Abstract
FnCpf1-mediated genome-editing technologies have enabled a broad range of research and medical applications. Recently, we reported that FnCpf1 possesses activity in human cells and recognizes a more compatible PAM (protospacer adjacent motif, 5′-KYTV-3′), compared with the other two commonly used Cpf1 enzymes (AsCpf1 and LbCpf1), which requires a 5′-TTTN-3′ PAM. However, due to the efficiency and fidelity, FnCpf1-based clinical and basic applications remain a challenge. The direct repeat (DR) sequence is one of the key elements for FnCpf1-mediated genome editing. In principle, its engineering should influence the corresponding genome-editing activity and fidelity. Here we showed that the DR mutants [G(−9)A and U(−7)A] could modulate FnCpf1 performance in human cells, enabling enhancement of both genome-editing efficiency and fidelity. These newly identified features will facilitate the design and optimization of CRISPR-Cpf1-based genome-editing strategies.
Keywords: FnCpf1, direct repeat, genome editing
Lin et al. show that engineered direct repeat sequence of crRNA modulates Cpf1 performance in human cells, enabling enhancement of both genome-editing efficiency and fidelity. This study provides an important insight into the design and optimization of CRISPR-Cpf1-based genome-editing strategies.
Introduction
CRISPR and their associated Cas proteins form widespread adaptive immune systems in prokaryotes that have been harnessed as powerful tools for biological research, as well as providing a potential avenue for therapy of genetic diseases.1, 2 According to the configuration of their effector modules, CRISPR-Cas systems can be classified into two groups: class 1 effectors, which utilize multi-protein complexes; and class 2 effectors, which rely on single-component effector proteins such as the well-characterized Cas9 nuclease.3 Streptococcus pyogenes Cas9 (SpCas9), as one of the class 2 effectors and the best characterized Cas9, induces double-stranded DNA breaks through complexation with two RNA molecules: CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA).4 A guanine-rich protospacer adjacent motif (NGG PAM) sequence at the 3′ end adjacent to the target site is essential for the cleavage by SpCas9. As genome-editing techniques using RNA-guided nucleases become more precise and diverse, there is an increasing need for genome-editing nucleases with different PAM requirements. The discovery of additional nucleases, including CRISPR-Cpf1 (also known as Cas12a), Staphylococcus aureus Cas9 analog (SaCas9), Streptococcus thermophilus Cas9 proteins (St1Cas9 and St3Cas9), and Neisseria meningitides Cas9 protein (NmCas9), which recognize different PAM sequences, offers additional targeting flexibility.2, 4
Cpf1, a class 2 CRISPR-Cas family of nucleases, was recently reported to be highly specific and programmable with efficiencies comparable with those of the SpCas9 nuclease.5 So far, three Cpf1 proteins from Acidaminococcus sp. (AsCpf1), Lachnospiraceae bacterium (LbCpf1), and Francisella novicida U112 (FnCpf1) have been reported to have DNA mutation-inducing activity in mammalian cells.5, 6, 7 Cpf1 requires only a single crRNA without a tracrRNA and recognizes a thymine-rich PAM sequence at the 5′ end of the protospacer, which increases the range of CRISPR-endonuclease-editable genomic sites that can be selected. Genome-wide analysis has suggested that Cpf1 may reduce the degree of off-target effects in comparison with Cas9.8, 9 So far, Cpf1-mediated genome editing has been harnessed for the generation of KO (knockout) mice and correction of pathogenic mutation in mouse models.10, 11, 12
FnCpf1 recognizes a 5′-KYTV-3′ PAM in human cells, which may allow great potential for further development as a gene therapy tool, compared with the PAM for AsCpf1 and LbCpf1.6 The crRNA for FnCpf1 is composed of a 19-nt direct repeat (DR) sequence followed by a 21-nt guide sequence (Figures 1A and 1B). Structural studies of FnCpf1 protein have revealed its bilobed molecular architecture with a recognition lobe (REC) and a nuclease lobe (NUC) connected by the wedge (WED) domain.13, 14, 15 The N-terminal REC lobe consists of two α-helical domains (REC1 and REC2) that have been shown to coordinate the crRNA-target DNA heteroduplex, whereas the C-terminal NUC lobe consists of the C-terminal RuvC and Nuc domains involved in target cleavage, the bridge helix (BH), and the PAM-interacting (PI) domain.13 The narrow minor groove, formed by the WED, REC1, and PI domains, interrogates the suitable PAM-containing DNA duplex. The FnCpf1-crRNA-DNA complex finally generates a staggered double-strand break (DSB) resulting in 5′ overhangs distal to the PAM site with a single catalytic site located at the interface of the RuvC and Nuc domains.5, 13, 16, 17
Figure 1.
