Abstract
Immunofluorescence on frozen tissue is the gold standard immunohistochemical technique for evaluation of immune deposits in the kidney. When frozen tissue is not available or lacks glomeruli, immunofluorescence can be performed on paraffin tissue after antigen retrieval (paraffin immunofluorescence). Excellent results can be obtained by paraffin immunofluorescence in most immune complex–mediated glomerulonephritides and dysproteinemia-associated kidney lesions, and thus this technique has become a valuable salvage technique in renal pathology. Furthermore, new data have emerged suggesting that paraffin immunofluorescence can be used as an unmasking technique, as it is more sensitive than frozen tissue immunofluorescence in some kidney lesions, such as crystalline light chain proximal tubulopathy and is needed to establish the diagnosis of certain unique lesions, such as membranous-like glomerulopathy with masked IgG kappa deposits and membranoproliferative glomerulonephritis with masked monotypic Ig deposits. However, it is important to recognize and be aware of the limitations and pitfalls associated with paraffin immunofluorescence. These include poor sensitivity for detection of C3 deposits and for the diagnosis of primary membranous nephropathy. Here, we summarize the available techniques of paraffin immunofluorescence, review its role and performance as a salvage and unmasking technique in renal pathology, address its limitations and pitfalls, and highlight unusual forms of glomerulopathy that require paraffin immunofluorescence for diagnosis.
Keywords: masked deposits, masked monoclonal deposits, paraffin immunofluorescence, pronase immunofluorescence
Interpretation of medical renal biopsies generally requires histological examination by light microscopy (LM), immunohistochemistry, and electron microscopy (EM). Immunofluorescence on frozen tissue (IF-F), invented by Coons et al.1 in 1942, has been the gold standard immunohistochemical technique for detecting Ig and complement components in the kidney for >50 years.2 However, IF-F is not always successful or possible, as cortical tissue with glomeruli may not be sampled in the portion of tissue submitted for immunofluorescence and fresh unfixed tissue may not be available. To overcome this limitation, immunofluorescence techniques on formalin-fixed, paraffin-embedded tissue (IF-P) have been developed and are currently used in many renal pathology laboratories. In addition to its important role as a salvage technique in renal pathology, recent data have shown that IF-P is more sensitive than IF-F in certain kidney lesions and could unmask Ig deposits. In this review, we summarize the currently available techniques of IF-P, address its role as a salvage and unmasking technique, review its limitations and pitfalls, and highlight the pathologic entities that require IF-P for diagnosis. Of note, immunoperoxidase-based immunohistochemistry following antigen retrieval, a less commonly used method in renal pathology than immunofluorescence and reportedly with comparable results,3, 4 is not addressed in the review.
Methodologies of Paraffin Immunofluorescence
In contrast to IF-F, which is performed on cryostat sections cut from unfixed frozen tissue, IF-P is performed on formalin-fixed, paraffin-embedded tissue. Because formalin fixation induces protein cross-linking, which generally blocks antigenicity, an antigen-retrieval step that allows for increased penetration of antibodies to the antigens “masked” by formalin fixation is required in IF-P.5, 6 This step involves incubating the paraffin sections with a proteolytic enzyme or heating the sections before incubation with fluorescein isothiocyanate–conjugated antibodies against Igs and complement components. Multiple proteolytic enzymes have been used in IF-P, including trypsin,7, 8, 9, 10, 11, 12 pronase E (protease XIV),13, 14, 15, 16, 17 proteinase XXIV,18 and proteinase K.13, 19, 20, 21, 22 Successful results were also obtained by heat treatment with Tris or citrate buffers15 and with dual microwave heating in EDTA antigen-retrieval solution.23 In our laboratory, we use the pronase technique, which was originally described by Fogazzi et al.17 and was introduced to the practice of renal pathology in the United States by Professor Vivette D’Agati (Columbia University, New York, NY).14 The procedure for the pronase technique is shown in Table 1. The procedure for the proteinase K technique can be found in a previous publication by Messias et al.20 from Arkana Laboratories (formerly Nephropath).
Table 1.
Pronase immunofluorescence procedure
|
|
|
|
|
In our laboratory, the deparaffinization and staining steps are automated on the Leica Bond III (Leica Microsystems Inc, Buffalo Grove, IL) in which we use the Bond wash solution instead of Tris buffer.
