days following hospital discharge, as captured from EHRs; and (ii) incident/progressive CKD, determined by comparing baseline eGFR (before index hospitalization) with the follow-up eGFR derived from the mean of the last 2 sCr measurements (91% of subjects) or last sCr measurement (9% of subjects) available in the EHRs, and obtained at least 90 days after hospital discharge. The definitions of incident and progressive CKD were adapted from the ones proposed in the Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) Study.46 Specifically, incident CKD was defined in no-CKD patients who had a reduction in eGFR of at least 25% relative to baseline and reached an eGFR <60 ml/min per 1.73 m2. Progressive CKD was defined in patients with pre-existent CKD as a decrease in eGFR of at least 50% relative to baseline or progression to CKD Stage 5 (eGFR <15 ml/min per 1.73 m2). The documentation of renal replacement therapy sessions reported in the EHRs during the follow-up period was adopted as an alternative criterion to adjudicate incident or progressive CKD. Death events were also captured from the EHRs.
Statistical Analyses
Study subjects were classified into 6 groups according to baseline eGFR (no-CKD, CKD) and AKI status during the ICU stay (no-AKI; AKI Stage 1; AKI Stages ≥2). Categorical data were reported as percentages, and continuous variables as mean ± SD or median (25th−75th percentile) according to data distribution. Comparisons between groups for categorical variables were made using the Fisher exact test. Continuous variables were compared with analysis of variance and model pairwise contrasts or with Kruskal−Wallis tests as per data distribution. Trends of variables across AKI categories were tested by Jonckheere−Terpstra and Cochran−Armitage trend tests for continuous and categorical variables, respectively. Two-sided P values <0.05 were considered statistically significant.
The CKD/AKI groups were modeled as categorical predictors for study outcomes in Cox regression hazard models. The interaction between CKD and AKI was assessed for both study outcomes and, since a significant interaction (AKI×CKD) was identified for 1 of the study outcomes (incident/progressive CKD, P = 0.079), we present all models using the no-CKD/no-AKI group as the reference group for comparison to the other 5 groups. The interaction between SOFA score and CKD/AKI groups was nonsignificant for both study outcomes. The proportional hazards assumption was satisfied for all study outcomes and tested by interaction of time and predictor and graphic assessment.
Candidate variables tested as confounders included demographic data, comorbidity-related factors, and indicators of critical illness severity. Two multivariable models were generated. Model 1 included the following variables: age, gender, race; the presence of diabetes, systolic heart failure and anemia; nonrenal SOFA score, the need for mechanical ventilation; the exposure to red blood cell transfusion or statins, and the length of hospital stay (the latter only for the outcome of incident/progressive CKD). Model 2 included variables deemed of particular clinical relevance (age, gender, race, diabetes), together with additional variables that were retained through backward selection (cut-off for retention: P < 0.10). In the event of collinearity between variables (SOFA and APACHE II scores), only 1 of 2 variables was included. Data analysis and figures were generated using SAS 9.4 (SAS Institute, Cary, NC) and GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA).
Sensitivity Analyses
Examination of AKI Stage 1 Based on Absolute and Relative Changes in sCr
We reclassified cases of AKI stage 1 into 2 groups: (i) AKI 1-absolute: patients who had an absolute increase in sCr ≥26.4 μmol/l (0.3 mg/dl) from baseline and only a relative increase in sCr <1.5 times baseline; and (ii) AKI 1-relative: patients with a relative increase in sCr 1.5 to 1.9 times baseline, independently of absolute sCr changes. When combined with CKD status and the other AKI groups, this reclassification of AKI stage 1 cases resulted in 8 CKD/AKI groups. These groups were used as categorical predictors for study outcomes in Cox regression hazard models, similarly to our primary analysis.
Examination of Potential Misclassification of AKI as CKD When Assessing CKD Outcome
We evaluated the reclassification of the CKD outcome when single last sCr versus the mean of the last 2 sCr measurements was used in patients with follow-up sCr measurements <7 days apart. We excluded the reclassified patients from the analysis in addition to patients with the upper and lower 2.5% of the distribution for percentage change in follow-up eGFR from baseline to avoid implausible extrapolation of associations at the extremes of the distribution where statistical power was lower.
Results
Clinical Characteristics
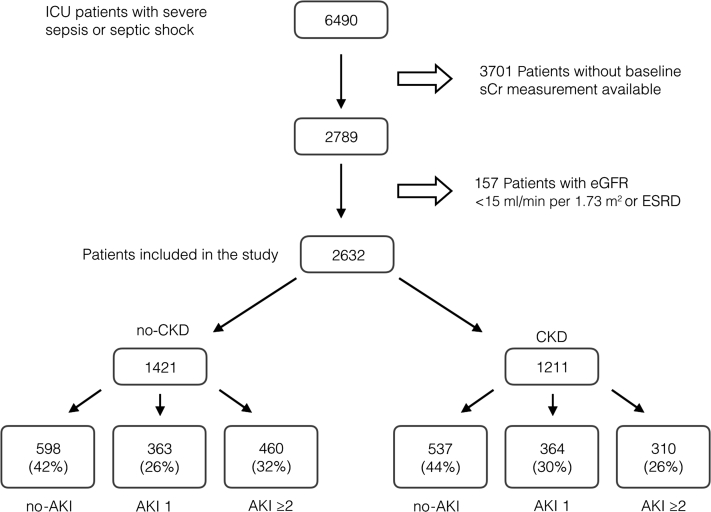
Figure 1 details how the cohort was generated. Of the 2632 patients included in the current analysis, 46% had baseline CKD and 57% developed AKI during the ICU stay. Within the CKD group, 77.8% of cases had a baseline eGFR between 30 and 60 ml/min per 1.73 m2. The overall incidence of AKI was similar in patients with and without baseline CKD (56% vs. 58%, respectively, P = 0.252).