The DR Loop Structure Is a Flexible Element for Engineering
(A) Each part of FnCpf1 direct repeat (DR). The DR harboring Stem, Loop, Finger, and 3′U. (B) Schematic diagram of the FnCpf1 crRNA-DNA-targeting complex. The target sequence is shown in blue, and the PAM sequence is shown in pink. (C) Schematic of the GFP-reporter system used to evaluate genome-editing efficiency and the percent of GFP-negative cells are detected by flow cytometry (FCM). (D–F) FnCpf1-mediated inactivation of GFP using FnCpf1 plus crRNA harboring wild-type or engineered DR. Guide sequences are shown above (B). DR reconstruction involves Loop-grafted (D), Stem-engineered (E), and Finger or 3′U pointed-mutated (F). All genome-editing efficiency data in this figure were obtained with GFP inactivation via flow cytometry. Relative activity was determined by the formula: (A − C)/(B − C), where A, B, and C represent the activity of the mutant DR, wild-type (WT) DR, and negative control, respectively. Error bars, SEM; n = 3. **p < 0.01.
It has been reported that the structure of single guide RNA (sgRNA, a fusion of crRNA and tracrRNA) is important for CRISPR-Cas9-mediated genome editing; modification of the sgRNA structure by extending the duplex length and mutating the fourth thymine of any continuous sequence of thymine to cytosine or guanine significantly improves KO efficiency in cells.18 In addition, chemically modified Cas9 sgRNA variants improve editing efficiency in vivo.19, 20 Also, the results of engineered crRNAs revealed excellent prospect for Cpf1 (FnCpf1, AsCpf1, and LbCpf1 included)-mediated genome editing.21, 22, 23 However, enhancement of the efficiency and fidelity of FnCpf1 via engineering the DR sequence has not been explored. In this study, we systematically investigated the effect of engineering the DR sequence on KO efficiency and fidelity of FnCpf1-mediated human genome editing. We found that after mutating specific nucleotides in the DR sequence, the corresponding KO efficiency and fidelity could be modulated.
Results
The Flexible Segment in the DR: Loop Structure
FnCpf1 can efficiently introduce indel mutations at multiple genomic loci in human cells.6 When targeted at a gene encoding GFP in 293-SC1 cells, this single crRNA-guided endonuclease leads to the loss of green fluorescence (Figure 1C).24 Our previous study suggested that the cleavage efficiencies of FnCpf1 could be maintained if we replaced its DR with other DR sequences from AsCpf1, LbCpf1, Lb2Cpf1 (Lachnospiraceae bacterium MA2020 Cpf1), PcCpf1 (Porphyromonas crevioricanis Cpf1) and PmCpf1 (Porphyromonas macacae Cpf1).6 Thus, we hypothesized that optimized performance of FnCpf1 may be achieved via engineering the DR sequence.
We first investigated whether the Loop of the DR of the crRNA could be replaced with the corresponding Loops from 15 additional Cpf1 family orthologs. To make it clear, here we annotated the DR structure in four parts, Loop [U(−10)-G(−9)-U(−8)-U(−7)], Stem [U(−15) to C(−11) and G(−6) to A(−2)], Finger [A(−19)-A(−18)-U(−17)-U(−16)] and 3′U, which fold into a handle-like structure (Figure 1A). Not surprisingly, we found the efficiency of DNA cleavage varied among groups. Specifically, we observed that the Loop from Lb2Cpf1/PcCpf1/PmCpf1 (these three share the same Loop) achieved higher activity (38%) than the wild-type (32%) (Figure 1D). Surprisingly, with the Loop from PeCpf1 (Peregrinibacteria bacterium GW2011_GWA_33_10), FnCpf1 could maintain partial activity (>40% of the wild-type activity; Figure 1D), compared with a complete loss of activity with the entire DR sequence from PeCpf1.6 This revealed that the Loop, which was more flexible to engineer than the entire DR, may have the potential to be engineered for improved traits.
The DR Sequence Except Loop Is Conserved
We then sought to determine the molecular consequences of engineering the other three parts of the FnCpf1 DR sequence (Stem, Finger, and 3′U). To address this, we introduced different mutation(s) into the Stem sequence. Specifically, single nucleotides or nucleotide pairs were mutated in this region. We found that genome-editing activities from all these Stem mutants, including pair mutations, were significantly reduced (Figure 1E). Specifically, as to paired mutants of the Stem, the mutants [A(−2)G&U(−15)C, U(−5)G&A(−12)C, and U(−5)C&A(−12)G] remain no more than 47% of the wild-type activity, whereas the rest mutants have no more than 8%. Our results revealed that the Stem sequence of FnCpf1 is highly conserved and any change in this part would lead to the impairment of FnCpf1 activity in human cells, which is not consistent with the previous study.5
We then investigated the effects of all possible single-nucleotide mutations in the Finger and 3′U region. All mutants abolished the genome-editing activity with the exception of one [U(−16)G, 53% of wild-type activity] (Figure 1F). This demonstrates the lack of flexibility of the Finger sequence and 3′U regions.
