Abstract
Knee Osteoarthritis (OA) is a progressive degenerative joint disease affecting the quality of life of the elderly population. There is considerable evidence that nutraceuticals from natural herbs may play a significant role in inflammation and joint destruction in OA. We review the current status of some of the commonly used nutraceuticals in Indian market – Boswellia, Aflapin, Chondroitin sulphate, Glucosamine sulphate, Collagen peptide, Curcumin, Fish Oil, Ginger, Green tea, and Rosehip extract. We have summarized their mechanism of action, biological effects, toxicities and efficacy in the management of Knee OA. These supplements have been found to be effective in knee OA in various studies. No serious side effects have been reported for any of these supplements. Overall, our study identifies and support the use of these nutraceuticals to provide symptomatic relief to patients with knee OA and justify their use as an adjunct therapy for the management. More good quality trials are needed to provide definitive answers to questions related to their efficacy and safety for OA prevention and treatment.
Keywords: Knee osteoarthritis, Nutraceuticals, Boswellia, Aflapin, Chondroitin sulphate, Glucosamine sulphate, Collagen peptide, Curcumin
1. Introduction
Osteoarthritis (OA) is a common degenerative disorder affecting elderly population and characterized by cartilage and synovium inflammation.1,2 Pathological changes in later stage of OA include softening, ulceration, and disintegration of the articular cartilage.3,4 The prevalence of OA of the knee in India is found to be 28.7%.5 The prevalence of OA increases with age.6,7 Almost, 45% of women over the age of 65 years are suffering from OA of knee.8,9 The recent high incidence of OA is observed in younger age group also.10 Analgesics and anti-inflammatory drugs are the most common agents in the management of knee OA.11 These only act as symptomatic treatment and do not provide a cure of OA12 and are associated with serious adverse events on gastrointestinal, renal and cardiovascular systems.
The ideal treatment should modify the natural history of OA and alter the articular cartilage destructive process. Such substances which protect the articular cartilage during OA are termed as ‘chondroprotective agents,’ and when these modify the course of the disease, these are called as ‘disease-modifying OA drugs’.13 In the recent times, Nutraceuticals are used commonly in the management of OA knee in India and abroad. The term ‘nutraceutical’ was coined from ‘nutrition’ and ‘pharmaceuticals’ in 1989 by DeFelicen14 and was described as food that provides medical or health benefits.
In the current study, we have investigated the role of 10 commonly used nutraceuticals in the management of knee OA in India, to evaluate their role and efficacy, based on the available literature. In this review article, extensive pubmed search has been done in each category and studies have been selected based on the level of the study and clinical relevance but the list may not be complete. The agents discussed herewith in the text and tables (Table 1) are in alphabetical order and they neither show the preference of our usage nor do these depict their popularity in the market.
Table 1.
Table showing the Nutraceuticals used in the management of knee osteoarthritis, their source, active ingredient, mechanism of action and side effects.
| Nutraceuticals | Source | Active ingredient | Mechanism of action | Side effects |
|---|---|---|---|---|
| Boswellia | Boswellia serrata gum resin | 3-O-Acetyl-11-keto-beta-boswellic acid18 | Inhibit 5-lipoxygenase,18 inhibit complement system at the level of conversion of C3 in to C3a and C3b, also inhibit proinflammatory cytokines17 | Gastrointestinal symptoms17 |
| Aflapin | Synergistic composition of Boswellia serrata extract enriched in AKBA and non-volatile oil portion of B.serrata gum resin29 | AKBA | 5-lipoxygenase inhibition and Matrix Metalloproteinase 3 inhibition26 | Nausea and Headache |
| Glucosamine | Glucosamine can be extracted from the chitosan and chitin exoskeleton of crustaceans such as shelfish and can be stabilized by salt32 | Glucosamine Sulfate, Glucosamine Hydrochloride |
GlcN penetrates into cells by means of glucose transporters. GlcN associate to O-GlcNAcylate proteins and modulates their activity, e.g. decrease nuclear factor-κB nuclear translocation. GlcN may also affect the transcription of pro-inflammatory cytokines by epigenetic mechanisms.34 |
Shelfish allergy,36,37 Affect glucose metabolism and can induce insulin resistance,35 Administered as a salt: Na+ and CL- can affect blood pressure and renal function in those pt.36,37 |
| Chondroitin | Can be obtained from shark or bovine cartlige33 | Chondroitin 4 and 6 sulfate | CS do not penetrate into chondrocytes, synoviocytes, and elicit the anti-inflammatory effect by engaging membrane receptors, e.g. CD44, TLR4, and ICAM1, with a resulting dual effect: impede the fragments of extracellular matrix engaging these receptors, cause of inflammatory reaction, and block the signal transduction pathways activated by the fragments and so diminish the nuclear translocation of pro-inflammatory transcription factors.34 | Epigastric pain, diarrhea, heart burn, nausea38 |
| Collagen peptide | Derived from gelatinization and subsequent enzymatic hydrolysis of native collagen and it contains small peptide with a molecular weight lower than 5000 Da53 | Collagenic animal tissue53 | Stimulates collagenic tissue regeneration by increasing collagen synthesis, glycosaminoglycans and hyaluronic acids53 | |
| Curcumin | Curcumin is derived from turmeric, a popular spice used in India, South Asia, and Japan, which is the grounded root and rhizome of the plant Curcuma Longa58,59 | Curcumin | Suppression of NF-kappaB mediated IL-1beta/TNF-alpha catabolic signaling pathways in chondrocytes60,61 | Dyspepsia, abdominal pain, nausea, loose stool67 |
| Fish Oil | Obtained from the body of fatty fish75 | n-3fatty acids, eicosapentaenoic acid, docosahaexaenoic acid75 |
Dose dependant decrease in inflammatory destruction of cartilage tissue75 | Intolerance, diarrhea and gastroesophageal reflux76 |
| Ginger | Ginger is rhizome of Z.officinale | Reduces inflammatory markers like nitric oxide, hs-C reactive protein,81 TNF-alpha and IL-1beta82 | ||
| Green tea | Polyphenols: epigallocatechin-3-gallate88 | Inhibit expression of TNF alpha, MMP-13 and NF-kappaB, inhibit IL-1beta90,91 and modulate miRNAs expressions92 | ||
| Rose Hip Extract | RHP, prepared from dried Rosa canina fruitsof a selected cultivar, obtained from Hyben Vital, Langeland, Denmark.94 | galactolipid (2 S)-1,2-di-O-[(9Z,12Z,15Z)-octadeca-9,12,15-trienoyl]-3-O-beta-d-galactopyranosyl glycerol95 | Proposed M/A: Rose hip extract inhibited the chemotaxis and chemiluminescence of peripheral blood polymorphonuclear leucocytes and also reduces the level of serum creatinine and acute phase protein CRP96 |
2. Review
2.1. Boswellia and Aflapin
The role of Boswellia in various health conditions like inflammatory diseases, cancers, wound healing and antimicrobial activity is well known. The gum resin is extracted from the ancient herb, Boswellia serrata (Fig. 1a). It has been found to be a potent anti-inflammatory, anti-arthritic and analgesic agent.15,16 Ammon HP et al.17 also reviewed literature for the side effect of Boswellia and concluded that the number and severity of side effects are meager. The most active component of Boswellia extract is 3-O-Acetyl-11-keto-beta-boswellic acid (AKBA), which inhibits 5-lipoxygenase (5-LOX) and complement system involved in cellular inflammatory cascade.18,19 These have also been found to reduce production of proinflammatory cytokines involved in the cartilage destruction.