Figure 1.
Study cohort derivation. AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; ICU, intensive care unit; sCr, serum creatinine.
Clinical characteristics of patients, stratified by the 6 CKD/AKI groups, are summarized in Table 1. The severity of AKI ≥2 was distributed as follows: 264 patients (34.3%) had AKI stage 2, 268 (34.8%) had stage 3, and 238 (30.9%) had stage 3 requiring renal replacement therapy. Relative to no-CKD, CKD subjects were older and more frequently had diabetes. Exposure to i.v. or intra-arterial iodinated contrast and aminoglycosides was significantly lower in CKD patients. In contrast, CKD patients were more likely to be prescribed diuretics and statins. The need for vasoactive drugs (pressors or inotropes) and for mechanical ventilation support and indexes of severity of illness increased in parallel with presence and severity of AKI, both in no-CKD and in CKD patients. There were no major differences in critical illness severity indicators between groups with the same AKI status, either with or without baseline CKD (Table 1).
Table 1.
Demographic and clinical characteristics of the study cohort by the 6 CKD/AKI groups
| Characteristics | no-CKD/no-AKI n = 598 | no-CKD/AKI 1 n = 363 | no-CKD/AKI≥2 n = 460 | CKD/no-AKI n = 537 | CKD/AKI 1 n = 364 | CKD/AKI ≥2 n = 310 |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, yr | 61.8 ± 16.5 | 64.4 ± 16.0a | 61.2 ± 15.3 | 70.5 ± 14.6b,c | 70.8 ± 14.3b,c | 66.9 ± 14.1b,c,d |
| Men, % | 55.9 | 60.1 | 56.5 | 45.1b,c | 46.4a,c | 58.1e |
| African American, % | 41.5 | 43.8 | 50.3a,d | 33.1a,f | 34.9a,f | 43.2d,f |
| Comorbidities | ||||||
| Baseline sCr, μmol/l | 80 (71–97) | 88 (71–97)a | 80 (62–97) | 141 (115–186)b,c | 133 (115–168)b,c | 150 (124–221)b,c |
| Baseline eGFR, ml/min per 1.73 m2 | 84.5 (70.8–105.1) | 80.7 (69.1–94.9)a | 88.1 (70.8–110.5) | 41.5 (30.3–50.6)b,c | 44.7 (37.4–51.3)b,c | 39.6 (28.1–50.7)b,c |
| Diabetes, % | 17.1 | 23.1a | 18.0 | 25.7b,c | 26.4b | 25.2a,f |
| Systolic heart failure, % | 3.0 | 4.7 | 1.7 | 2.6 | 4.1 | 5.2f |
| Anemia, % | 85.5 | 82.4 | 89.7a | 86.4 | 90.0a,f | 88.3 |
| Drug exposure | ||||||
| Diureticsg, % | 39.5 | 53.2b | 40.4 | 48.6a,f | 55.8b | 47.4a,f |
| Statinsg, % | 26.8 | 30.6 | 19.8a,d | 35.9b,c | 42.9b,c | 30.0f |
| Iodine contrastg, % | 34.3 | 33.6 | 20.4b,e | 21.2b,c | 15.9b,c | 14.8b,d,f |
| Aminoglycosidesg, % | 11.5 | 12.4 | 11.5 | 6.5a,f | 6.3a,f | 6.1a,f |
| Critical illness indicators | ||||||
| Oliguria, % | 3.3 | 5.7 | 21.8b,e | 5.4 | 9.3b | 32.2b,e,f |
| CFB at 72 h, L | 2.8 ± 5.4 | 3.5 ± 6.6 | 7.3 ± 7.7b,e | 2.8 ± 5.6 | 2.6 ± 6.0 | 6.1 ± 8.5b,e,f |
| LOS, d | 12 (7–22) | 11 (6–18)a | 13 (7–24) | 12 (7–20)a,f | 11 (6–18)b | 15 (7–26)d |
| Pressors or inotropesg, % | 28.8 | 34.4 | 51.3b,e | 27.7 | 35.4a | 50.3b,e |
| Mechanical ventilation, % | 38.1 | 43.0 | 52.6b,e | 35.6 | 40.1 | 48.4a,e |
| RBC transfusiong, % | 3.2 | 3.3 | 3.5 | 3.5 | 2.7 | 2.9 |
| APACHE II | 11.2 ± 6.2 | 12.2 ± 6.2a | 14.3 ± 7.2b,e | 12.3 ± 5.9a,f | 13.4 ± 6.5b,f | 15.6 ± 7.8b,f,e |
| Nonrenal SOFA | 3 (1–6) | 4 (2–7)b | 6 (3–9)b,e | 4 (2–6)a,f | 4 (2–7)b | 6 (3–10)b,e |
APACHE II, Acute Physiology and Chronic Health Evaluation II; CFB, cumulative fluid balance; eGFR, estimated glomerular filtration rate; LOS, length of stay in the hospital; RBC, red blood cell; sCr, serum creatinine; SOFA, Sequential Organ Failure Assessment.