Single-Base Mutation of the Loop Sequence
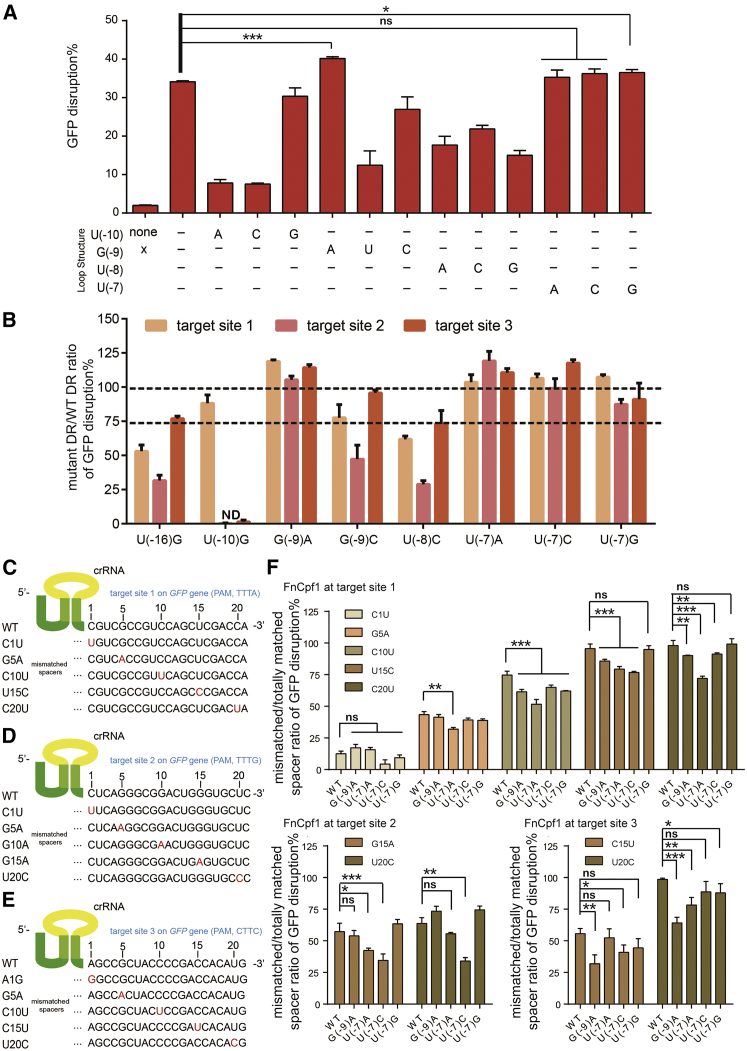
Next, we focused on the Loop sequence of FnCpf1. We mutated each nucleotide of 4-nt units (5′-UGUU-3′) and found that the cleavage efficiency of three mutants [U(−7)A/C/G] was similar to that of the wild-type Loop (Figure 2A). Another mutant [G(−9)A] showed higher activity (about 120% of the wild-type), which is the exact Loop sequence from Lb2Cpf1/PcCpf1/PmCpf1 (Figure 2A). It suggests that the mutant possessing the maximum efficiency among all the tested ones still exists in nature. We then sought to know the performance of FnCpf1 with “man-made” mutants, i.e., additional indel mutations in the Loop sequence. After generation and examination of 38 additional mutants, we observed all the indel mutants lost the majority of their activity (Figure S1). These results highlight that indel mutations are not tolerated in the Loop sequence.
Figure 2.
Activity and Fidelity of the Loop Mutant
(A) Inactivation of GFP with point mutation in the Loop sequence. The guide sequence is shown in Figure 1B. (B) Inactivation of GFP using DR mutants. Sequences for guides are shown in (C)–(E). (C–E) Schematic of FnCpf1 crRNAs at sites 1 (C), 2 (D), and 3 (E) targeting GFP. Single-nucleotide mismatches are marked in red. (F) FnCpf1-mediated inactivation of GFP using FnCpf1 plus crRNA harboring DR mutants or DR wild-type with guides containing single mismatches at different positions. Inactivation of GFP is measured by FCM (flow cytometry). Relative activity (ratio) was determined by the formula (A − C)/(B − C) × 100%, where A, B, and C represent the activity of the mutant DR, wild-type (WT) DR, and negative control, respectively. As to the fidelity test (F), ratio was determined by the formula (A′ − C)/(B′ − C) × 100%, where A′, B′, and C represent the activity of the mismatched spacer, totally matched spacer, and negative control, respectively. Error bars, SEM; n = 3. *p < 0.05; **p < 0.01; ***p < 0.001. WT, wild-type.