Fig. 1.
Figure showing various nutraceuticals a) Aflapin, b) Curcumin, c) Fish Oil, d) Ginger, e) Green Tea, f) Rose Hip.
Blain EJ et al.20 concluded that Boswellia decreases MMP-9 and MMP-13 mRNA levels; inhibit MMP9 expression and activation. It also decreases the production of nitrite (the stable end product of nitric oxide), prostaglandin E2 and cyclooxygenase-2. Sengupta et al.21 found that 5-Loxin which is a novel Boswellia Serrata extract enriched with 30% 3-O-acetyl-11-keto-beta-boswellic acid (AKBA) is efficient and safe in OA patients. It was also observed that the MMP-3 of synovial fluid was also reduced significantly.
Belcaro G et al.22 also found that at the end of 4 weeks, the Karnofski Scale was improved more in the Boswellia group compared to control group. The WOMAC Score considering pain, stiffness and physical functions were decreased significantly more in the treatment group in comparison with controls. Belcaro G et al.23 did another similar study with 12 weeks follow-up instead of 4 weeks of the previous study. These findings were similar to the earlier survey.
Different studies have shown that the Boswellia extracts exhibit poor intestinal absorption.24,25 (Table 2) Aflapin is a synergistic composition derived from Boswellia serrata gum resin.26, 27, 28, 29 Aflapin contains B. serrata extract enriched in AKBA and non-volatile oil portion of B. Serrata gum resin. The bioavailability of AKBA increased when given in the form of Aflapin.
Table 2.
Table showing the review of literature of commonly used nutraceuticals used in the management of knee osteoarthritis.
| Nutraceuticals | Author and year | Type of Study | Number of patients | Outcome scoring system used | Results | Conclusion | Remarks |
|---|---|---|---|---|---|---|---|
| Boswellia | |||||||
| Krishanu Sengupt,21 et al. | Randomized double-blind placebo controlled study | 75 | VAS, Lequesne's Functional Index, WOMAC, Cartilage degrading enzyme from synovial fluid |
Statistically and clinically significant improvement in pain score and physical function score, and reduction of cartilage degradation enzyme in synovial fluid | 5-Loxin® reduces pain and improves physical functioning significantly in OA patients; and it is safe for human consumption. 5-Loxin® may exert its beneficial effects by controlling inflammatory responses through reducing proinflammatory modulators, and it may improve joint health by reducing the enzymatic degradation of cartilage in OA patients. | 5-Loxin is Boswellia serrata extract enriched with 30% 3-O-acetyl-11-keto-beta-boswellic acid. | |
| Belcaro G,22 et al. | Supplement registry | 55 | Kamofsky scale, WOMAC score, Treadmill test | The effect of supplement is significant higher than only using standard medicine | The difference between standard medicine and suppl. To SM was significant in favor of suppl. For all target measurement used in registry | ||
| Belcaro G,23 et al. | A comparative study | 66 | WOMAC Score, treadmill test | In supplement plus standard medicine group WOMAC score reduced significantly. | The difference between SM and the flexiqule + SM was in favor of the management with supplement. | Flexiqule: Boswellia extract in capsule: safe and well tolerated. | |
| Aflapin | |||||||
| Krishanu Sengupt30 et al. | Randomized double-blind placebo controlled trial | 60 | VAS, Lequesene's Functional Index, WOMAC | Significant improvement in pain score and physical function score. Significant improvement in pain score and functional ability were recorded at the 7th day of treatment. | Aflapin and 5-Loxin reduce pain and improve physical functions significantly in OA subjects. Aflapin exhibited better efficacy compared to 5-Loxin. Both were safe. | ||
| Amar A,31 et al. | Randomized double-blind placebo controlled trail | 152 | VAS score, Lequesne's functional index, WOMAC score | Significant reduction in all the pain score is observed in aflapin group by day 30. Significne reduction of VAS and LFI observed by day 5 | Aflapin is effective and safe in treatment of OA pt. and its effect shows as early as 5th day of starting treatment. | ||
| Collagen paptide | |||||||
| Kumar S55 et al. | Double-blind, placebo-controlled randomized trial | WOMAC, VAS, Quality of life (QOL) | Scores reduced significantly in collagen peptide group compared to placebo group | Collagen peptide found to be effective in reducing pain of OA knee | Collagen paptide was isolated from pork skin and bovine bone | ||
| Lugo JP,56 et al. | Multicenter, randomized, placebo-controlled, double-blind trail | 191 | WOMAC, VAS, Lequesne Functional Index (LFI) | Significant improvement in UC II group compared to other GS and placebo group | UC=II improved symptoms in OA patients and well tolerated | Undenatured type II collagen obtained from chiken sternum cartilage However, further studies required for establishing its effect and mechanism of action |
|
| Figueres Juher T53 et al. | Review study | 60 scientific studies | Hydrolyzed collagen reduces collagen damage and loss causing reduction in joint pain | Hydrolyzed collagen found to be effective in OAknee | |||
| Curcumin | |||||||
| Madhu K65et al. | A Randomized placebo-controlled trail | WOMAC subscalesand total score | Improved WOMAC score, joint temderness, crepitation, effusion, limitation of move,ents | Curcumin found effective in treatment of OA knee patients | Single blinded, small sample size, short duration | ||
| Kuptniratsaikul V,67 et al.(2014) | Comparative study between curcuma domestica extract and ibuprofen | 345 | WOMAC score | Both group showed significant improvement in WOMAC score | Curcuma domestic a extract is an effective in treatment of OA with less side effects | Large sample size, proper blinding and randomization but short duration | |
| Henrotin Y,68 et al. | Exploratory clinical trail | 22 | VAS score and blood markers | Significantly reduced circulating markers of collagen degradation, Coll2-1,Fib3-1,Fib3-2, Myeloperoxidase and VAS score reduced significantly | Flexofytol reduce inflation and thus reduce pain in OA patient | Flexofytol: another optimized curcumin formation with emulsifier polysorbate 80, Sample size of this study was small |
|
| Nakagawa Y,71 et al. | Randomized, double-blind, placebo-controlled prospective study | Kellgren and Lawrence scale. Japanese Knee Osteoarthritis Measure |
Reduced severity of pain and rate of concomitant celecoxib use. No difference in JKOM | Theracurmin reduces pain significantly. | Large sample and longer duration required. | ||
| Panahi Y,73 et al. | Randomized double-blind placebo-controlled trial | WOMAC,VAS, Lequesne's pain functional index | Significant reduction in all the score compare to placebo | Curcuminoids represent an effective and safe alternative treatment in OA. | |||
| Kok-Yong Chin,62 et al. | Review study | Reduction in pain and improvement in physical function | Patients has better quality of life after taking curcumin | More well planned randomized control trails and enhanced curcumin formulation required | |||
| Daily JW74et al. | Systematic review | 8 RCTs | Pain Visual Analog score, WOMAC score | Reduction of PVAS compared to placebo (p < .00001) in 3 RCTs, Reduction of WOMAC score in 4 RCTs, No significant difference in PVAS in 5 RCTs |