Data are presented as mean ± SD, median (25th–75th percentile), or percentage. APACHE II and nonrenal SOFA scores were calculated with data from the first 24 hours of ICU stay.
Significant difference (P < 0.05) relative to reference group no-CKD/no-AKI.
Significant difference (P < 0.001) relative to reference group no-CKD/no-AKI.
Significant difference (P < 0.001) between the CKD group and the correspondent no-CKD group with the same AKI status (CKD/no-AKI vs. no-CKD/no-AKI; CKD/AKI 1 vs. no-CKD/AKI 1; CKD/AKI ≥2 vs. no-CKD/AKI ≥2).
Significant P for trend (P < 0.05) across the different AKI status groups, either in no-CKD or CKD (trend across no-CKD/no-AKI, no-CKD/AKI 1 and no-CKD/AKI ≥2 or across CKD/no-AKI, CKD/AKI 1 and CKD/AKI ≥2).
Significant P for trend (P < 0.001, respectively) across the different AKI status groups, either in no-CKD or CKD (trend across no-CKD/no-AKI, no-CKD/AKI 1 and no-CKD/AKI ≥2 or across CKD/no-AKI, CKD/AKI 1 and CKD/AKI ≥2).
Significant difference (P < 0.05) between the CKD group and the correspondent no-CKD group with the same AKI status (CKD/no-AKI vs. no-CKD/no-AKI; CKD/AKI 1 vs. no-CKD/AKI 1; CKD/AKI ≥2 vs. no-CKD/AKI ≥2).
Exposure to drugs/RBC transfusions was based on the first 72 hours of intensive care unit admission.
Ninety-Day Mortality Outcome
The crude 90-day mortality rate was 26.7%. Crude mortality proportions and time-to-event analysis in the 6 CKD/AKI study groups are shown in Table 2 and Figure 2, respectively. The 90-day mortality was lowest in the no-CKD/no-AKI group (17.4%), closely followed, in ascending order, by CKD/no-AKI (19.7%), CKD/AKI 1 (22.5%), and no-CKD/AKI 1 (25.6%). Groups with AKI ≥2, either with or without CKD, had the highest mortality, which was above 40% in both groups (Table 2).
Table 2.
Crude proportion of study outcomes stratified by the 6 CKD/AKI groups
| Group | 90-Day mortality | Incident/progressive CKD |
|---|---|---|
| no-CKD | ||
| no-AKI | 104/598 (17.4%) | 33/321 (10.3%) |
| AKI 1 | 93/363 (25.6%) | 42/183 (22.9%) |
| AKI ≥2 | 193/460 (42.0%) | 51/175 (29.1%) |
| CKD | ||
| no-AKI | 106/537 (19.7%) | 24/259 (9.3%) |
| AKI 1 | 82/364 (22.5%) | 20/181 (11.0%) |
| AKI ≥2 | 125/310 (40.3%) | 33/116 (28.4%) |
AKI, acute kidney injury; CKD, chronic kidney disease.
The 90-day mortality denotes all-cause death during index hospital admission or within 90 days of hospital discharge. Incident/progressive CKD (in patients without and with baseline CKD, respectively) was adjudicated in survivors during median follow-up of 15.3 months.
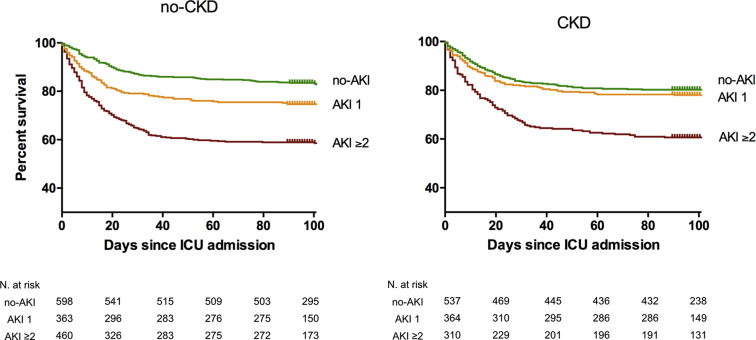
Figure 2.
Ninety-day mortality across chronic kidney disease (CKD)/acute kidney injury (AKI) groups. For the purpose of clarity, the no-CKD and CKD groups are represented on the left and right chart, respectively. no-CKD: baseline eGFR ≥60; CKD: baseline eGFR <60; no-AKI: no AKI occurrence; AKI 1: KDIGO-sCr stage 1 AKI; AKI ≥2: KDIGO-sCr stage 2 or higher. ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes; sCr, serum creatinine.