In total, eight mutants [G(−9)A, U(−7)A/C/G, U(−8)C, G(−9)C, U(−10)G, and U(−16)A] possessed more than half of the activity of the wild-type (Figures 1F and 2A). We then wanted to determine FnCpf1 performance with all of these eight mutants at additional sites. To do this, we selected two additional sites in the GFP gene (marked as sites 2 and 3) (Figures 2B–2E; Table S1) and generated the corresponding plasmids. The latter four mutants [U(−8)C, G(−9)C, U(−10)G, and U(−16)A] have a relatively lower activity (below half of the wild-type) at site 2 and/or site 3, which excludes these four modified DR mutants for universal FnCpf1-mediated genome editing (Figure 2B). In contrast, we observed robust efficiency of the four mutants [G(−9)A and U(−7)A/C/G], exceeding the original activity or at least displaying the majority (>75%) of the wild-type activity at the additional sites (Figure 2B). Remarkably, two mutants [G(−9)A and U(−7)A] exhibited higher activity than the wild-type at all of these three sites, which may facilitate the generation of CRISPR-FnCpf1-based gene KOs. Thus, we selected these four mutants for further studies of editing fidelity.
FnCpf1 Fidelity with These DR Mutants
We designed single-nucleotide mismatches (+1, +5, +10, +15, +20, respectively) within the spacer region of crRNAs targeting sites 1, 2, and 3 in the GFP gene (Figures 2C–2E; Table S1). We found the mutant [G(−9)A and U(−7)A/C] exhibited greatly decreased tolerance of mismatches at sites distal to the PAM (Figure 2F; Figures S2A and S2B). Specifically, single-nucleotide mismatches at guide 2 (G15A, U20C) mediated by the mutants [U(−7)A/C] had only 34%–55% activity of the totally matched spacer, whereas the same single-nucleotide mismatches mediated by the wild-type DR had 57%–64% activity (Figure 2F). Moreover, with single-nucleotide mismatches at guide 3 (C15U, U20C), the mutant [G(−9)A] possessed 32%–64% of the activity of the totally matched spacer, compared with 56%–99% activity mediated by the wild-type sequence (Figure 2F). Then we sought to determine whether the mutations at G(−9) and U(−7) could have synergistic effects when used in combination. We found that its activity of G(−9) and U(−7) resembled that of the wild-type DR sequence, whereas this combined mutant severely hampered the fidelity of FnCpf1 (Figures S2C and S2D). Here we showed that with the engineered Loop, the fidelity of FnCpf1 improved.
The Performance of DR Mutants for Endogenous Genes
The above results were obtained with the GFP reporter system; we sought to know the performance of endogenous genes. We designed the crRNA to target three endogenous genes (CCR5, DNMT1, and HBB), respectively, in HEK293 cells with these DR mutants (Table S2).
First, as we expected, with the mutants G(−9)A and U(−7)A/C, the higher activity could be obtained (Figures S3A and S3B). The mutant G(−9)A possesses higher on-target editing activity than the wild-type. We then investigated the fidelity of these mutants. The results showed that all of the mutants [G(−9)A and U(−7)A/C/G] have lower off-target effects than the wild-type (Figures S3A and S3C).
To confirm the results of DR mutants at endogenous loci, we took advantage of another two additional systems, the puromycin resistance-positive selection system (Figure S4A) and ATP1A1-negative selection system (Figure S5A). In the puromycin selection system, FnCpf1-mediated puromycin-resistance gene editing would result in the inactivation of puromycin-resistance gene. Under the selection of puromycin, the effectively edited cells with the out-frame of puromycin-resistance gene would be killed. It showed that G(−9)A and U(−7)G have the higher activity (65.97% and 69.40%, respectively) than the wild-type (46.00%) (Figures S4B–S4D and S6A). U(−7)A could significantly increase the fidelity if there are 2-nt mismatches between the target sequence and the crRNA (Figures S4B–S4D and S6A). In the ouabain selection system, cells would be dead without the editing of ATP1A1 under the ouabain selection.25 Compared with the wild-type DR (19 colonies survived), G(−9)A has higher activity (73 colonies survived) (Figures S5B–S5D and S6B). If we introduced 2-nt mismatches between the target sequence and the crRNA, no cells could have survived (Figures S5B–S5D and S6B). All of these data illustrated that the mutations at the DR of crRNA modulate the activity and fidelity of FnCpf1 in human cells.
Newly Identified DR Features for AsCpf1 and Variant of FnCpf1
The above results suggested the mutants [G(−9)A and (U(−7)A/C] have higher activity and fidelity. We then wondered whether it is also applicable with AsCpf1, another well-characterized member of the Cpf1 family with highly similar DR sequence to FnCpf1.5 We introduced the mutation at AsCpf1 DR and tested it with AsCpf1. Not surprisingly, we found the presence of higher on-target activity as we observed with FnCpf1 (Figure 3A). Specifically, we found C(−9)A and U(−7)A/C improved efficiency of AsCpf1 (134%, 109%, and 122%, respectively, compared with wild-type [100%]). All of these mutants [C(−9)A and U(−7)A/C] improved AsCpf1 fidelity with mismatches (C1U, C1A, C1G, and C20U; Figures 3B–3D). With the single-base mismatch (C1U), these three mutants showed only 15%–24% activity of their completely matched spacer, compared with 85% activity with the wild-type DR (Figure 3B). Furthermore, the combination of mutations at G(−9) and U(−7) also results in almost no improvement of fidelity but maintenance of cleavage activity (Figures 3A and 3B). These results reveal that these DR mutant sequences may be applied to the engineering of different species of Cpf1.