1000 mg/day is effective for treatment of arthritis. It is difficult to draw definitive conclusion due to total sample size, quality of primary study |
More rigorous and larger studies are needed to confirm therapeutic efficacy of turmeric for arthritis | |
| Fish Oil | |||||||
| Nuria Caturla,77 et al. | Randomized, double-blinded, placebo-controlled study | 45 | WOMAC, Lequesne's score | WOMAC, Lequesne's total score reduced 53% and 78% respectively | Standardized lemon verbena extract and Fish oil omega-3 fatty acid reduced pain and stiffness significantly | May be considered for further investigation as a alternative treatment. | |
| Peanpadungrat P,78 et al. | Comparative study | 75 | VAS score, 100 m walking velocity, three steps walking time | Average score of patient satisfaction was 9.06 of 10. all parameters improved significantly | Safe and effective in mild to moderate OA knee pts. | ||
| Hill CL,79 et al. | Randomized clinical trial of low dose versus high dose of fish oil | WOMAC pain and function score | Improvement in both the group, greater improvement in pain and functions score at 2 years in low-dose patients. No difference in cartilage volume loss. | Fish oil is an effective treatment in OAknee | |||
| Boe C,75 et al. | Review study | In vitro studies: anti-inflammatory action, Canine trial: reduction in symptoms Human clinical trial: Not consistently sigmificant |
Long-term, well-designed studies required, and standardization of fish oil industry required | ||||
| Senftleber NK,80 et al. | Systematic review | 42 | Grading of Recommendation Assessment, Development, and Evaluation (GRADE) | The standardized mean difference suggested unfavorable effect in OA patients | Evidence of marine oil using in alleviate pain arthritis patients was over all of the low quality | ||
| Ginger | |||||||
| Naderi Z81 et al. | double-blind randomized placebo-controlled clinical trial | 120 | Serum concentration of nitric oxide (NO) and hs-C reactive protein (hs-CRP | concentration of these markers declined more in the Ginger containing group | Ginger powder supplementation can reduce inflammatory markers | ||
| Mozafarri – Khosravi82 et al. | randomized double-blind clinical trial | 120 | serum TNF-α and IL-1β level | both cytokines decreased in the Ginger containing group relative to the Placebo group | benefit in reducing inflammatory biomarkers | ||
| Bartels EM83 et al. | Meta analysis of randomised placebo controlled trials | 593 | Hedges' standardized mean difference (SMD), and safety by risk ratio (RR) | Statistically significant pain reduction and a statistically significant reduction in disability were seen, both in favor of ginger. | modestly efficacious and reasonably safe but moderate quality evidence | ||
| Paramdeep G84et al. | randomized open label study | 60 | VAS SCORE, WOMAC SCORE |
statistically significant improvement with time in all groups with patients who received both ginger and diclofenac treatments | Ginger powder has add-on effect with acceptable safety profile. | ||
| Amorndoljai P85 et al. | comparative study comparing paired t score before and after treatment | 60 | KOOS, ISOA, PGA | statistically significant improved patient's global assessment, knee joint pain, symptoms, daily activities, sports activities, and quality of life | Application of Ginger extract nanoparticles relieves joint pain with symptomatic and improved quality of life | ||
| Rondanelli M,86 et al. | A pilot study | Tegner Lysholm Knee Scoring,VAS,SF-36), anthropometric parameters,hydration | significant improvement of pain by Lysholm scale score, SF-36 | This study shows feasibility and safety data for the use of highly standardized ginger. | |||
| Green tea | |||||||
| Hashempur MH,93 et al. | Randomized open-label active-controlled clinical trial, Intervention group: green tea extract + diclofenac Control group: diclofenac |
VAS, total WOMAC | Mean difference of VAS pain, total WOMAC, and WOMAC physical functional score shows significant reduction compared with the control group. No significant difference between two groups in mean differences of WOMAC pain and stiffness scores. | Green tea extract can be considered as an adjunctive treatment for control of pain and betterment of knee joint physical function in OA knee pt. | Duration and sample size of this study is small. | ||
| Rose Hip Extract | |||||||
| Winther99 K et al. | Randomized, double-blind, placebo-controlled trail | WOMAC pain, stiffness, global assessment of severity of the disease | Significant reduction in WOMAC pain, and global assessment of severity of the disease | Reduces symptom of osteoarthritis | |||
| Rossnagel K,100 et al. | Meta-analysis of RCT | 2 RCTs revied | 1st RCT:no improvement in knee flexion 2nd RCT: reduction of pain in RHP group |
In both studies RHP has moderate effect in OA patients | 1st RCT: parallel design 2nd RCT: crossover design In both studies sample size was small |
||
| Christensen R,101 et al. | Meta analysis of RCT | 3 RCT reviewed | Reduction of pain score in RHP compared to placebo | Although sparse amount of data available, RHP reduces pain in OA pt. | In future, large-scale/long term trail require | ||
Sengupta K et al.30 did 90-days, randomized, double-blind, placebo-controlled study to evaluate the efficacy of 5-Loxin and Aflapin in osteoarthritis (OA) of the knee. Both 5-Loxin and Aflapin showed significant improvements in pain scores and physical function scores in patients with knee OA. Vishal et al.31 also found similar results.
2.2. Chondroitin sulfate and glucosamine sulfate
Glucosamine sulfate (GS), and Chondroitin sulfate (CS) are glycosaminoglycans (GAGs) synthesized by chondrocytes and synoviocytes. These are essential components of the extracellular matrix and synovial fluid. GS is extracted from the chitosan and chitin exoskeleton of crustaceans such as shellfish and is stabilized by a salt.32 The CS is obtained from shark or bovine cartilage.33
The proposed mechanism of GlcN is by penetration into cells by glucose transporters. GS associated with O-GlcNAcylate proteins are responsible for modulating the inflammatory process like decreasing nuclear factor-κB nuclear translocation. GlcN also affects the transcription of pro-inflammatory cytokines by epigenetic mechanisms. The mechanism of action of CS differs from that of GlcN. Being large molecules; CS does not penetrate into cells,e.g., chondrocytes and activates the anti-inflammatory effect by associating membrane receptors, e.g., CD44, TLR4, and ICAM1. It obstructs the fragments of extracellular matrix engaging these receptors, and blocks the signal transduction pathways activated by the fragments to reduce the nuclear translocation of proinflammatory transcription factors.34
Dostrovsky NR et al.35 noted that GS can affect glucose metabolism and may induce insulin resistance. GlcN may also cause shellfish allergy (Table 3). The GS is administered as salt and may affect the hypertensive and renal patients.36,37 Other side effects include epigastric pain, heartburn, diarrhea, and nausea.38 Kahan et al.39 concluded that CS has structure and symptom modifying effect in patients with knee OA. Gruenwald J et al.40 Also found that GS reduces pain symptoms significantly in patients with knee OA.
Table 3.
Table showing the various studies related to the Glucosamine.