Paraffin Immunofluorescence as a Salvage Technique
Studies performed as early as the 1970s have shown that IF-P is a valuable salvage technique in renal pathology when frozen tissue is inadequate (e.g., lacks glomeruli) or not available.8, 10, 14, 18, 20, 21 Overall, diagnostic results by IF-P can be obtained in >80% of cases,14, 18, 20, 21 but the diagnostic yield varies depending on 3 factors: (i) The antigen-retrieval method used: proteinase K, pronase, proteinase XXIV, and dual microwave heating appear to be more sensitive than the other methodologies, although a systematic study comparing the diagnostic yield of these methods side by side on the same cohort of cases has yet to be performed. (ii) The disease type: the best results are obtained in dysproteinemia-associated kidney diseases (Table 2). In a study from Columbia University using the pronase technique, diagnostic results were obtained in 96% of cases, including all cases of AL amyloidosis (Figure 1g), myeloma cast nephropathy, and light chain proximal tubulopathy (LCPT) (Figure 1h), and in 80% of cases of light chain deposition disease14 (Figure 1f). Similar excellent results also can be obtained using the proteinase K technique.20, 22 We have also observed good results in other paraprotein-associated glomerulopathies, including immunotactoid glomerulonephritis, type 1 cryoglobulinemic glomerulonephritis (Supplementary Figure S1), and proliferative glomerulonephritis with monoclonal Ig deposits (Figure 1e). Good results can also be obtained in most immune-complex–mediated proliferative glomerulonephritides, including lupus nephritis (96%–100%)14, 17, 18, 21 (Figure 1b), IgA nephropathy (88%)14 (Figure 1a), cryoglobulinemic glomerulonephritis types II and III (100%)14 (Figure 1d), C1q nephropathy,21 and fibrillary glomerulonephritis (100%)14 (Figure 1c; Table 2). IF-P has a lower sensitivity than IF-F in primary membranous nephropathy (50%–78%),14, 21 C3 glomerulonephritis,20, 21 and anti–glomerular basement membrane (GBM) nephritis.14, 20 In our laboratory, we use a case of diffuse proliferative lupus nephritis as our positive control for IF-P. (iii) The antigen tested: Good results can be obtained for IgG, kappa, and lambda (aside from cases of primary membranous nephropathy and anti-GBM nephritis), IgA, IgM, and C1q. IF-P is not sensitive for detecting C3 deposits.14, 20 Therefore, C3 antibody is not included in our pronase IF-P staining panel. The IF-P method also is used in some laboratories for phospholipase A2 receptor immunostaining on kidney biopsies.24
Table 2.
Sensitivity of paraffin immunofluorescence (compared with frozen tissue immunofluorescence)
| Significantly less sensitive | Slightly less sensitive | Comparable | More sensitive | Needed for diagnosis “masked deposits” |
|---|---|---|---|---|
| - C3 GN - Bacterial infection- associated GN - Primary membranous nephropathy - Anti-GBM nephritis |
- IgA nephropathy - Lupus nephritis - AL amyloidosis - MIDD - PGNMID |
- Myeloma cast nephropathy - Immunotactoid GN - C1q nephropathy |
- LCPT - Crystalglobulin- induced nephropathy - Cryoglobulinemic GN - Fibrillary GN |
- MGMID - MPGN with masked monoclonal deposits |
GBM, glomerular basement membrane; GN, glomerulonephritis; LCPT, crystalline light chain proximal tubulopathy; MGMID, membranous-like glomerulopathy with masked IgG kappa deposits; MIDD, monoclonal Ig deposition disease; MPGN, membranoproliferative glomerulonephritis; PGNMID, proliferative glomerulonephritis with monoclonal Ig deposits.
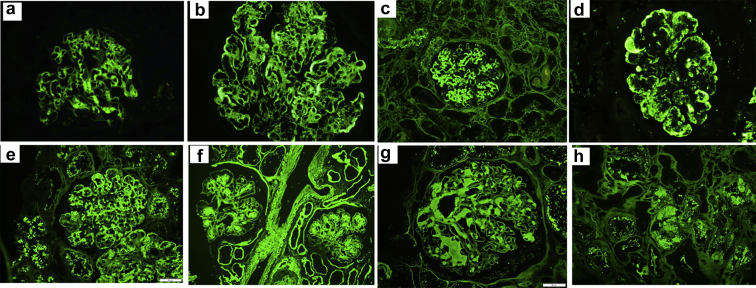
Figure 1.
Representative immunofluorescence on formalin-fixed paraffin-embedded tissue images of various kidney diseases. (a) Global mesangial staining for IgA in a case of IgA nephropathy. (b) Bright global glomerular capillary wall and mesangial staining for C1q in a case of lupus nephritis class IV and V. (c) Bright smudgy mesangial and segmental glomerular capillary wall staining for IgG in a case of fibrillary glomerulonephritis. (d) Global semilinear to granular glomerular capillary wall staining for IgM in a case of cryoglobulinemic glomerulonephritis type II. (e) Global glomerular capillary wall and mesangial staining for IgG in a case of proliferative glomerulonephritis with monoclonal Ig deposits. (f) Diffuse linear staining of the basement membranes of glomeruli, tubules, and vascular myocytes as well as nodular mesangial staining for kappa in a case of kappa-type light chain deposition disease. (g) Smudgy mesangial staining for lambda in a case of lambda-type AL amyloidosis. (h) Staining of intratubular cytoplasmic crystals/inclusions for kappa in a case of kappa-type light chain proximal tubulopathy.