Figure 3.
The Loop Mutants for AsCpf1
(A) Inactivation of GFP with AsCpf1 plus mutant DR. The guide sequence is shown above (Figure 1B). (B) Inactivation of GFP by AsCpf1 plus crRNA harboring DR mutants with guides containing single mismatches at different positions (+1, +5, +10, +15, +20) across the spacer sequence. (C and D) Inactivation of GFP by AsCpf1 plus crRNA harboring DR wild-type or mutants with guides containing two different single mismatches at (+1): C1A (C) and C1G (D). Inactivation of GFP is measured by FCM. As to the fidelity test (B–D), ratio was determined by the formula, (A′ − C)/(B′ − C) × 100%, where A′, B′, and C represent the activity of the mismatched spacer, totally matched spacer, and negative control, respectively. Error bars, SEM; n = 3; *p < 0.05; **p < 0.01; ***p < 0.001.
Recent studies revealed that two AsCpf1 variants carrying the mutations S542R/K607R and S542R/K548V/N552R have a more flexible PAM;26 however, it has not been well explored to engineer FnCpf1 protein for the modulation of activity. Structural analysis of the FnCpf1-mediated cleavage suggested that there are certain hydrogen bonding contacts formed between FnCpf1 residue [Tyr(865)] and the DR nucleotide [A(−18)].13 Thus, we speculated that benzene ring of Tyr(865) may play an important role. Here we introduced two types of mutations; one is Tyr(865)Ala, which is the absence of benzene ring, and the other is Tyr(865)Phe, which keeps benzene ring, respectively. As we expected, the mutant Tyr(865)Ala lost the majority of cleavage activity, whereas the mutant Tyr(865)Phe maintained the majority of activity (Figure S7), which may be due to the presence of the benzene ring. Next, we sought to know whether the mutation in DR could restore the activities of the FnCpf1 variants [Tyr(865)Ala and Tyr(865)Phe]. We found that G(−9)A could improve efficiency for both mutants (Figure S7). As to the mutant Tyr(865)Phe, with the G(−9)A DR mutant, its activity could be restored, which is confirmed with additional sites (Figures S7D and S7F). Collectively, these results demonstrate that a specific nucleotide mutation or mutations in the DR sequence of crRNA affect the structure of the FnCpf1-crRNA-DNA complex, which results in the modulation of cleavage activity both for wild-type FnCpf1 and for mutant FnCpf1.
Discussion
Recently our group reported that FnCpf1 can trigger efficient genome editing in human cells.6 However, enhancing its efficiency and fidelity has not been systematically explored. In this study, we sought to engineer the DR sequence, which is one of the key elements in the formation of the FnCpf1-crRNA complex. Specifically, the intramolecular hydrogen bonds induce the crRNA 5′ handle as a “pseudoknot” structure that is a prerequisite for its bonds at the groove between the WED and RuvC domains.13 As to the interpseudoknot hydrogen bonds, some base moieties of the crRNA 5′ handle form stacking interactions with certain amino acid residues in the WED and RuvC domains, whereas its phosphodiester backbone forms an extensive network of interactions with the WED, RuvC, and REC2 domains, highlighting the functional relevance between FnCpf1 protein and the DR sequence.13 Thus, introduction of the mutation in the DR sequence would result in changes of interpseudoknot and intrapseudoknot hydrogen bonds, or even generation of new hydrogen bonding, which modulates the formation of ternary FnCpf1-crRNA-DNA complexes and then affects its efficiency and fidelity. As we observed, the mutants G(−9)A and U(−7)A/C/G could modulate the performance of FnCpf1 in human cells. Generally, the mutants G(−9)A and U(−7)A have the best performance at endogenous and exogenous genes in human cells, in terms of their genome-editing efficiency and fidelity (Figure S8). These findings are encouraging and may pave a way for the further exploitation of FnCpf1-mediated genome editing. Our data also suggested that the specific mutations in the DR are also applicable for another genome-editing enzyme, AsCpf1 (Figure 3). Thus, our engineering strategy may provide a reference for engineering some of the additional genome-editing enzymes.
We observed that the majority of the DR mutants abolished FnCpf1 activity (Figures 1D–1F and 2A; Figure S1). We speculated it may be due to destruction of the effective interaction between FnCpf1 protein and the DR sequence, which has been validated with the introduction of mutation at the coding sequence for FnCpf1 [Tyr(865)Ala]. It highlights that hydrogen bonds participate in the formation of the FnCpf1-crRNA complex. Once the nucleotide replacement in the DR sequence involves the destruction of interpseudoknot and intrapseudoknot hydrogen bonds, the formation of the cleavage complex may be hampered.