| Nutraceuticals | Author | Type of study | Number of patients | Outcome scoring system used | Result | Conclusion | Remarks |
|---|---|---|---|---|---|---|---|
| Glucosamine and Chondroitin Sulphate | |||||||
| Sherman AL,38 et al., | Review article | GL and CS showed anti-inflammatory action in in vitro study on human chondrocyte, Beneficial effect of CS and GL on pain and function. Small but significant reduction in rate of joint space narrowing. | This review clarifies the role of these compounds in the therapeutic arsenal for OA knee pt. | ||||
| Kahan A,39 et al., | Randomized, double-blind, placebo-controlled trial | Assessed medial compartment of tibio-femoral joint | Significant minimum loss of joint space, pain also improved significantly in OA knee Pt. | CS has structure and symptom modifying effect in pt. with knee OA. | |||
| Gruenwald J,40 et al., | RCT | Glucosamine reduces pain symptoms significantly. | |||||
| Kanzaki N,41 et al., | Randomized, double-blind, placebo-controlled study | 100 | Japanese Knee Osteoarthritis Measure, VAS, Normal walking speed, knee- extensor strength | Knee extensor strength and walking speed is better in treatment group. | This supplement is effective for relieving knee pain and improving locomotor function. | However, along with GL, CS: type II collagen peptide, quercetin glycoside, imidazole, vitamin D also used. | |
| Kanzaki N,42 et al., | Pilot study of gait analysis | Gait analysis | Supplement increases walking speed through increased stride length and angle of kicking from the ground during steps. | Reduction of knee pain leads to improvement in locomotor function | However, along with GL, CS: type II collagen peptide, quercetin glycoside, imidazole also used. | ||
| Jorge A. Roman-Blas,43 et al., | Multicenter, randomized, double-blind,placebo-controlled trail | 164 | Global pain score, VAS, WOMAC | 19% reduction in VAS global pain score compare to 33% reduction in placebo group. Similar improvement in WOMAC score in both group |
CS/GS combination of therapy was not superior to placebo in controlling pain and functional limitation in pt. with knee OA. | Sample was small, confounding factor of analgesic effect conferred by using pain killer as a rescue medication. | |
| Vangsness CT,45 et al. | Review Article | All trials have found the safety of these compounds to be equal to placebo. Inconsistent efficacy in reducing OA pain and improving joint function. |
Because of many studies confirmed OA pain relief with GL + GS and their excellent safety, these supplements may serve a role as an initial treatment modality for OA knee pt. | ||||
| Bishnoi M,47 et al. | Review study | CS, either alone or in combination with other drugs has potential to be effective in treatment of OA knee | |||||
| Mantovani V,48 et al. | Review arthicle | CS and GL can modify the disease progression | No absolute certainities on their efficacy in modifying the course of the disease. | ||||
| Bruyere O,50 et al. | Compared Patented crystalline GS(PGS) with other GS and Glucosamine hydrochloride | PCG found superior over other GS and Glucosamine hydrochloride. Also alter the disease course when started in early stage of disease |
Various formulation of Glucosamine present in market but standardization of formulation is required | ||||
| Raynauld JP,51 et al., | Jonckheere-Terpstra trend test, Multivariant analysis | Significantly reduced the cartilage volume loss in the global knee. The protective effect at 6 years being significant in participants exposed to 2 or more years of treatment. | These findings provide future support for the long-term protective structure-modifying effects of GL/CS treatment in OA knee pt. | ||||
| Haris S.,52 et al., | Review study | 1)trial should to methodological standard (CONSORT) 2)systematic review should follow similar standards (MECIR) |
The best dosage, duration of dosage that provide symptom relief is still unknown. More advanced tools (e.g. MRI) should be used to assess the joint. The quality and quantity of cartilage should also be more accurately defined. (DGRMRIC) Group of pt. who get benefit should be clearly defined. |
||||
There are variable reports about the efficacy of GS and CS in knee OA. Kanzaki et al.41 in a comparative study of 16 weeks duration over 100 patients found improvement in the treatment group. Kanazaki et al.42 in a pilot study concluded that these supplement increases walking speed in the patient of OA knee patients. Roman-Blas JA et al.43 in their study found results of GS and CS to be inferior compared to placebo therapy. Provenza JR et al.44 found that any of the combinations provide clinically significant pain relief in knee OA irrespective of dose fractionation and capsule or sachet formulations. Vangsness CT Jr et al.45 reviewed literature for the same and found that both the drugs were found safe compared to placebo. As an individual drug, there are inconsistent results, but in combination, they found to be effective.46, 47, 48 Henrotin et al.49 also reviewed the literature and concluded that there is an evidence of a reduction in the rate of joint space narrowing.
There are several reasons for these inconsistent results which include that current treatment dose of GlcNbarely reaches the required therapeutic concentration in plasma and tissue. There is no standard formulation available in the market for these supplements. Bruyère et al.50 examined patented crystalline GS (PCG) formulation and found it to be superior to other GS formulations. PCGs also showed a delay in joint structural changes in various studies, indicating potential benefit in altering disease course of OA knee. Raynauld JP et al.51 indicated that treatment with GS/CS significantly reduces the cartilage volume loss in the knee. Haris S Vassiliadis et al.52 observed that despite a significant number of available RCTs, the question of the effectiveness of GS and CS is still not answered. They also noted that which group of patients with the specific grading of OA gets the most benefit of this supplement is not clear.
2.3. Collagen peptide
Hydrolysate Collagen (HC) is derived from gelatinization and enzymatic hydrolysis of native collagen derived from collagenic animal tissue and contained small peptide with a molecular weight lower than 5000 Da.53 In preclinical studies, it is found that HC stimulates collagenic tissue regeneration by increasing collagen synthesis and also by increasing glycosaminoglycans and hyaluronic acids.53 Poole et al.54 found that HC has a therapeutic target for controlling degeneration of articular cartilage and also have analgesic and anti-inflammatory properties.55
Kumar et al.55 used collagen peptides in their study and results were evaluated by WOMAC, VAS and Quality of Life (QOL) score from starting of study to 13 weeks of the study. These scores reduced significantly in collagen peptide group compared to placebo group. Lugo et al.56 carried out a study to evaluate the Undenatured type II collagen (UC II) derived from chicken sternum cartilage in modulating knee OA symptoms. UC II was found to be effective in patients with OA knee and was well tolerated. Figueres Juher T et al.53 did a review of the effect of hydrolyzed collagen on the joint in 60 scientific studies and found that HC intake reduces collagen damage and loss causing a reduction in joint pain. Schadow et al.57 found that the pharmacological effect of the various compositions is different on human chondrocytes. So, standardization of CHs formulation is required.
2.4. Curcumin
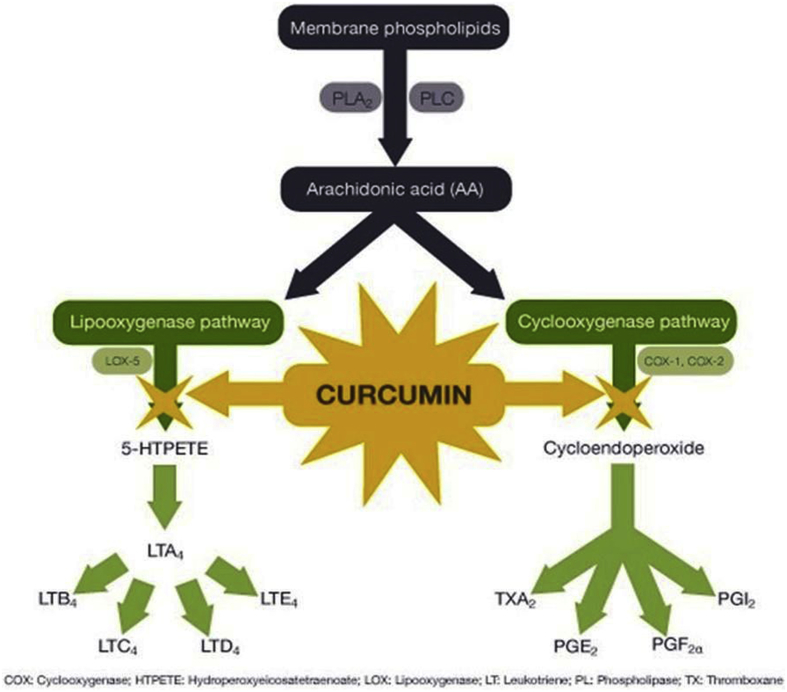
The grounded root and rhizome of the plant Curcuma longa provides Turmeric which is used to treat the biliary digestive disorder, healing wounds and in rheumatic diseases (Fig. 1b). Curcumin (77%) is the main constituent of Turmeric but also contains bisdemethoxycurcumin (17%), and bisdemethoxycurcumin (3%). All these together are called “curcuminoids”.58,59 Curcumin inhibits NF-kappa B mediated IL-1beta/TNF-alpha catabolic signaling pathway in chondrocytes and acts as an anti-inflammatory agent.60,61 Curcumin acts as a chondroprotective agent by inhibiting apoptosis of chondrocytes; proteoglycans and metal metalloproteases release inhibition and inhibition of cyclooxygenase, prostaglandin E-2, and inflammatory cytokines expression in chondrocytes.62 (Fig. 2).