Paraffin Immunofluorescence as an Unmasking Technique
Aside from being a valuable salvage tool, IF-P can be used as an unmasking technique because it is more sensitive than IF-F in certain renal diseases. The first study highlighting its role in unmasking deposits reported higher sensitivity than IF-F in LCPT.14 In this study, diagnostic kappa light chain staining of proximal tubular crystals was obtained in all 10 (100%) cases of LCPT analyzed versus only 4 (40%) cases by IF-F.14 Several subsequent studies from Arkana Laboratories13, 19, 20, 24 and other centers13, 25, 26, 27 have highlighted an important role for IF-P as an unmasking tool. In a recent study by Stokes et al.,27 the sensitivity of detection of LCPT by pronase IF-P was 97% (37 of 38 cases) versus 35% (15 of 43 cases) by IF-F. In the study by Messias et al.,20 proteinase K IF-P was performed on 304 cases (6.1% of native kidney biopsies). In 207 (68%) of these cases, it was used as a salvage technique and in these cases it was necessary or significantly contributed to the diagnosis in 42%. In the remaining 97 (32%) cases, it was used as an unmasking technique (to unmask immune complex or monoclonal deposits), and in these cases it was necessary or significantly contributed to the diagnosis in 23% of cases. Importantly, 13 of 20 cases with masked deposits in this study had C3-dominant staining on IF-F. Thus, these cases could have been misdiagnosed as C3 glomerulopathy if IF-P had not been performed.20
Pathologic Entities That Require Paraffin Immunofluorescence for Diagnosis
There are 2 recently described entities that require IF-P for diagnosis: membranous-like glomerulopathy with masked IgG kappa deposits (MGMID) and membranoproliferative glomerulonephritis (MPGN) with masked monotypic Ig deposits. MGMID was first described in 2014 by Chris Larsen and colleagues19 at Arkana Laboratories who reported a series of 14 cases characterized by large subepithelial deposits, which, on IF-F stained with C3 with negative or weak staining for Igs but on IF-P using proteinase K showed strong staining for IgG and kappa. These investigators have recently expanded their experience with MGMID to 41 cases.24 Patients with MGMID are typically young women with positive autoimmune serologies but without well-defined autoimmune disease, who present with proteinuria (within the nephrotic range in 35%) and hematuria.24 The vast majority of patients do not have clinical evidence of monoclonal gammopathy or hypocomplementemia. Prognosis is variable. Within a mean follow-up of 22 months in 27 patients, 41% had complete remission, 15% partial remission, 33% persistent renal dysfunction, and 11% progressed to end-stage renal disease.24 LM in MGMID shows segmental GBM “spikes” and/or “pinholes.” Mesangial hypercellularity, segmental sclerosis, and crescents are seen in 20%, 17%, and 15% of cases, respectively. The subepithelial deposits frequently exhibit a hump-shaped appearance and preferential localization to the hinge region. Glomerular staining for phospholipase A2 receptor and THSD7A is negative,24 favoring a secondary form of membranous nephropathy.
MPGN with masked monotypic Ig deposits is a rare lesion characterized by an MPGN pattern of injury on LM and EM, with monoclonal Ig deposits on IF-P and false-negative staining for monoclonal deposits on IF-F. The clinicopathologic characteristics of 16 patients with this lesion were described in a recent series from Arkana Laboratories and the Mayo Clinic.13 In contrast to MGMID, patients are typically elderly and have clinical evidence of monoclonal gammopathy (monoclonal gammopathy of renal significance or less commonly multiple myeloma or lymphoma) in whom the circulating monoclonal protein matches the glomerular monoclonal protein detected in glomeruli by IF-P.13 Patients present with hematuria (100%), full nephrotic syndrome (81%), renal insufficiency (88%), and hypocomplementemia (67%). Chemotherapy directed against the underlying hematologic condition generally results in improvement of proteinuria and serum creatinine.13 IF-F in these cases frequently shows glomerular staining for C3, and, therefore, this lesion can be misdiagnosed as monoclonal gammopathy–associated C3 glomerulonephritis if IF-P is not performed to unmask these monoclonal deposits. Cases of glomerulonephritis with masked monoclonal deposits but without an MPGN pattern of injury also have been recently reported, including diffuse endocapillary proliferative and exudative glomerulonephritis (mimicking bacterial infection–associated glomerulonephritis)25 and pure mesangial proliferative glomerulonephritis.26
Indications for Paraffin Immunofluorescence
IF-F remains the gold standard immunohistochemical technique in renal pathology and should not be replaced by IF-P. Thus, IF-P is not necessary to perform in most renal biopsies. In our laboratory, we performed IF-P on 303 of 5946 (5%) native kidney biopsies that we accessioned between August 1, 2016, and August 31, 2017. In Arkana Laboratories, IF-P was performed on 324 of 4969 (6.5%) of their native biopsies accessioned between January 2013 and September 2013.20 Our indications for IF-P are listed in Table 3 and detailed as follows.
Table 3.
Indications for paraffin immunofluorescence
|
|
|
|
|
EM, electron microscopy; GN, glomerulonephritis; IF-F, immunofluorescence on frozen tissue; MPGN, membranoproliferative glomerulonephritis.