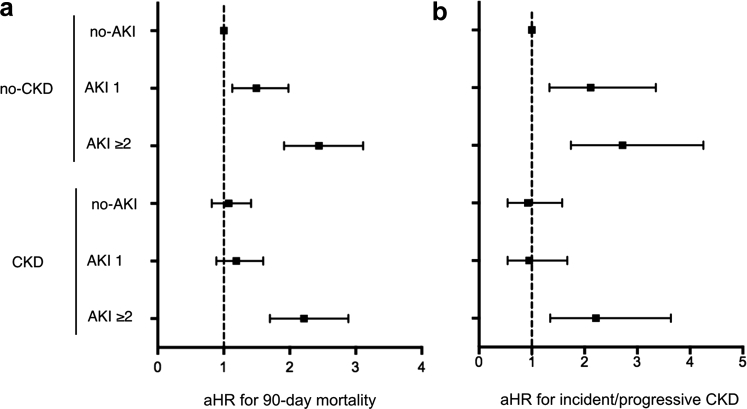
After multivariable adjustment for demographic and clinical variables relative to the reference group of no-CKD/no-AKI, the risk for 90-day mortality significantly increased in a stepwise incremental fashion according to the presence and severity of AKI in the no-CKD groups (Table 3 and Figure 3a). The CKD/no-AKI and CKD/AKI 1 groups were associated with a slight, but statistically non-significant, increase in the risk of 90-day mortality. Importantly, the risk for 90-day mortality significantly increased in the CKD/AKI ≥2 group (adjusted hazard ratio = 2.23, 95% confidence interval = 1.71−2.91), similarly to the corresponding no-CKD counterparts (adjusted hazard ratio = 2.45, 95% confidence interval = 1.92−3.12) (Table 3).
Table 3.
Multivariable models for 90-day mortality (dependent variable) in the 6 CKD/AKI groups (independent variable)
| Variable | Model 1 aHR (95% CI) | P | Model 2 aHR (95% CI) | P |
|---|---|---|---|---|
| no-CKD | ||||
| no-AKI | Reference | Reference | ||
| AKI 1 | 1.50 (1.13–1.99) | 0.005 | 1.50 (1.13–1.98) | 0.005 |
| AKI ≥2 | 2.45 (1.92–3.12) | <0.001 | 2.44 (1.92–3.11) | <0.001 |
| CKD | ||||
| no-AKI | 1.06 (0.81–1.40) | 0.657 | 1.07 (0.82–1.41) | 0.609 |
| AKI 1 | 1.19 (0.89–1.60) | 0.238 | 1.19 (0.89–1.60) | 0.245 |
| AKI ≥2 | 2.23 (1.71–2.91) | <0.001 | 2.22 (1.70–2.89) | <0.001 |
| Age, per 1 yr | 1.02 (1.01–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| African American | 0.88 (0.75–1.02) | 0.098 | 0.89 (0.76–1.04) | 0.127 |
| Male gender | 1.08 (0.93–1.26) | 0.325 | 1.08 (0.93–1.26) | 0.321 |
| Diabetes | 0.89 (0.74–1.08) | 0.239 | 0.89 (0.74–1.08) | 0.239 |
| Systolic heart failure | 0.68 (0.43–1.09) | 0.111 | — | |
| Anemia | 1.29 (1.01–1.66) | 0.046 | 1.29 (1.00–1.65) | 0.049 |
| Nonrenal SOFA ≥4 | 1.38 (1.16–1.66) | <0.001 | 1.39 (1.16–1.66) | <0.001 |
| Mechanical ventilation | 2.22 (1.88–2.62) | <0.001 | 2.21 (1.87–2.60) | <0.001 |
| RBC transfusion | 1.32 (0.89–1.99) | 0.171 | — | |
| Exposure to statins | 0.68 (0.57–0.81) | <0.001 | 0.67 (0.56–0.80) | <0.001 |
APACHE II score was not included in the models due to collinearity with the SOFA score; aHR, adjusted hazard ratio; AKI, acute kidney injury; APACHE II, Acute Physiology and Chronic Health Evaluation II; CI, confidence interval; CKD, chronic kidney disease; RBC, red blood cell; SOFA, Sequential Organ Failure Assessment.
Data are aHRs and 95% CIs for 90-day mortality by Cox proportional hazard regression. Model 1 includes all tested variables, whereas model 2 includes variables of deemed particular clinical relevance (age, race, gender, diabetes), together with additional variables that were retained through backward selection (cut-off for retention: P < 0.10).
Figure 3.
Adjusted hazard ratios (aHRs) for study outcomes. Summary of adjusted hazard ratios (dots) and 95% confidence intervals (lines) for (a) 90-day mortality (model 1) and (b) incident/progressive chronic kidney disease (CKD) (model 1). no-CKD: baseline eGFR ≥60; CKD: baseline eGFR <60; no-AKI: no AKI occurrence; AKI 1: KDIGO-sCr stage 1 AKI; AKI ≥2: KDIGO-sCr stage 2 or higher. AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes; sCr, serum creatinine.
Other variables independently associated with 90-day mortality were age, nonrenal SOFA score ≥4, mechanical ventilation, anemia at ICU admission (all positive associations), and statin exposure in the first 72 hours of ICU stay (negative association) (Table 3).
Incident/Progressive CKD Outcome
Follow-up sCr was available in 1235 patients, corresponding to 64.0% of ICU survivors at 90 days postdischarge. Median follow-up time was 15.3 (5.7−29.2) months. The proportions of the incident/progressive CKD outcome and the corresponding adjusted hazard ratios for the CKD/AKI groups, in reference to no-CKD/no-AKI, are summarized in Tables 2 and 4 and Figure 3b.
Table 4.