Fig. 2.
Figure showing the mechanism of action of Curcumin.
The oral bioavailability of curcumin is low63 which can be increased by delivering curcumin in liposomes or solid lipid nanoparticles, polymeric micelles, or nanoparticles.64 Madhu K et al.65 observed that Curcumin was effective in improving all WOMAC score and other clinical outcomes in patients with knee OA. Kertia et al.66 compared curcumin with Diclofenac Sodium, and they found curcumin to be equally efficient in suppressing the synthesis of COX-2. Kuptniratsaikul et al.67 did a randomized multicentric study and observed similar results. Adverse effect profile observed include dyspepsia, abdominal pain, nausea, loose stool, and edema. Henrotin et al.68 found significant improvement in their study.
In a study, it was found that the peak plasma Curcumin concentration of Theracurmin (Curcumin formulation dispensed with colloidal submicron-particles) was higher compared to other formulations in the market.69, 70, 71 In humans, concurrent administration of Curcumin and Piperine enhanced the bioavailability of Curcumin by 2000%.72 Panahi et al.73 and Kok-Yong Chin62 et al.also concluded that the improvements in the treatment group were better significantly after taking curcumin. Daily et al.74 systematically reviewed all RCTs and found that three RCTs showed a reduction of PVAS with curcumin in comparison with placebo.
2.5. Fish oil
The effect of fish oil in knee OA patients is still not well understood. It is postulated that the fatty acids present in fish oil alter metabolic pathways by reducing the inflammatory process (Fig. 1c). Various studies showed the reduction in inflammatory destruction of cartilage tissue.75 The anti-inflammatory actions of n-3 fatty acids, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) from fish oil were observed on human cartilage cells. Some adverse effect like intolerance, diarrhea, and gastroesophageal reflux have reported76 with its use.
Caturla et al.77 in their study found improved physical function in patients with knee OA. Peanpadungrat et al.78 also concluded that the fish oil is efficient and safe in mild to moderate stages of knee OA patients, however, the higher dose of 2000 mg did not show greater efficacy than 1000 mg of fish oil. Hill et al. and March et al.79 also concluded that the low-dose fish oil group showed better pain and functional score improvement. Standardization of the fish oil formulations is required for consistency of therapy. Senftleberet al.80 searched database systematically and suggested unfavorable effect in knee OA patients. They concluded that the evidence for using marine oil to alleviate pain in arthritis patients was over all of the low quality.
2.6. Ginger
Ginger is one of the ancient herbs used in India for cooking and for the treatment of different diseases. Ginger has anti-inflammatory action which helps in treating knee OA (Fig. 1d). Naderi et al.81 in their study found that inflammatory markers like nitric oxide and C- reactive protein were reduced significantly in the serum of patients who were given ginger as a treatment compared to placebo. The similar study was done by Mozaffari-Khosravi et al.82 assessing the levels of proinflammatory after ginger supplementation and suggested that Cytokines were decreased in the Ginger Group relative to the Placebo Group. The efficacy and safety of ginger are evaluated in various studies. Meta-analyses of randomized placebo-controlled trials by Bartels EM et al.83 showed statistically significant pain reduction and disability, both in favor of ginger. Paramdeep G et al.84 did randomized, open-label study and found that the group which received both ginger and diclofenac showed better improvement than the individual treatments. The exact dosage and the duration of treatment with Ginger extract still need to be validated. Local application of ginger is also found to be effective in reducing symptoms of OA knee. Amorndoljai P et al.85 concluded that local application of ginger extract relieves joint pain and improves quality of life. Rondinelli et al.86 also found significant improvement in pain relief in patients with Ginger with knee OA who have a poor response with NSAIDs.
2.7. Green tea
The tea is one of the most commonly consumed beverage worldwide. Green tea is 'non-fermented,' and contains more Catechins which are potent antioxidants as compared to black tea (Fig. 1e). There is an increasing interest to evaluate the role of green tea in various diseases including knee OA. “Polyphenols” present in green tea inhibits the inflammatory response at cellular levels. Epigallocatechin-3-gallate (EGCG), is the most important type of polyphenol which inhibits enzyme activities and signal transduction pathways.87,88 Rasheed et al.89 have done in vitro study on human chondrocytes. EGCG significantly reduces advanced glycation end products (AGEs) which induce pro-inflammatory substances in chondrocytes through various mechanisms. EGCG inhibits expression of TNF alpha, MMP-13 and NF-kappaB and also IL-1beta-induced glycosaminoglycan (GAG) release from human cartilage explants.90,91 Newer studies have shown that the role of EGCG in OA might be related to its ability to inhibit inflammatory response by modulating micro RNAs expressions.92,93Green tea has shown potent anti-inflammatory action in various in vitro studies.
2.8. Rosehip extract
Rosehip is derived from dried Rosa canina fruits obtained from Hyben Vital, Langeland, Denmark.94 Rosehip extract95 contains Galactolipid (2 S)-1, 2-di-O-[(9Z, 12Z, 15Z)-octadeca-9, 12, 15-trienoyl]-3-O-β-d-galactopyranosyl glycerol, Mono-galactosyl diglyceride, Di-galactosyl diglyceride, Betulinic acid, oleanolic acid, ursolic acid, vitamin C, vitamin E, β-Carotene, Lycopene, Linoleic acid, EPA, and DHA. These agents modulate inflammatory response and prevent cartilage destruction (Fig. 1f).
Kharazmi A et al.96 found that rose hip extract inhibits the peripheral blood polymorphonuclear leucocytes (PMNs) and also reduce the level of acute phase protein CRP and serum creatinine. It was observed that the gene expression of CCL5/RANTES, CCL20/MIP-3α, CXCL2/MIP-2 and CXCL10/IP-10 on target cells like chondrocytes, was reduced by Rosehip. The expression of genes that degrade ECM was also reduced, and thus RHP showed a chondroprotective effect on the cartilage tissue. Jäger AK et al.97 reported from an in vitro study that component of rose hip powder: (Linoleic acid and alpha-linolenic acid) inhibit COX-1 and COX-2 and contribute anti-inflammatory property. Saaby Let al98 evaluated the immunomodulatory effect of Rosehip powder and found that it inhibits the lipopolysaccharide-induced interleukin-6 release. Winther K et al.99 noticed a reduction in pain, stiffness, and severity of the disease, with the use of Rose-hip. Rossnagel K et al.100 and Christensen R et al.101 found that Rose hip powder is an effective nutraceutical for the treatment of OA knee patients. Chrubasik C et al.102 also conducted a comprehensive review, according to which anti-oxidative and anti-inflammatory properties of various preparations of the Rosehip have been demonstrated. Chrubasik-Hausmann S et al.103 performed a 3-month investigation and found the rose hip shell powder to be as effective as pseudo-fruit powder Litozin (®). However, future research is required to elaborate the importance of the reported promising experimental effects in clinical use.