Atypical findings for C3 GN include presence of predominately subendothelial and/or intraluminal deposits, presence of organized deposits, lack of subepithelial humps, and lack of deposits with only slight electron density.
As indicated earlier, IF-P is generally performed when the frozen tissue portion of renal biopsy sample lacks glomeruli and in renal biopsies in which frozen tissue is not available (such as in archived tissue and referral cases).12, 14, 20 In cases in which EM is already done and does not show granular electron-dense or organized deposits, IF-P may not be necessary, with the exception of cases of crescentic glomerulonephritis; despite the known lower sensitivity of IF-P compared with IF-F in diagnosing anti-GBM nephritis, we have encountered several cases of crescentic glomerulonephritis with no glomeruli sampled on IF-F in which bright linear IgG staining of the GBM was observed on IF-P, distinguishing anti-GBM nephritis from antineutrophil cytoplasmic antibody–associated pauci-immune crescentic glomerulonephritis. IF-P is also useful to exclude immune complex–mediated glomerulopathies and monoclonal gammopathy-related kidney diseases on nephrectomy specimens done for tumor resection and on postmortem specimens, in which unfixed/frozen tissue is frequently not stored due to cost.11, 28, 29
We routinely perform IF-P on suspected cases of crystalline LCPT and crystalglobulin-induced nephropathy. In most but not all cases of crystalline LCPT, staining of at least some intracytoplasmic tubular crystals for 1 light chain (typically kappa) can be demonstrated by IF-P, more frequently than by IF-F.27, 30, 31 Likewise, IF-P is more likely than IF-F to demonstrate the monoclonal staining of extracellular (intraglomerular and/or intravascular) crystals composed of monoclonal Ig heavy and light chains or light chains only in crystalglobulin-induced nephropathy associated with crystalglobulinemia or cryocrystalglobulinemia.32 Monoclonal staining of extrarenal crystals in the latter syndromes also can be demonstrated by IF-P; for example, in corneal biopsy samples (in which frozen tissue is typically not available), which help establishing the diagnosis of paraproteinemic keratopathy. In some cases of mild myeloma cast nephropathy, the atypical casts are very focal and may not be sampled in the tissue allocated for IF-F. In these cases, unequivocal preferential light chain staining of the same atypical casts seen on LM can be demonstrated by IF-P. Likewise, IF-P is useful in cases of early AL amyloidosis in which the focal glomerular or vascular amyloid deposits are not sampled in the tissue allocated for IF-F.
To exclude MGMID, we recommend performing IF-P in cases with a predominant membranous glomerulonephritis pattern of injury on LM and EM in which IF-F shows negative or weak staining for Igs and complement components, or shows C3-dominant staining.24 We also recommend performing IF-P in patients with clinical evidence of monoclonal gammopathy or autoimmune disease in whom the kidney biopsy shows C3 glomerulonephritis (in cases with positive C4d staining and in cases with EM findings that are atypical for C3 glomerulonephritis) or MPGN with negative IF-F.13, 33 In our experience, roughly 10% to 20% of cases of C3 glomerulonephritis associated with monoclonal gammopathy (based on the findings on LM, EM, and IF-F) turned out to be examples of MPGN with masked monoclonal deposits when IF-P was performed.13 The EM findings can assist in distinguishing true C3 glomerulonephritis with monoclonal gammopathy (caused by continuous activation of the alternative pathway of complement by the monoclonal protein34, 35) from MPGN with masked monoclonal deposits (resulting from deposition of monoclonal protein in glomeruli). The findings of subepithelial humps, intramembranous deposits, or deposits with only slight electron density favor C3 glomerulonephritis with monoclonal gammopathy, whereas the lack of these findings, the presence of predominantly subendothelial and/or intraluminal deposits, or the presence of any organization of deposits favor MPGN with masked monoclonal deposits.13 Interestingly, we have not observed this “masking” phenomenon in dense deposit disease associated with monoclonal gammopathy,13 and thus IF-P is not warranted in this situation.