Among the four different parts of the DR, the Stem is the most susceptible part to mutations because it locates in the center of the complex network of interpseudoknot and intrapseudoknot hydrogen-bonding interactions. Our study revealed that FnCpf1 loses its majority cleavage activity with all the tested mutations in the Stem sequence (Figure 1E). However, the nearly complete loss of FnCpf1 genome-editing activity in human cells mediated by some paired mutants of the Stem is unexpected, because the paired mutants [A(−2)G&U(−15)C, A(−4)G&U(−13)C, and U(−5)C&A(−12)G] in the Stem have been reported to retain their activity in vitro.5 We speculated that the additional factors in human cells may account for it. Also, the different spacer sequences used in the present study and the literature may be another factor for the inconsistency. But additional studies are still needed to provide insights into these observations. Notably, we found that four mutants [U(−8)C, G(−9)C, U(−10)G, U(−16)A] could retain more than half of the wild-type activity at certain loci, but not the other (Figure 2B), which revealed the target sequence itself may also play a role in the formation of the FnCpf1-crRNA-DNA complex.
It is generally accepted that one cannot enhance both activity and fidelity simultaneously.27 Specifically, if the Cas9-RNA complex is easy to be escaped from its target DNA strand (i.e., mismatch), its corresponding on-target efficiency would be lower and off-target effects would be decreased, which has been adopted for Cas9 engineering.27 Surprisingly, our present data do not fit it exactly. The results demonstrated the mutants’ [G(−9)A and (U(−7)A/C] DRs have higher activity and fidelity at the same time, at least at some sites [i.e., G(−9)A at sites 1 and 3 of GFP, CCR5, DNMT1, and HBB] (Figures 2B and 2F; Figure S8). Here we proposed an explanation for the synchronous improvement of Cpf1 activity and fidelity. With the mutation in DR sequence, the interaction of the crRNA-FnCpf1-DNA complex may be changed, which facilitates and increases the FnCpf1 cleavage activity. Also, it may require “rigorous binding” for target DNA, which leads to the reduced off-target effects. It suggested that RNA-based engineering for Cpf1-mediated genome editing may be utilized for both activity and fidelity modulation.
Interestingly, all of the possible mutants [U(−7)A/C/G] at U(−7) possess higher activity and/or fidelity (Figures 2B and 2F; Figure S8). Structural studies showed that U(−7) may not participate in the formation of intrapseudoknot or interpseudoknot hydrogen bonds.13 Thus, we speculate that the U(−7)A/C/G mutations may create the molecular interactions in FnCpf1 complexes, and then improved binding could lead to more efficient and/or more accurate genome editing.
In conclusion, our study provides molecular insights of the DR in FnCpf1-mediated DNA cleavage in human cells. Our engineering of the DR sequence to create mutant [G(−9)A and U(−7)A] equips FnCpf1 with improved cleavage activity and fidelity. These newly identified features will facilitate the design of efficient and accurate CRISPR-FnCpf1-based human genome editing.
Materials and Methods
Plasmids Encoding Cpf1 and crRNA
Plasmids for the expression of FnCpf1 and AsCpf1 were obtained from Addgene (Addgene plasmids 69976 and 69982, respectively). The crRNA expression cassette (Figure 1B) was generated by insertion of PCR products into vector pJET1.2 (CloneJET PCR Cloning Kit; Thermo Fisher Scientific). The direction of crRNA expression cassette was selected by primer-specific PCR. The oligonucleotide sequences used are summarized in Tables S1–S3 and S4. Plasmid DNA and genomic DNA were isolated by standard techniques. DNA sequencing confirmed the desired specific sequence in the constructs.
Cell Culture and Flow Cytometry Analysis
HEK293 cells were obtained from ATCC (catalog no. CRL-1573) and grown at 37°C in 5% CO2 in DMEM (Life Technologies, Carlsbad, CA, USA), 10% heat-inactivated fetal bovine serum, and 1% PS (penicillin and streptomycin). HEK293 cells expressing GFP were generated by lentiviral transduction as previously described.24 Drug-resistant single colonies of transduced HEK293 cells were isolated and named 293-SC1.24 To maintain GFP expression, the medium for 293-SC1 culture included puromycin. The flow cytometry protocol was described previously.24 In brief, on day 0, 1.8 × 105 293-SC1 cells/well were seeded in 12-well plates and transfected with Cpf1 expression plasmid (750 ng) and crRNA expression plasmids (250 ng) by the Transfection Reagent (TurboFect; Thermo Scientific) on day 1. Fresh medium was added to the transfected 293-SC1 cells on day 2. Cells were harvested for flow cytometry (Figure S9) or genomic DNA isolation on day 3.
T7E1 Nuclease Assay for Genome Editing
Fragments harboring deletion or insertion of nucleotides (indel) were amplified by PCR using the primer sets listed in Table S2. For T7 endonuclease I (T7E1) assay, 500 ng of purified PCR products was mixed with 1 μL of 10 × NEB#2 buffer and ultrapure water to a final volume of 9.5 μL and were subjected to a re-annealing process to enable heteroduplex formation. After re-annealing, products were treated with T7E1. Quantification was based on relative band intensities. Indel percentage was determined by the formula 100 × (1− sqrt[b + c]/[a + b + c]), where a is the integrated intensity of the undigested PCR product, and b and c are the integrated intensities of the cleavage product.