3. Conclusion
Knee OA is one of the most prevalent diseases in the elderly population. Lifestyle modification and physical therapy forms the first line of management follows by Analgesics and NSAIDs, but these agents only give symptomatic relief and do not affect the natural history of the disease. Nutraceuticals are dietary compounds that are considered to alter inflammatory process and change the natural course of the disease process of OA. However, the term nutraceuticals is not recognized by the US Food and Drug Administration (FDA), which uses the term ‘dietary supplements’ instead. The responsibility of framing and regulating standards for nutraceuticals rests with the Food Safety and Standards Authority of India (FSSAI) as outlined in the Food Safety Act 2006.
Anti-inflammatory, anti-arthritic and analgesic action of Boswellia has been observed in many studies, but the bio-availability is found to be low. Aflapin have shown better bio-availability than Boswellia. The effect of Aflapin was observed as early as the 5th day of starting treatment. Ginger and green tea extract are also found to be effective and safe for OA knee patients. However, further studies required confirming these results. Collagen peptide is also found to be effective in the treatment of OA but different formulations are available in the market, and each formulation has a different pharmacological effect, so standardization of collagen peptide formulation required before using it in the treatment of OA knee.
GS and CH supplements are found to be safe, but results of their effects were inconsistent. However, the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis has recommended them as first-line therapy in the treatment algorithm for knee OA. In future, more specific studies are required to evaluate the exact dosage of these drugs, which formulation is most effective, which group and stage of patients get most benefited, duration of treatment required, when to stop medicine if no response and the exact role of GS/CH in modifying disease process.
Rosehip powder is an effective nutraceutical for treatment of OA patients because of its anti-inflammatory, chondroprotective and immune-modulatory action but the only sparse amount of data is available. In future, more extensive studies are required for establishing its efficacy. Curcumin also has shown positive results but the bio-availability of curcumin found to be low. In future, well-planned RCTs required with enhanced Curcumin formulation to overcome low bio-availability. The low dose of fish oil (1000 mg) is found to be more efficacious than the higher dose. Overall, it is found to be safe, but some side effects like diarrhea, intolerance and gastro-esophageal reflux also have been observed.
Contributor Information
Raju Vaishya, Email: raju.vaishya@gmail.com.
Amit Kumar Agarwal, Email: amitkumar_a@apollohospitalsdelhi.com.
Amish Shah, Email: amish104@gmail.com.
Vipul Vijay, Email: dr_vipulvijay@yahoo.com.
Abhishek Vaish, Email: drabhishekvaish@gmail.com.
References
- 1.Musumeci G., Aiello F.C., Szychlinska M.A., et al. Osteoarthritis in the XXIst century: risk factors and behaviors that influence disease onset and progression. Int J Mol Sci. 2015;16:6093–6112. doi: 10.3390/ijms16036093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szychlinska M.A., Trovato F.M., di Rosa M., et al. Co-expression and co-localization of cartilage glycoproteins CH13L1 and lubricin in osteoarthritic cartilage: morphological, immunohistochemical and gene expression profile. Int J Mol Sci. 2016;17:359. doi: 10.3390/ijms17030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinpelu A.O., Alonge T.O., Adekanla B.A., et al. Prevalence and pattern of symptomatic knee osteoarthritis in Nigeria: a community-based study. Internet J Allied Health Sci Pract. 2009;7(3):10. [Google Scholar]
- 4.Litwic A., Edwards M., Dennison E. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal C.P., Singh P., Chaturvedi S. Epidemiology of knee osteoarthritis in India and related factors. Indian J Orthop. 2016;50:518–522. doi: 10.4103/0019-5413.189608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmona L., Ballina J., Gabriel R., et al. The burden of musculoskeletal disease in the general population of Spain: results from a national survey. Ann Rheum Dis. 2001;60:1040–1045. doi: 10.1136/ard.60.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felson D.T. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev. 1988;10:1–28. doi: 10.1093/oxfordjournals.epirev.a036019. [DOI] [PubMed] [Google Scholar]
- 8.Solomon L., Beighton P., Valkenburg H.A. Rheumatic disorder in the South African Negro. Part I. Rheumatoid arthritis and ankylosing spondylitis. S Afr Med J. 1975;49(32):1292–1296. [PubMed] [Google Scholar]
- 9.Davis M.A., Ettinger W.H., Neuhaus J.M., et al. Sex differences in osteoarthritis of the knee. The role of obesity. Am J Epidemiol. 1988;127:1019–1030. doi: 10.1093/oxfordjournals.aje.a114878. [DOI] [PubMed] [Google Scholar]
- 10.Jordan J.M., Helmick C.G., Renner J.B., et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in african american caucasians: the johnston county osteoarthritis project. J Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 11.Wielage R.C., Myers J.A., Klein R.W., et al. Cost-effectiveness analyses of osteoarthritis oral therapies: a systematic review. Appl Health Econ Health Pol. 2013;11:593–618. doi: 10.1007/s40258-013-0061-x. [DOI] [PubMed] [Google Scholar]
- 12.Towheed T.E., Maxwell L., Judd M.G., et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1) doi: 10.1002/14651858.CD004257.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manno R.L., Bingham C.O., Paternotte S., et al. OARSI-OMERACT initiative: defining thresholds for symptomatic severity and structural changes in disease-modifying osteoarthritis drug (DMOAD) clinical trial. Osteoarthritis Cartilage. 2012;20:93–101. doi: 10.1016/j.joca.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalra E.K. Nutraceutical – definition, and introduction. AAPS PharmSci. 2003;5(3):E25. doi: 10.1208/ps050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh G.B., Atal C.K. Pharmacology of an extract of salai guggal ex-Boswellia serrata, a new non-steroidal anti-inflammatory agent. Agents Actions. 1986;18(3–4):407–412. doi: 10.1007/BF01965005. [DOI] [PubMed] [Google Scholar]
- 16.Ethan B., Heather B., Theresa D.H., et al. Boswellia: an evidence-based systematic review by the natural standard research collaboration. J Herb Pharmacother. 2004;4:63–83. [PubMed] [Google Scholar]
- 17.Ammon H.P. Boswellic acids and their role in chronic inflammatory diseases. Adv Exp Med Biol. 2016;928:291–327. doi: 10.1007/978-3-319-41334-1_13. [DOI] [PubMed] [Google Scholar]
- 18.Safayhi H., Mack T., Sabieraj J., et al. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exp Therapeut. 1992;26:1143–1146. [PubMed] [Google Scholar]
- 19.Sailer E.R., Subramanian L.R., Rall B., et al. Acetyl-11-keto-β-boswellic acid (AKBA): structure requirements or binding and 5-lipoxygenase inhibitory activity. Br J Pharmacol. 1996;117:615–618. doi: 10.1111/j.1476-5381.1996.tb15235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blain E.J., Ali A.Y., Duance V.C. Boswellia frereana (frankincense) suppresses cytokine-induced matrix metalloproteinase expression and production of pro-inflammatory molecules in articular cartilage. Phytother Res. 2010;24(6):905–912. doi: 10.1002/ptr.3055. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta K., Alluri K.V., Satish A.R., et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Res Ther. 2008;10(4) doi: 10.1186/ar2461. R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belcaro G., Dugall M., Luzzi R., et al. FlexiQule (Boswellia extract) in the supplementary management of osteoarthritis: a supplement registry. Minerva Med. 2014;105(6 Suppl 2):9–16. [PubMed] [Google Scholar]