Another important indication of IF-P is cases in which the findings by LM and EM are consistent with cryoglobulinemic glomerulonephritis but with negative IF-F for Igs (with or without C3 deposition). IF-P in these cases usually establishes the composition of glomerular and vascular cryoglobulin deposits (Supplementary Figure S1) and thus distinguishes type 1 cryoglobulinemia from mixed cryoglobulinemia.13 We have also observed that in some cases of fibrillary glomerulonephritis with apparent light chain restriction of IgG deposits on IF-F (particularly in cases with lambda restriction) and without clinical evidence of monoclonal gammopathy, IF-P shows positive staining for both kappa and lambda36 (Supplementary Figure S2). IF-P is important in this situation, as it excludes monoclonal fibrillary glomerulonephritis, which is currently considered a monoclonal gammopathy of renal significance lesion.31
Limitations and Pitfalls of Paraffin Immunofluorescence
There are several limitations and pitfalls for IF-P (Table 4). (i) As mentioned previously, IF-P is less sensitive than IF-F in some glomerular lesions, including primary membranous nephropathy, C3 glomerulonephritis, and anti-GBM nephritis. (ii) The intensity of staining for IgG, IgA, kappa, and lambda is generally weaker (≈1+ order of intensity) compared with IF-F. Thus, cases of IgA nephropathy with weak staining for IgA (e.g., only 1+ on IF-F) occasionally show false-negative staining for IgA, kappa, and lambda on IF-P. (iii) In contrast to IF-F in which the glomerular staining is generally diffuse, there is variable staining among glomeruli in IF-P: some glomeruli may show bright staining, whereas others may show weak or even negative staining. Thus, false-negative results by IF-P can be encountered if the paraffin tissue contains only a few glomeruli. (iv) The granular texture of deposits is less appreciable by IF-P than IF-F in some cases that may show a more smudgy appearance instead22 (Figure 1a). (v) Occasionally, there is positive staining of serum in glomerular capillaries (retained due to formalin fixation) by IF-P (Figure 2), which may lead to a false-positive diagnosis if careful examination on high power to determine the exact location of staining is not done.13, 20 Furthermore, the potential for false-positive staining of glomerular immune deposits by IF-P remains to be excluded. For example, false-positive reactions in pronase-treated T-cell flow cytometric cross-match tests have been reported.37 (vi) The various IF-P techniques described are based on formalin fixation.4, 9, 14, 15, 20, 21 The performance characteristics of IF-P on non–formalin-based fixatives (Bouin, Duboscq-Brasil, and other fixatives) used in approximately 25% of renal pathology laboratories38, 39 are not known.
Table 4.
Limitations and pitfalls of paraffin immunofluorescence
|
|
|
|
|
GBM, glomerular basement membrane; GN, glomerulonephritis; IF-F, immunofluorescence on frozen tissue.
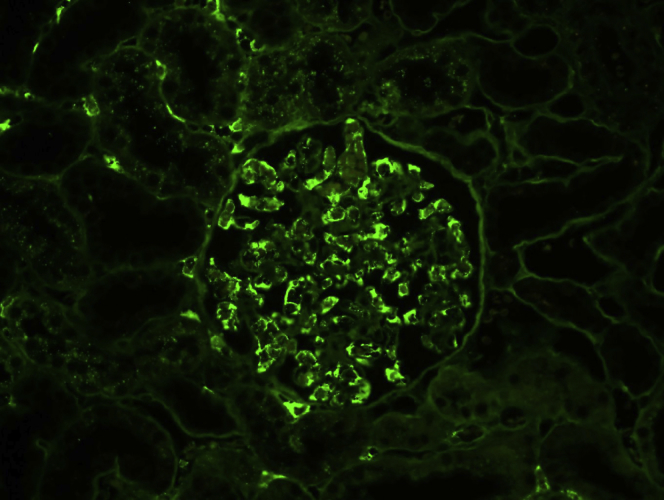
Figure 2.
Artifact intraluminal serum staining on immunofluorescence on formalin-fixed paraffin-embedded tissue. There is bright positivity for lambda at the periphery of capillary spaces, likely representing serum staining. This nonspecific (artifact) staining can be distinguished from true immune staining by (i) its intraluminal location, (ii) its staining of most or all immune reactants (IgG, IgM, IgA, C1q, C3, kappa, lambda, albumin, fibrinogen), and (iii) the presence of similar staining of peritubular capillary serum (as evident in the left upper portion of the image).
Mechanisms of “Masked” Deposits
The reason why some deposits stain by IF-P and not by IF-F remains to be determined. Multiple theories have been proposed. (i) The antigenic epitopes may not be available for antibody binding by IF-F. This could be due to loss of Igs in the tissue during the washing steps of IF-F (whereas they are retained in the tissue during IF-P due to formalin-induced protein-crosslinking) or due to alterations in the tertiary or quaternary structure of proteins that prevent the antibodies from reaching their target epitopes without an antigen-retrieval step.13, 20 (ii) In cases of paraprotein crystalline nephropathies such as crystalline LCPT and crystalglobulin-induced nephropathy, the highly crystallized structure of monoclonal proteins (and the intracellular localization of crystals in LCPT) could render the antigenic sites inaccessible to antibody binding. The antigen-retrieval step in IF-P could open up the antigenic sites sequestered within the crystalline lattice and in cases of LCPT denature the cell membranes allowing for the antibodies to reach the intracellular crystals.14 (iii) Because most cases of glomerulopathies with masked deposits have been reported from renal pathology laboratories that perform IF-F on biopsies placed in Michel (Zeus) transport media before freezing,13, 24 it has been hypothesized that the phenomenon of masked glomerular deposits could potentially be due to interference of the transport media with Ig reactivity on IF-F. Regardless of the mechanism, the phenomenon of masked deposits does exist and should be kept in mind when there is discordance between the findings on LM and EM and the findings on IF-F.
Disclosure
All the authors declared no competing interests.