Multivariable models for incident/progressive CKD (dependent variable) in the 6 CKD/AKI groups (independent variable)
| Variable | Model 1 aHR (95% CI) | p | Model 2 aHR (95% CI) | P |
|---|---|---|---|---|
| no-CKD | ||||
| no-AKI | Reference | Reference | ||
| AKI 1 | 2.11 (1.33–3.35) | 0.001 | 2.13 (1.34–3.37) | 0.001 |
| AKI ≥2 | 2.72 (1.74–4.25) | <0.001 | 2.79 (1.79–4.34) | <0.001 |
| CKD | ||||
| no-AKI | 0.92 (0.54–1.58) | 0.769 | 0.94 (0.55–1.61) | 0.835 |
| AKI 1 | 0.95 (0.54–1.67) | 0.863 | 0.96 (0.54–1.68) | 0.878 |
| AKI ≥2 | 2.21 (1.35–3.64) | 0.002 | 2.33 (1.43–3.80) | <0.001 |
| Age, per 1 yr | 1.01 (1.00–1.02) | 0.093 | 1.01 (1.00–1.02) | 0.112 |
| African American | 1.28 (0.97–1.71) | 0.083 | 1.28 (0.96–1.70) | 0.087 |
| Male gender | 0.98 (0.74–1.30) | 0.880 | 0.98 (0.74–1.29) | 0.867 |
| Diabetes | 0.98 (0.71–1.36) | 0.924 | 0.98 (0.71–1.36) | 0.921 |
| Systolic heart failure | 0.85 (0.40–1.82) | 0.678 | — | |
| Anemia | 1.87 (1.10–3.18) | 0.021 | 1.88 (1.11–3.19) | 0.019 |
| Nonrenal SOFA ≥4 | 1.57 (1.14–2.15) | 0.005 | 1.48 (1.11–1.98) | 0.008 |
| Mechanical ventilation | 0.83 (0.60–1.15) | 0.259 | — | |
| RBC transfusion | 0.87 (0.35–2.13) | 0.760 | — | |
| LOS, per 1 d | 1.11 (0.81–1.51) | 0.505 | — | |
| Exposure to statins | 0.93 (0.69–1.27) | 0.671 | — |
APACHE II score was not included in the models due to collinearity with the SOFA score; aHR, adjusted hazard ratio; AKI, acute kidney injury; APACHE II, Acute Physiology and Chronic Health Evaluation II; CI, confidence interval; CKD, chronic kidney disease; LOS, length of stay in the hospital; RBC, red blood cells; SOFA, Sequential Organ Failure Assessment.
Data are aHRs and 95% CIs for incident/progressive CKD by Cox proportional hazard regression. Model 1 included all tested variables, while model 2 includes variables of deemed particular clinical relevance (age, race, gender, diabetes), together with additional variables that were retained through backward selection (cut-off for retention: P < 0.10).
Similar to the 90-day mortality outcome, in patients without baseline CKD, the risk for incident CKD increased in a stepwise fashion by AKI severity. In the presence of pre-existing CKD, however, the risk of progressive CKD was significantly higher only in patients with AKI ≥2 (Table 4, Figure 3b). Factors independently associated with incident/progressive CKD were nonrenal SOFA ≥4 and anemia at ICU admission (all positive associations) (Table 4). Crude mortality during follow-up was 6.5% and 9.3% among patients with and without baseline CKD, respectively.
Sensitivity Analyses
Examination of AKI Stage 1 Based on Absolute and Relative Changes in sCr
We reclassified patients with AKI stage 1 into 2 groups according to whether they met the criterion of relative increase in sCr 1.5 to 1.9 times baseline (AKI 1-relative) or not (AKI 1-absolute). The main purpose of this sensitivity analysis was to test whether, in the presence of baseline CKD, the 2 groups of stage 1 AKI were differentially associated with study outcomes. Similar to our primary analysis, the adjusted hazard ratios (95% confidence intervals) for 90-day mortality were 1.16 (0.82−1.64) and 1.25 (0.84−1.85) in CKD/AKI 1-absolute and CKD/AKI 1-relative, respectively (reference no-CKD/no-AKI). Concordant findings were observed for incident/progressive CKD. The complete results are reported in Supplementary Tables S1 and S2.
Examination of Potential Misclassification of AKI as CKD When Assessing CKD Outcome
We found that only 10 patients had 2 follow-up SCr measurements <7 days apart and were reclassified in relation to their CKD outcome if single last SCr or the mean of the last 2 sCr measurements was used. After excluding these 10 patients in addition to patients with the upper and lower 2.5% of the distribution for percentage change in follow-up eGFR from baseline (n = 36), the multivariable analysis showed concordant findings with our primary analysis (Supplementary Table S3).
Discussion
We studied how prevalent CKD and incident AKI jointly affect the prognosis of critically ill patients with sepsis. Our key finding is that, in the presence of underlying CKD, AKI stage 2 or 3, but not stage 1, was independently associated with increased risk of 90-day mortality and progressive CKD during the median observation period of 15.3 months. In contrast, in patients without pre-existing CKD, the risk of 90-day mortality and incident CKD significantly increased in a stepwise incremental fashion according to the stages of AKI severity.
Previous reports have suggested that pre-existing CKD might attenuate the adverse prognosis of patients with AKI.28, 29, 31, 32 For instance, in the Program to Improve Care in Acute Renal Disease (PICARD) study, a prospective cohort of critically ill patients with AKI, prior history of CKD was independently associated with reduced in-hospital mortality and shorter length of ICU stay in AKI patients.32 Our results seem congruent with the observation that prevalent CKD does not significantly worsen prognosis in critically ill patients with mild AKI. However, these findings are not fully concordant with those of other studies. For example, Thakar et al. described a cohort of more than 30,000 subjects undergoing elective or emergent cardiac surgery and, after adjustment for confounders, found that AKI patients with underlying CKD had a higher risk of hospital mortality than those without CKD.47 It is interesting that in that cohort, any degree of CKD, independently of AKI, conferred an excess risk of mortality. On the contrary, we found that prevalent CKD without incident AKI was not independently associated with an increased risk of 90-day mortality. This divergence could be due to differences in the study populations, with CKD per se playing a key role in more stable cardiac surgery patients but a less relevant role in our cohort of critically ill septic patients (2.2% vs. 26.7% all-cause mortality, respectively).