- 23.Belcaro G., Dugall M., Luzzi R., et al. Management of osteoarthritis (OA) with the pharma-standard supplement FlexiQule (Boswellia): a 12-week registry. Minerva Gastroenterol Dietol. 2015 Oct 22 ([Epub ahead of print]) [PubMed] [Google Scholar]
- 24.Kruger P., Daneshfar R., Eckert G.P., et al. Metabolism of boswellic acids in vitro and in vivo. Drug Metabol Dispos. 2008;36:1135–1142. doi: 10.1124/dmd.107.018424. [DOI] [PubMed] [Google Scholar]
- 25.Kruger P., Kanzer J., Hummel J., et al. Permeation of Boswellia extract in the Caco-2 model and possible interactions of its constituents KBA and AKBA with OATP1B3 and MRP2. Eur J Pharmaceut Sci. 2009;36:275–284. doi: 10.1016/j.ejps.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Sengupta K., Kolla J.N., Krishnaraju A.V., et al. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: a novel Boswellia serrata extract. Mol Cell Biochem. 2011;354:189–197. doi: 10.1007/s11010-011-0818-1. [DOI] [PubMed] [Google Scholar]
- 27.Krishnaraju A.V., Sundararaju D., Vamsikrishna U., et al. Safety and toxicological evaluation of Aflapin®: a novel Boswellia-derived anti-inflammatory product. Toxicol Mech Meth. 2010;20:556–563. doi: 10.3109/15376516.2010.497978. [DOI] [PubMed] [Google Scholar]
- 28.Sengupta K., Krishnaraju A.V., Vishal A.A., et al. Comparative efficacy and tolerability of 5-loxin® and Aflapin® against osteoarthritis of the knee: a double-blind, randomized, placebo-controlled clinical study. Int J Med Sci. 2010;7(6):366–377. doi: 10.7150/ijms.7.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sengupta K., Kolla J.N., Krishnaraju A.V., et al. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: a novel Boswellia serrata extract. Mol Cell Biochem. 2011;354(1–2):189–197. doi: 10.1007/s11010-011-0818-1. [DOI] [PubMed] [Google Scholar]
- 30.Sengupta K., Krishnaraju A.V., Vishal A.A., et al. Comparative efficacy and tolerability of 5-loxin® and Aflapin® against osteoarthritis of the knee: a double-blind, randomized, placebo-controlled clinical study. Int J Med Sci. 2010;7(6):366–377. doi: 10.7150/ijms.7.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vishal A.A., Mishra A., Raychaudhuri S.P. A double-blind, randomized, placebo-controlled clinical study evaluates the early efficacy of Aflapin® in subjects with osteoarthritis of KneeInt. J Med Sci. 2011;8(7):615–622. doi: 10.7150/ijms.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henrotin Y., Mobasheri A., Marty M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Res Ther. 2012;14(1):201. doi: 10.1186/ar3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos G.R., Piquet A.A., Glauser B.F., et al. Systemic analysis of pharmaceutical preparations of chondroitin sulfate combined with glucosamine. Pharmaceuticals. 2017;10(2) doi: 10.3390/ph10020038. pii:E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.du Souich P. Absorption, distribution and mechanism of action of SYSADOAS. Pharmacol Ther. 2014;142(3):362–374. doi: 10.1016/j.pharmthera.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Dostrovsky N.R., Towheed T.E., Hudson R.W., et al. The effect of glucosamine on glucose metabolism in humans: a systematic review of the literature. Osteoarthritis Cartilage. 2011;19:375–380. doi: 10.1016/j.joca.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Adrogue H.J., Madias N.E. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356:1966–1978. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 37.Kurtz T.W., Al-Bander H.A. M orris RC Jr. “Salt-sensitive” essential hypertension in men. Is the sodium ion alone important? N Engl J Med. 1987;317:1043–1048. doi: 10.1056/NEJM198710223171702. [DOI] [PubMed] [Google Scholar]
- 38.Sherman A.L., Ojeda-Correal G., Mena J. Use of glucosamine and chondroitin in persons with osteoarthritis. PMR. 2012;4(5):S110–S116. doi: 10.1016/j.pmrj.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Kahan A., Uebelhart D., De Vathaire F., Delmas P.D., Reginster J.Y. Long-term effects of chondroitin 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized,double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60(2):524–533. doi: 10.1002/art.24255. [DOI] [PubMed] [Google Scholar]
- 40.Gruenwald J., Petzold E., Busch R., Petzold H.P., Graubaum H.J. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858–871. doi: 10.1007/s12325-009-0060-3. [DOI] [PubMed] [Google Scholar]
- 41.Kanzaki N., Ono Y., Shibata H., et al. Glucosamine-containing supplement improves locomotor functions in subjects with knee pain: a randomized, double-blind, placebo-controlled study. Clin Interv Aging. 2015 Oct 28;10:1743–1753. doi: 10.2147/CIA.S93077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanzaki N., Otsuka Y., Izumo T., et al. Glucosamine-containing supplement improves locomotor functions in subjects with knee pain - a pilot study of gait analysis. Clin Interv Aging. 2016;11:835–841. doi: 10.2147/CIA.S103943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roman-Blas J.A., Castaneda S., Sanchez-Pernaute O., et al. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo:a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheum. 2017;69(1):77–85. doi: 10.1002/art.39819. [DOI] [PubMed] [Google Scholar]
- 44.Provenza J.R., Shinjo S.K., Silva J.M., et al. Combined glucosamine and chondroitin sulfate, once or three times daily, provides clinically relevant analgesia in knee osteoarthritis. Clin Rheumatol. 2015;34(8):1455–1462. doi: 10.1007/s10067-014-2757-1. [DOI] [PubMed] [Google Scholar]
- 45.Vangsness C.T., Jr., Spiker W., Erickson J. A review of evidence-based medicine for glucosamine and chondroitin sulfate use in knee osteoarthritis. Arthroscopy. 2009;25(1):86–94. doi: 10.1016/j.arthro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 46.Rainsford K.D. Importance of pharmaceutical composition and evidence from clinical trials and pharmacological studies in determining effectiveness of chondroitin sulphate and other glycosaminoglycans: a critique. J Pharm Pharmacol. 2009;61(10):1263–1270. doi: 10.1211/jpp/61.10.0001. [DOI] [PubMed] [Google Scholar]
- 47.Bishnoi M., Jain A., Hurkat P., et al. Chondroitin sulphate: a focus on osteoarthritis. Glycoconj J. 2016;33(5):693–705. doi: 10.1007/s10719-016-9665-3. [DOI] [PubMed] [Google Scholar]
- 48.Mantovani V., Maccari F., Volpi N. Chondroitin sulfate and glucosamine as disease modifying anti- osteoarthritis dru gs (DMOADs) Curr Med Chem. 2016;23(11):1139–1151. doi: 10.2174/0929867323666160316123749. [DOI] [PubMed] [Google Scholar]
- 49.Henrotin Y., Marty M., Mobasheri A. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas. 2014;78(3):184–187. doi: 10.1016/j.maturitas.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 50.Bruyère O., Altman R.D., Reginster J.Y. Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4):S12–S17. doi: 10.1016/j.semarthrit.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Raynauld J.P., Pelletier J.P., Abram F., et al. Long-term effects of glucosamine and chondroitin sulfate on the progression of structural changes in knee osteoarthritis: six-year follow-up data from the osteoarthritis initiative. Arthritis Care Res. 2016;68(10):1560–1566. doi: 10.1002/acr.22866. [DOI] [PubMed] [Google Scholar]