Footnotes
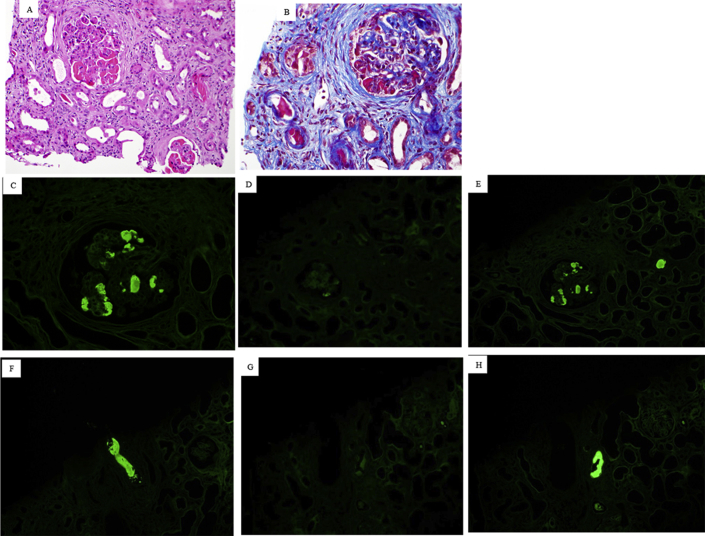
Figure S1. Cryoglobulinemic glomerulonephritis with masked monoclonal deposits. The biopsy is from a 52-year-old man with an IgG lambda monoclonal protein in the serum and urine and a history of positive serum cryoglobulin test. The biopsy revealed membranoproliferative glomerulonephritis with large cryoglobulin-type deposits in glomeruli and vessels ([A] hematoxylin and eosin; [B] trichrome). The deposits showed trace staining for IgM and were negative for IgG, IgA, C1q, C3, kappa, and lambda on immunofluorescence on frozen tissue. On immunofluorescence on formalin-fixed paraffin-embedded tissue, the glomerular (C,D,E) and vascular (F,G,H) cryoglobulin deposits stained brightly for IgG (C,F) and lambda (E,H) with negative staining for kappa (D,G,), establishing the diagnosis of type 1 cryoglobulinemic glomerulonephritis.
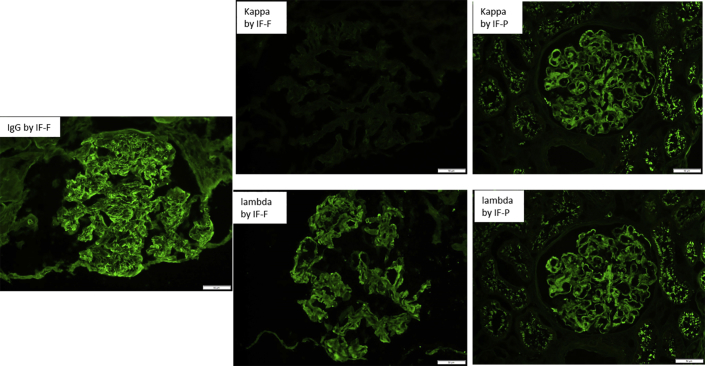
Figure S2. Unmasking polyclonal deposits by immunofluorescence on formalin-fixed paraffin-embedded tissue (IF-P) in fibrillary glomerulonephritis. The biopsy is from a 68-year-old woman with fibrillary glomerulonephritis and without clinical evidence of monoclonal gammopathy. Immunofluorescence on frozen tissue (IF-F) showed bright smudgy glomerular staining for IgG and lambda with negative kappa (left and middle panels), whereas staining for kappa and lambda by IF-P showed bright smudgy glomerular staining for both kappa and lambda (right panel). The findings on IF-P exclude monoclonal fibrillary glomerulonephritis and thus exclude monoclonal gammopathy of renal significance.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Figure S1.
Cryoglobulinemic glomerulonephritis with masked monoclonal deposits. The biopsy is from a 52-year-old man with an IgG lambda monoclonal protein in the serum and urine and a history of positive serum cryoglobulin test. The biopsy revealed membranoproliferative glomerulonephritis with large cryoglobulin-type deposits in glomeruli and vessels ([A] hematoxylin and eosin; [B] trichrome). The deposits showed trace staining for IgM and were negative for IgG, IgA, C1q, C3, kappa, and lambda on immunofluorescence on frozen tissue. On immunofluorescence on formalin-fixed paraffin-embedded tissue, the glomerular (C,D,E) and vascular (F,G,H) cryoglobulin deposits stained brightly for IgG (C,F) and lambda (E,H) with negative staining for kappa (D,G,), establishing the diagnosis of type 1 cryoglobulinemic glomerulonephritis.
Figure S2.
Unmasking polyclonal deposits by immunofluorescence on formalin-fixed paraffin-embedded tissue (IF-P) in fibrillary glomerulonephritis. The biopsy is from a 68-year-old woman with fibrillary glomerulonephritis and without clinical evidence of monoclonal gammopathy. Immunofluorescence on frozen tissue (IF-F) showed bright smudgy glomerular staining for IgG and lambda with negative kappa (left and middle panels), whereas staining for kappa and lambda by IF-P showed bright smudgy glomerular staining for both kappa and lambda (right panel). The findings on IF-P exclude monoclonal fibrillary glomerulonephritis and thus exclude monoclonal gammopathy of renal significance.