Puromycin and Ouabain Selection System
In the puromycin selection system, the 293-SC1 cells were used, which harbor a single copy of puromycin-resistance cassette. At day 0, 1.8 × 105 293-SC1 cells/well were seeded in 12-well plates. Plasmids were transfected into 293-SC1 in 12-well plates at day 1. Cells were transferred into two wells in six-well plates at day 3. One well was for puromycin selection, and the other was without selection (negative control). The total survived cells were counted with an automatic cell counter to cell numbers at day 6. In the ouabain selection system, cell culture and plasmid transfection protocols were similar to puromycin selection. With 3 days’ selection, cells were counted with an automatic cell counter. Ouabain-selection cells were cultured and replaced to get single individual colonies in a 10-cm dish. Single individual colonies were stained with Coomassie brilliant blue and then counted for the number. Ouabain selection was performed as described in the literature.25
Off-Target Analysis for FnCpf1
We examined the possibility that FnCpf1 induced off-target mutations. The potential off-target sites were predicted using online software (http://www.rgenome.net/cas-offinder/). The fragments harboring potential off-target sites have been amplified (primer information in Table S2) and tested with T7E1.
Statistics
All data were expressed as mean ± SEM. Differences were determined by two-tailed Student’s t test between two groups, or one-way ANOVA followed by post hoc Bonferroni test for multiple groups. The criterion for statistical significance was *p < 0.05, **p < 0.01, or ***p < 0.001.
Author Contributions
F.G. conceived the idea; L.L., X.H., T.Z., L.G., Y.L., X.L., H.L., F.Y., M.T., and L.T. performed the experiments; X.G., C.L., J.Z., Z.S., J.Q., and F.G. performed data analyses; and F.G. wrote the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
All authors declare that they have no competing interests.
Acknowledgments
We appreciate comments from Prof. Caixia Gao (Center for Genome Editing, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China). This work was supported by grants from the Natural Science Foundation of China (81201181 to F.G., 81700885 to X.G., and 81473295 and 81670882 to Z.S.), the Zhejiang Provincial & Ministry of Health research fund for medical sciences (2016KYA145 to X.G. and WKJ-ZJ-1828 to J.Z.),the Science Technology Project of Zhejiang Province (2017C37176 to F.G.), the Zhejiang Provincial Fund for Collegiate Technological Innovation Program (2017R413001 to L.L.), Wenzhou city (Y20160008 to J.Z), the Eye Hospital at Wenzhou Medical University (YNZD201602 to F.G.), and the Research Fund for Lin He’s Academician Workstation of New Medicine and Clinical Translation (17331209 to C.L.).
Footnotes
Supplemental Information includes nine figures and four tables and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.08.021.
Supplemental Information
References
- 1.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E., Norville J.E., Church G.M. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makarova K.S., Zhang F., Koonin E.V. SnapShot: Class 2 CRISPR-Cas Systems. Cell. 2017;168:328–328.e1. doi: 10.1016/j.cell.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 4.Komor A.C., Badran A.H., Liu D.R. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017;168:20–36. doi: 10.1016/j.cell.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zetsche B., Gootenberg J.S., Abudayyeh O.O., Slaymaker I.M., Makarova K.S., Essletzbichler P., Volz S.E., Joung J., van der Oost J., Regev A. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu M., Lin L., Cheng Y., He X., Sun H., Xie H., Fu J., Liu C., Li J., Chen D. A ‘new lease of life’: FnCpf1 possesses DNA cleavage activity for genome editing in human cells. Nucleic Acids Res. 2017;45:11295–11304. doi: 10.1093/nar/gkx783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie H., Tang L., He X., Liu X., Zhou C., Liu J., Ge X., Li J., Liu C., Zhao J. SaCas9 requires 5′-NNGRRT-3′ PAM for sufficient cleavage and possesses higher cleavage activity than SpCas9 or FnCpf1 in human cells. Biotechnol. J. 2018;13 doi: 10.1002/biot.201800080. e1700561. [DOI] [PubMed] [Google Scholar]
- 8.Kleinstiver B.P., Tsai S.Q., Prew M.S., Nguyen N.T., Welch M.M., Lopez J.M., McCaw Z.R., Aryee M.J., Joung J.K. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat. Biotechnol. 2016;34:869–874. doi: 10.1038/nbt.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D., Kim J., Hur J.K., Been K.W., Yoon S.H., Kim J.S. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat. Biotechnol. 2016;34:863–868. doi: 10.1038/nbt.3609. [DOI] [PubMed] [Google Scholar]