Another study reporting CKD/AKI-associated outcomes was conducted in more than 43,000 hospitalized patients in Canada.48 Notably, prevalent CKD without incident AKI was found to be independently associated with in-hospital mortality, but only in patients with baseline eGFR <30 ml/min per 1.73 m2. This may help in interpreting our findings, as only one-fourth of our CKD patients had a baseline eGFR <30 ml/min per 1.73 m2, possibly attenuating the putative association. Similar to our study, the authors reported that the risk of in-hospital mortality for AKI patients with pre-existing CKD was similar or lower than their counterparts with the same AKI severity, but no CKD.48 However, discrepant with our analysis is that stage 1 AKI was associated with excess risk of in-hospital mortality across all strata of baseline eGFR. It is possible that the smaller sample size of our study limited statistical power and the ability to detect small effects. Alternatively, our findings might suggest that the prognostic impact of stage 1 AKI is attenuated in patients with underlying CKD. This phenomenon may be specific to critically ill septic patients, as opposed to a generic group of hospitalized patients in the Canadian study.48
Interestingly, the analysis of the outcome of incident or progressive CKD during the median observation period of 15.3 months revealed a pattern similar to the 90-day mortality outcome. In patients without prevalent CKD, any level of AKI worsened outcomes in a stepwise incremental fashion according to AKI severity, whereas in the presence of baseline CKD, only AKI stage 2 or above conferred a significant excess in risk. These findings reinforce the notion that, in the setting of severe sepsis or septic shock, the diagnosis of stage 1 AKI, when superimposed on CKD, did not significantly worsen prognosis, in either the short- or long-term follow-up. One explanation for this is that, in the presence of CKD, the definition of stage 1 AKI may capture erratic oscillations in sCr of limited clinical significance. This was shown to be the case in a cohort of hospitalized veterans, in which an increase in sCr of 26.4 to 35.2 μmol/l (0.3−0.4 mg/dl) during hospital admission was associated with excess in-hospital mortality only in patients with baseline eGFR above 30 ml/min per 1.73 m2, but not in those with CKD stages 4 to 5.49 Similarly, Lin et al. demonstrated that, in the setting of higher baseline sCr levels, the inherent biological and laboratory variation in sCr measurement contributes to a substantial rate of false-positive cases of AKI, especially when the criterion of absolute increase in sCr is applied to define AKI.50 However, our sensitivity analysis suggests that the use of absolute sCr increases to classify AKI stage 1 did not influence our findings.
An alternative explanation is that, in the setting of sepsis, AKI on CKD, specifically stage 1 AKI, is pathophysiologically distinct from that found in patients without underlying CKD. Transient renal hypoperfusion frequently occurs in sepsis and is likely to result in appreciable increases in sCr in patients with CKD who already have diminished renal reserve. In contrast, transient renal hypoperfusion can remain subclinical in subjects with well-preserved baseline renal function. Therefore, cases of stage 1 AKI on CKD, compared with their counterparts without CKD, may reflect lesser degrees of underlying renal/systemic injury and entail a greater component of prerenal pathophysiology, with limited or no de novo intrinsic renal damage. An additional observation supporting this hypothesis was made by Kellum et al. when they reported that patients with sepsis-associated AKI who recover partial or complete renal function had long-term survival similar to that in patients without AKI.51 Interestingly, experimental data suggest that pre-existing CKD could limit further kidney damage in response to renal insults, which could also contribute to mitigate post-AKI outcomes in CKD patients.52, 53 For instance, following an ischemia/reperfusion challenge, rats that had undergone a significant ablation of renal mass developed a blunted degree of acute tubular necrosis and less parenchymal infiltration by inflammatory cells, compared to control animals with normal kidneys.53 It has been proposed that this may be the result of complex pathophysiological adaptations that occur in the setting of chronically reduced nephron mass, culminating in a protective influence of prior kidney injury on acute parenchymal damage.54 As shown by measurement55 or modeling,56 chronically low GFR and corresponding low tubular Na+ reabsorption reduce O2 consumption in the kidney, and potentially protect it from ischemia. It is also possible that pre-existing kidney damage may favorably alter the response to sepsis-associated renal injury to a greater extent than to other nephrotoxic insults. However, the current understanding of CKD-related renal adaptations is still very limited, and this remains merely a hypothesis at present.
Our study has several strengths. Unlike most of the previous analyses focusing on the joint prognostic effects of CKD and AKI, we adopted a consensus sCr-based definition of AKI and incorporated AKI severity in the analysis, which makes our results more relevant to clinical practice. Another asset is that we did not only examine 90-day mortality, but also considered adverse renal outcomes over a prolonged follow-up period. Finally, we performed sensitivity analyses to examine the differential effect of absolute and relative changes in sCr (used to define AKI stage 1) on study outcomes and to address the potential misclassification of AKI as CKD when assessing the CKD outcome.