References
- 1.Coons A.H., Creech H.J., Jones R.N., Berliner E. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159–170. [Google Scholar]
- 2.McCluskey R.T., Vassalli P., Gallo G., Baldwin D.S. An immunofluorescent study of pathogenic mechanisms in glomerular diseases. N Engl J Med. 1966;274:695–701. doi: 10.1056/NEJM196603312741301. [DOI] [PubMed] [Google Scholar]
- 3.Molne J., Breimer M.E., Svalander C.T. Immunoperoxidase versus immunofluorescence in the assessment of human renal biopsies. Am J Kidney Dis. 2005;45:674–683. doi: 10.1053/j.ajkd.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair R.A., Burns J., Dunnill M.S. Immunoperoxidase staining of formalin-fixed, paraffin-embedded, human renal biopsies with a comparison of the peroxidase-antiperoxidase (PAP) and indirect methods. J Clin Pathol. 1981;34:859–865. doi: 10.1136/jcp.34.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason J.T., O'Leary T.J. Effects of formaldehyde fixation on protein secondary structure: a calorimetric and infrared spectroscopic investigation. J Histochem Cytochem. 1991;39:225–229. doi: 10.1177/39.2.1987266. [DOI] [PubMed] [Google Scholar]
- 6.Fowler C.B., Evers D.L., O'Leary T.J., Mason J.T. Antigen retrieval causes protein unfolding: evidence for a linear epitope model of recovered immunoreactivity. J Histochem Cytochem. 2011;59:366–381. doi: 10.1369/0022155411400866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qualman S.J., Keren D.F. Immunofluorescence of deparaffinized, trypsin-treated renal tissues. Preservation of antigens as an adjunct to diagnosis of disease. Lab Invest. 1979;41:483–489. [PubMed] [Google Scholar]
- 8.Yabuki A., Sawa M., Kohyama M. Paraffin immunofluorescence for detection of immune complexes in renal biopsies: an efficient salvage technique for diagnosis of glomerulonephritis in dogs. BMC Vet Res. 2017;13:371. doi: 10.1186/s12917-017-1287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi Y.J., Reiner L. Immunofluorescence of renal lesions in paraffin-embedded and fresh-frozen sections. Am J Clin Pathol. 1980;73:116–119. doi: 10.1093/ajcp/73.1.116. [DOI] [PubMed] [Google Scholar]
- 10.Huang S.N., Minassian H., More J.D. Application of immunofluorescent staining on paraffin sections improved by trypsin digestion. Lab Invest. 1976;35:383–390. [PubMed] [Google Scholar]
- 11.Perrone M.E., Chang A., Henriksen K.J. Medical renal diseases are frequent but often unrecognized in adult autopsies. Mod Pathol. 2018;31:365–373. doi: 10.1038/modpathol.2017.122. [DOI] [PubMed] [Google Scholar]
- 12.Arias L.F., Henao J., Giraldo R.D. Glomerular diseases in a Hispanic population: review of a regional renal biopsy database. Sao Paulo Med J. 2009;127:140–144. doi: 10.1590/S1516-31802009000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen C.P., Messias N.C., Walker P.D. Membranoproliferative glomerulonephritis with masked monotypic immunoglobulin deposits. Kidney Int. 2015;88:867–873. doi: 10.1038/ki.2015.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasr S.H., Galgano S.J., Markowitz G.S. Immunofluorescence on pronase-digested paraffin sections: a valuable salvage technique for renal biopsies. Kidney Int. 2006;70:2148–2151. doi: 10.1038/sj.ki.5001990. [DOI] [PubMed] [Google Scholar]
- 15.Mubarak M., Kazi Javed I., Kulsoom U., Ishaque M. Detection of immunoglobulins and complement components in formalin fixed and paraffin embedded renal biopsy material by immunoflourescence technique. J Nephropathol. 2012;1:91–100. doi: 10.5812/nephropathol.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sreedharanunni S., Joshi K., Duggal R. An analysis of transplant glomerulopathy and thrombotic microangiopathy in kidney transplant biopsies. Transpl Int. 2014;27:784–792. doi: 10.1111/tri.12331. [DOI] [PubMed] [Google Scholar]
- 17.Fogazzi G.B., Bajetta M., Banfi G., Mihatsch M. Comparison of immunofluorescent findings in kidney after snap-freezing and formalin fixation. Pathol Res Pract. 1989;185:225–230. doi: 10.1016/S0344-0338(89)80256-0. [DOI] [PubMed] [Google Scholar]
- 18.van der Ven K., Nguyen T.Q., Goldschmeding R. Immunofluorescence on proteinase XXIV-digested paraffin sections. Kidney Int. 2007;72:896. doi: 10.1038/sj.ki.5002495. [DOI] [PubMed] [Google Scholar]
- 19.Larsen C.P., Ambuzs J.M., Bonsib S.M. Membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int. 2014;86:154–161. doi: 10.1038/ki.2013.548. [DOI] [PubMed] [Google Scholar]