- 10.Hur J.K., Kim K., Been K.W., Baek G., Ye S., Hur J.W., Ryu S.M., Lee Y.S., Kim J.S. Targeted mutagenesis in mice by electroporation of Cpf1 ribonucleoproteins. Nat. Biotechnol. 2016;34:807–808. doi: 10.1038/nbt.3596. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y., Cheong S.A., Lee J.G., Lee S.W., Lee M.S., Baek I.J., Sung Y.H. Generation of knockout mice by Cpf1-mediated gene targeting. Nat. Biotechnol. 2016;34:808–810. doi: 10.1038/nbt.3614. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Long C., Li H., McAnally J.R., Baskin K.K., Shelton J.M., Bassel-Duby R., Olson E.N. CRISPR-Cpf1 correction of muscular dystrophy mutations in human cardiomyocytes and mice. Sci. Adv. 2017;3:e1602814. doi: 10.1126/sciadv.1602814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swarts D.C., van der Oost J., Jinek M. Structural basis for guide RNA processing and seed-dependent DNA targeting by CRISPR-Cas12a. Mol. Cell. 2017;66:221–233.e4. doi: 10.1016/j.molcel.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamano T., Nishimasu H., Zetsche B., Hirano H., Slaymaker I.M., Li Y., Fedorova I., Nakane T., Makarova K.S., Koonin E.V. Crystal structure of Cpf1 in complex with guide RNA and target DNA. Cell. 2016;165:949–962. doi: 10.1016/j.cell.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong D., Ren K., Qiu X., Zheng J., Guo M., Guan X., Liu H., Li N., Zhang B., Yang D. The crystal structure of Cpf1 in complex with CRISPR RNA. Nature. 2016;532:522–526. doi: 10.1038/nature17944. [DOI] [PubMed] [Google Scholar]
- 16.Fagerlund R.D., Staals R.H., Fineran P.C. The Cpf1 CRISPR-Cas protein expands genome-editing tools. Genome Biol. 2015;16:251. doi: 10.1186/s13059-015-0824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao P., Yang H., Rajashankar K.R., Huang Z., Patel D.J. Type V CRISPR-Cas Cpf1 endonuclease employs a unique mechanism for crRNA-mediated target DNA recognition. Cell Res. 2016;26:901–913. doi: 10.1038/cr.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang Y., Jia G., Choi J., Ma H., Anaya E., Ye C., Shankar P., Wu H. Optimizing sgRNA structure to improve CRISPR-Cas9 knockout efficiency. Genome Biol. 2015;16:280. doi: 10.1186/s13059-015-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendel A., Bak R.O., Clark J.T., Kennedy A.B., Ryan D.E., Roy S., Steinfeld I., Lunstad B.D., Kaiser R.J., Wilkens A.B. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin H., Song C.Q., Suresh S., Wu Q., Walsh S., Rhym L.H., Mintzer E., Bolukbasi M.F., Zhu L.J., Kauffman K. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat. Biotechnol. 2017;35:1179–1187. doi: 10.1038/nbt.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow R.D., Kim H.R., Chen S. Programmable sequential mutagenesis by inducible Cpf1 crRNA array inversion. Nat. Commun. 2018;9:1903. doi: 10.1038/s41467-018-04158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong W., Zhang J., Cui G., Wang L., Wang Y. Multiplexed CRISPR-Cpf1-mediated genome editing in Clostridium difficile toward the understanding of pathogenesis of C. difficile infection. ACS Synth. Biol. 2018;7:1588–1600. doi: 10.1021/acssynbio.8b00087. [DOI] [PubMed] [Google Scholar]
- 23.Sun H., Li F., Liu J., Yang F., Zeng Z., Lv X., Tu M., Liu Y., Ge X., Liu C. A single multiplex crRNA array for FnCpf1-mediated human genome editing. Mol Ther. 2018;26:2070–2076. doi: 10.1016/j.ymthe.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Ge X., Yang F., Zhang L., Zheng J., Tan X., Jin Z.B., Qu J., Gu F. Comparison of non-canonical PAMs for CRISPR/Cas9-mediated DNA cleavage in human cells. Sci. Rep. 2014;4:5405. doi: 10.1038/srep05405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agudelo D., Duringer A., Bozoyan L., Huard C.C., Carter S., Loehr J., Synodinou D., Drouin M., Salsman J., Dellaire G. Marker-free coselection for CRISPR-driven genome editing in human cells. Nat. Methods. 2017;14:615–620. doi: 10.1038/nmeth.4265. [DOI] [PubMed] [Google Scholar]
- 26.Gao L., Cox D.B.T., Yan W.X., Manteiga J.C., Schneider M.W., Yamano T., Nishimasu H., Nureki O., Crosetto N., Zhang F. Engineered Cpf1 variants with altered PAM specificities. Nat. Biotechnol. 2017;35:789–792. doi: 10.1038/nbt.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinstiver B.P., Pattanayak V., Prew M.S., Tsai S.Q., Nguyen N.T., Zheng Z., Joung J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529:490–495. doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.