We also acknowledge that our study has limitations. First, it is retrospective in nature and derived using ICD-9-CM codes to determine sepsis diagnosis. We relied on EHR data obtained in routine clinical care, with the inherent possibility of selection bias and attrition due to missing data. Second, prevalent CKD was defined on the basis of only 1 sCr measurement, which may have led to misclassification. Nonetheless, we defined CKD using exclusively sCr measurements within 3 months before hospitalization and did not consider admission sCr for the purpose of CKD determination, thus avoiding a major reason for possible misclassification of AKI as CKD. Third, the determination of the CKD outcome was based on 1 or 2 sCr measurements obtained during the observation period, which may be susceptible to misclassification of AKI as CKD. However, we performed sensitivity analyses to further validate our findings. Finally, we defined AKI based only on changes in sCr and did not incorporate the KDIGO urine output criterion. However, this is the case also for the vast majority of other retrospective clinical studies of AKI, and thus makes the findings more comparable across different cohorts.
Conclusion
We studied how prevalent CKD and incident AKI interact to affect outcomes in critically ill septic patients. We found that, in the presence of pre-existing CKD, AKI stage 1 did not significantly increase the risk of 90-day mortality and progressive CKD. However, AKI stage 2 or higher in those patients with baseline CKD, and each stage of AKI in those without baseline CKD, significantly increased adverse outcomes. These findings raise the hypothesis that stage 1 AKI on prevalent CKD in critically ill septic patients could reflect distinct AKI pathophysiology. In particular, functional or transient forms of AKI, with a limited extent of intrinsic damage, could be represented primarily among septic patients with stage 1 AKI superimposed on CKD. Further collection of comprehensive prospective clinical data characterizing AKI severity, duration, and recovery phenotypes and incorporating biomarker information, tissue interrogation, and novel imaging technologies will hopefully offer fresh insights into the pathophysiology of sepsis-associated AKI and its complex interplay with CKD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was presented in part as an abstract at the 2016 annual meeting of the American Society of Nephrology in Chicago, IL. Research reported in this publication was supported by the University of Texas Southwestern Medical Center O’Brien Kidney Research Core Center (NIH, P30 DK079328-06), the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH, UL1TR001105), and the Division of Nephrology and Hypertension of Henry Ford Hospital. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health, the University of Texas Southwestern, or Henry Ford Hospital. JAN was supported by the Ben J. Lipps Research Fellowship Program of the American Society of Nephrology Foundation for Kidney Research and the Truelson Fellowship Fund at UT Southwestern Charles and Jane Pak Center of Mineral Metabolism and Clinical Research. JAN is currently supported by an Early Career Pilot Grant from the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR001998.
Footnotes
Table S1. Multivariable model for 90-day mortality (dependent variable) in the CKD/AKI groups redefined for sensitivity analysis (independent variable).
Table S2. Multivariable model for incident/progressive CKD (dependent variable) in the CKD/AKI groups redefined for sensitivity analysis (independent variable).
Table S3. Multivariable model for incident/progressive CKD (redefined for sensitivity analysis, dependent variable) in the CKD/AKI groups (independent variable).
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Multivariable model for 90-day mortality (dependent variable) in the CKD/AKI groups redefined for sensitivity analysis (independent variable).
Multivariable model for incident/progressive CKD (dependent variable) in the CKD/AKI groups redefined for sensitivity analysis (independent variable).
Multivariable model for incident/progressive CKD (redefined for sensitivity analysis, dependent variable) in the CKD/AKI groups (independent variable).
References
- 1.Rewa O., Bagshaw S.M. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10:193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 2.Hoste E.A., Bagshaw S.M., Bellomo R. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw S.M., Laupland K.B., Doig C.J. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchino S., Bellomo R., Morimatsu H. Continuous renal replacement therapy: a worldwide practice survey. The Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) investigators. Intensive Care Med. 2007;33:1563–1570. doi: 10.1007/s00134-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 5.VA/NIH Acute Renal Failure Trial Network. Palevsky P.M., Zhang J.H. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.RENAL Replacement Therapy Study Investigators. Bellomo R., Cass A. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 7.Amdur R.L., Chawla L.S., Amodeo S. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089–1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 8.Bihorac A., Yavas S., Subbiah S. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 9.Coca S.G., Yusuf B., Shlipak M.G. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobson C.E., Yavas S., Segal M.S. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 11.Wald R., Quinn R.R., Luo J. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 12.Lafrance J.P., Miller D.R. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucaloiu I.D., Kirchner H.L., Norfolk E.R. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81:477–485. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 14.Wald R., Quinn R.R., Adhikari N.K. Risk of chronic dialysis and death following acute kidney injury. Am J Med. 2012;125:585–593. doi: 10.1016/j.amjmed.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Wu V.C., Wu C.H., Huang T.M. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnaoutakis G.J., Bihorac A., Martin T.D. RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg. 2007;134:1554–1561. doi: 10.1016/j.jtcvs.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 17.Hsu C.Y., Ordonez J.D., Chertow G.M. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grams M.E., Astor B.C., Bash L.D. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21:1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James M.T., Hemmelgarn B.R., Wiebe N. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376:2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 20.Lafrance J.P., Djurdjev O., Levin A. Incidence and outcomes of acute kidney injury in a referred chronic kidney disease cohort. Nephrol Dial Transplant. 2010;25:2203–2209. doi: 10.1093/ndt/gfq011. [DOI] [PubMed] [Google Scholar]