- 20.Messias N.C., Walker P.D., Larsen C.P. Paraffin immunofluorescence in the renal pathology laboratory: more than a salvage technique. Mod Pathol. 2015;28:854–860. doi: 10.1038/modpathol.2015.1. [DOI] [PubMed] [Google Scholar]
- 21.Singh G., Singh L., Ghosh R. Immunofluorescence on paraffin embedded renal biopsies: experience of a tertiary care center with review of literature. World J Nephrol. 2016;5:461–470. doi: 10.5527/wjn.v5.i5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nada R., Kumar A., Kumar V.G. Unmasking of complements using proteinase-K in formalin fixed paraffin embedded renal biopsies. Indian J Nephrol. 2016;26:182–187. doi: 10.4103/0971-4065.159558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S., Cheng Q., Zhang P. Immunofluorescence with dual microwave retrieval of paraffin-embedded sections in the assessment of human renal biopsy specimens. Am J Clin Pathol. 2013;139:71–78. doi: 10.1309/AJCPRZG8EXN7BAID. [DOI] [PubMed] [Google Scholar]
- 24.Larsen C.P., Boils C.L., Cossey L.N. Clinicopathologic features of membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int Rep. 2016;1:299–305. doi: 10.1016/j.ekir.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howlader A., Thajudeen B., Sussman A.N. Proliferative glomerulonephritis with masked monoclonal deposits responsive to myeloma therapy. Kidney Int Rep. 2017;2:1233–1237. doi: 10.1016/j.ekir.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd I.E., Khalighi M.A. Glomerulonephritis with masked monotypic immunoglobulin deposits and concurrent lymphomatous infiltration. Am J Kidney Dis. 2016;68:640–644. doi: 10.1053/j.ajkd.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Stokes M.B., Valeri A.M., Herlitz L. Light chain proximal tubulopathy: clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol. 2016;27:1555–1565. doi: 10.1681/ASN.2015020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bijol V., Batal I. Non-neoplastic pathology in tumor nephrectomy specimens. Surg Pathol Clin. 2014;7:291–305. doi: 10.1016/j.path.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Henriksen K.J., Meehan S.M., Chang A. Non-neoplastic renal diseases are often unrecognized in adult tumor nephrectomy specimens: a review of 246 cases. Am J Surg Pathol. 2007;31:1703–1708. doi: 10.1097/PAS.0b013e31804ca63e. [DOI] [PubMed] [Google Scholar]
- 30.Said S.M., Assaad A.M., Cerda J., Nasr S.H. Light chain tubulopathy without Fanconi syndrome. Nephrol Dial Transplant. 2006;21:3589–3590. doi: 10.1093/ndt/gfl363. [DOI] [PubMed] [Google Scholar]
- 31.Bridoux F., Leung N., Hutchison C.A. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87:698–711. doi: 10.1038/ki.2014.408. [DOI] [PubMed] [Google Scholar]
- 32.Gupta V., El Ters M., Kashani K. Crystalglobulin-induced nephropathy. J Am Soc Nephrol. 2015;26:525–529. doi: 10.1681/ASN.2014050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander M.P., Fervenza F.C., De Vriese A.S. C3 glomerulonephritis and autoimmune disease: more than a fortuitous association? J Nephrol. 2016;29:203–209. doi: 10.1007/s40620-015-0218-9. [DOI] [PubMed] [Google Scholar]
- 34.Chauvet S., Fremeaux-Bacchi V., Petitprez F. Treatment of B-cell disorder improves renal outcome of patients with monoclonal gammopathy-associated C3 glomerulopathy. Blood. 2017;129:1437–1447. doi: 10.1182/blood-2016-08-737163. [DOI] [PubMed] [Google Scholar]
- 35.Ravindran A., Fervenza F.C., Smith R.J.H., Sethi S. C3 glomerulopathy associated with monoclonal Ig is a distinct subtype. Kidney Int. 2018;94:178–186. doi: 10.1016/j.kint.2018.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasr S.H., Vrana J.A., Dasari S. DNAJB9 is a specific immunohistochemical marker for fibrillary glomerulonephritis. Kidney Int Rep. 2018;3:56–64. doi: 10.1016/j.ekir.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park H., Lim Y.M., Han B.Y. Frequent false-positive reactions in pronase-treated T-cell flow cytometric cross-match tests. Transplant Proc. 2012;44:87–90. doi: 10.1016/j.transproceed.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 38.Pullman J.M., Ferrario F., Nast C.C. Actual practices in nephropathology: a survey and comparison with best practices. Adv Anat Pathol. 2007;14:132–140. doi: 10.1097/PAP.0b013e31803250d8. [DOI] [PubMed] [Google Scholar]
- 39.Walker P.D., Cavallo T., Bonsib S.M. Ad Hoc Committee on Renal Biopsy Guidelines of the Renal Pathology Society. Practice guidelines for the renal biopsy. Mod Pathol. 2004;17:1555–1563. doi: 10.1038/modpathol.3800239. [DOI] [PubMed] [Google Scholar]