- 21.Chawla L.S., Eggers P.W., Star R.A. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James M.T., Grams M.E., Woodward M. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66:602–612. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta R.L., Pascual M.T., Soroko S. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 24.Uchino S., Kellum J.A., Bellomo R. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 25.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 26.Chronic Kidney Disease Prognosis Consortium. Matsushita K., van der Velde M. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Velde M., Matsushita K., Coresh J. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 28.Groeneveld A.B., Tran D.D., van der Meulen J. Acute renal failure in the medical intensive care unit: predisposing, complicating factors and outcome. Nephron. 1991;59:602–610. doi: 10.1159/000186651. [DOI] [PubMed] [Google Scholar]
- 29.Mehta R.L., Pascual M.T., Gruta C.G. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–1357. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- 30.Chertow G.M., Soroko S.H., Paganini E.P. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int. 2006;70:1120–1126. doi: 10.1038/sj.ki.5001579. [DOI] [PubMed] [Google Scholar]
- 31.Waikar S.S., Curhan G.C., Wald R. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 32.Khosla N., Soroko S.B., Chertow G.M. Preexisting chronic kidney disease: a potential for improved outcomes from acute kidney injury. Clin J Am Soc Nephrol. 2009;4:1914–1919. doi: 10.2215/CJN.01690309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali T., Khan I., Simpson W. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 34.Hsu C.Y., Chertow G.M., McCulloch C.E. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishani A., Xue J.L., Himmelfarb J. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James M.T., Ghali W.A., Knudtson M.L. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409–416. doi: 10.1161/CIRCULATIONAHA.110.970160. [DOI] [PubMed] [Google Scholar]
- 37.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 38.Bagshaw S.M., Lapinsky S., Dial S. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–881. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 39.Poukkanen M., Vaara S.T., Pettila V. Acute kidney injury in patients with severe sepsis in Finnish intensive care units. Acta Anaesthesiol Scand. 2013;57:863–872. doi: 10.1111/aas.12133. [DOI] [PubMed] [Google Scholar]
- 40.Alobaidi R., Basu R.K., Goldstein S.L. Sepsis-associated acute kidney injury. Semin Nephrol. 2015;35:2–11. doi: 10.1016/j.semnephrol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heung M., Koyner J.L. Entanglement of sepsis, chronic kidney disease, and other comorbidities in patients who develop acute kidney injury. Semin Nephrol. 2015;35:23–37. doi: 10.1016/j.semnephrol.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Angus D.C., Linde-Zwirble W.T., Lidicker J. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 44.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 45.Knaus W.A., Draper E.A., Wagner D.P. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 46.Go A.S., Parikh C.R., Ikizler T.A. The Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) study: design and methods. BMC Nephrol. 2010;11:22. doi: 10.1186/1471-2369-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thakar C.V., Worley S., Arrigain S. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67:1112–1119. doi: 10.1111/j.1523-1755.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 48.Pannu N., James M., Hemmelgarn B.R. Modification of outcomes after acute kidney injury by the presence of CKD. Am J Kidney Dis. 2011;58:206–213. doi: 10.1053/j.ajkd.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 49.Lafrance J.P., Miller D.R. Defining acute kidney injury in database studies: the effects of varying the baseline kidney function assessment period and considering CKD status. Am J Kidney Dis. 2010;56:651–660. doi: 10.1053/j.ajkd.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Lin J., Fernandez H., Shashaty M.G. False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol. 2015;10:1723–1731. doi: 10.2215/CJN.02430315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kellum J.A., Chawla L.S., Keener C. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193:281–287. doi: 10.1164/rccm.201505-0995OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayes J.M., Boonshaft B., Maher J.F. Resistance to glycerol induced hemoglobinuric acute renal failure. Nephron. 1970;7:155–164. doi: 10.1159/000179817. [DOI] [PubMed] [Google Scholar]
- 53.Vercauteren S.R., Ysebaert D.K., De Greef K.E. Chronic reduction in renal mass in the rat attenuates ischemia/reperfusion injury and does not impair tubular regeneration. J Am Soc Nephrol. 1999;10:2551–2561. doi: 10.1681/ASN.V10122551. [DOI] [PubMed] [Google Scholar]
- 54.Singh P., Rifkin D.E., Blantz R.C. Chronic kidney disease: an inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol. 2010;5:1690–1695. doi: 10.2215/CJN.00830110. [DOI] [PubMed] [Google Scholar]
- 55.Khatir D.S., Pedersen M., Jespersen B. Evaluation of renal blood flow and oxygenation in CKD using magnetic resonance imaging. Am J Kidney Dis. 2015;66:402–411. doi: 10.1053/j.ajkd.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Lee C.J., Gardiner B.S., Ngo J.P. Accounting for oxygen in the renal cortex: a computational study of factors that predispose the cortex to hypoxia. Am J Physiol Renal Physiol. 2017;313:F218–F236. doi: 10.1152/ajprenal.00657.2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariable model for 90-day mortality (dependent variable) in the CKD/AKI groups redefined for sensitivity analysis (independent variable).
Multivariable model for incident/progressive CKD (dependent variable) in the CKD/AKI groups redefined for sensitivity analysis (independent variable).
Multivariable model for incident/progressive CKD (redefined for sensitivity analysis, dependent variable) in the CKD/AKI groups (independent variable).