- 52.Vasiliadis H.S., Tsikopoulos K. Glucosamine and chondroitin for the treatment of osteoarthritis. World J Orthoped. 2017;8(1):1–11. doi: 10.5312/wjo.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Figueres Juher T., Basés Pérez E. An overview of the beneficial effects of hydrolysed collagen intake on joint and bone health and on skin ageing. Nutr Hosp. 2015;32(1):62–66. doi: 10.3305/nh.2015.32.sup1.9482. [DOI] [PubMed] [Google Scholar]
- 54.Poole A.R., Ha N., Bourdon S., et al. Ability of a urine assay of type II collagen cleavage by collagenases to detect early onset and progression of articular cartilage degeneration: results from a population-based cohort study. J Rheumatol. 2016;43(10):1864–1870. doi: 10.3899/jrheum.150917. [DOI] [PubMed] [Google Scholar]
- 55.Kumar S., Sugihara F., Suzuki K., et al. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J Sci Food Agric. 2015;95(4):702–707. doi: 10.1002/jsfa.6752. [DOI] [PubMed] [Google Scholar]
- 56.Lugo J.P., Saiyed Z.M., Lane N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: a multicenter randomized, double-blind, placebo-controlled study. Nutr J. 2016;15:14. doi: 10.1186/s12937-016-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schadow S., Siebert H.C., Lochnit G., et al. Collagen metabolism of human osteoarthritic articular cartilage as modulated by bovine collagen hydrolysates. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0053955. e53955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prasad S., Gupta S.C., Tyagi A.K., et al. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32(6):1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Pari L., Tewas D., Eckel J. Role of curcumin in health and disease. Arch Physiol Biochem. 2008;114(2):127–149. doi: 10.1080/13813450802033958. [DOI] [PubMed] [Google Scholar]
- 60.Shakibaei M., John T., Schulze-Tanzil G., et al. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclooxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73(9):1434–1445. doi: 10.1016/j.bcp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Schulze-Tanzil G., Mobasheri A., Sendzik J., et al. Effects of curcumin (diferuloylmethane) on nuclear factor kappaB signaling in interleukin-1beta-stimulated chondrocytes. Ann N Y Acad Sci. 2004;1030:578–586. doi: 10.1196/annals.1329.067. [DOI] [PubMed] [Google Scholar]
- 62.Chin Kok-Yong. The spice for joint inflammation: anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des Dev Ther. 2016;10:3029–3042. doi: 10.2147/DDDT.S117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang K.Y., Lin L.C., Tseng T.Y., et al. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853(1–2):183–189. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Liu W., Zhai Y., Heng X., et al. Oral bioavailability of curcumin: problems and advancements. J Drug Target. 2016;24(8):694–702. doi: 10.3109/1061186X.2016.1157883. [DOI] [PubMed] [Google Scholar]
- 65.Madhu K., Chanda K., Saji M.J. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: a randomized placebo-controlled trial. Inflammopharmacology. 2013;21(2):129–136. doi: 10.1007/s10787-012-0163-3. [DOI] [PubMed] [Google Scholar]
- 66.Kertia N., Asdie A.H., Rochmah W. Marsetyawan Ability of curcuminoid compared to diclofenac sodium in reducing the secretion of cyclooxygenase-2 enzyme by synovial fluid's monocytes of patients with osteoarthritis. Acta Med Indones. 2012;44(2):105–113. [PubMed] [Google Scholar]
- 67.Kuptniratsaikul V., Dajpratham P., Taechaarpornkul W., et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451–458. doi: 10.2147/CIA.S58535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henrotin Y., Gharbi M., Dierckxsens Y., et al. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Compl Alternative Med. 2014;14:159. doi: 10.1186/1472-6882-14-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sunagawa Y., Hirano S., Katanasaka Y., et al. Colloidal submicron-particle curcumin exhibits high absorption efficiency-a double-blind, 3-way crossover study. J Nutr Sci Vitaminol. 2015;61(1):37–44. doi: 10.3177/jnsv.61.37. [DOI] [PubMed] [Google Scholar]
- 70.Morimoto T., Sunagawa Y., Katanasaka Y., et al. Drinkable preparation of Theracurmin exhibits high absorption efficiency – a single-dose, double-blind, 4-way crossover study. Biol Pharm Bull. 2013;36(11):1708–1714. doi: 10.1248/bpb.b13-00150. [DOI] [PubMed] [Google Scholar]
- 71.Nakagawa Y., Mukai S., Yamada S., et al. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci. 2014;19(6):933–939. doi: 10.1007/s00776-014-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shoba G., Joy D., Joseph T., et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 73.Panahi Y., Rahimnia A.R., Sharafi M., et al. Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytother Res. 2014;28(11):1625–1631. doi: 10.1002/ptr.5174. [DOI] [PubMed] [Google Scholar]
- 74.Daily J.W., Yang M., Park S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials. J Med Food. 2016;19(8):717–729. doi: 10.1089/jmf.2016.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boe C., Vangsness C.T. Fish oil and osteoarthritis: current evidence. Am J Orthoped. 2015;44:302–305. [PubMed] [Google Scholar]
- 76.Fortin P.R., Lew R.A., Liang M.H., et al. Validation of a meta-analysis: the effect of fish oil in rheumatoid arthritis. J Clin Epidemiol. 1995;48:1379–1390. doi: 10.1016/0895-4356(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 77.Caturla N., Funes L., Pérez-Fons L., Micol V. A randomized, double-blinded, placebo-controlled study of the effect of a combination of lemon verbena extract and fish oil Omega-3 fatty acid on joint management. J Alternative Compl Med. 2011;17(11):1051–1063. doi: 10.1089/acm.2010.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peanpadungrat P. Efficacy and safety of fish oil in treatment of knee osteoarthritis. J Med Assoc Thai. 2015;98(Suppl 3):S110–S114. [PubMed] [Google Scholar]
- 79.Hill C.L., March L.M., Aitken D., et al. Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann Rheum Dis. 2016;75(1):23–29. doi: 10.1136/annrheumdis-2014-207169. [DOI] [PubMed] [Google Scholar]
- 80.Senftleber N.K., Nielsen S.M., Andersen J.R., et al. Marine oil supplements for arthritis pain: a systematic review and meta-analysis of randomized trials. Nutrients. 2017;9(1) doi: 10.3390/nu9010042. E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naderi Z., Mozaffari-Khosravi H., Dehghan A., et al. Effect of ginger powder supplementation on nitric oxide and C-reactive protein in elderly knee osteoarthritis patients: a 12-week double-blind, randomized placebo-controlled clinical trial. J Tradit Complement Med. 2015;6(3):199–203. doi: 10.1016/j.jtcme.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mozaffari-Khosravi H., Naderi Z., Dehghan A., et al. Effect of ginger supplementation on proinflammatory cytokines in older patients with osteoarthritis: outcomes of a randomized controlled clinical trial. J Nutr Gerontol Geriatr. 2016;35(3):209–218. doi: 10.1080/21551197.2016.1206762. [DOI] [PubMed] [Google Scholar]
- 83.Bartels E.M., Folmer V.N., Bliddal H., et al. Efficacy and safety of ginger in osteoarthritis patients: a meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage. 2015;23(1):13–21. doi: 10.1016/j.joca.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 84.Paramdeep G. Efficacy and tolerability of ginger (Zingiber officinale) in patients of osteoarthritis of the knee. Indian J Physiol Pharmacol. 2013;57(2):177–183. [PubMed] [Google Scholar]
- 85.Amorndoljai P., Taneepanichskul S., Niempoog S., et al. Improving of knee osteoarthritic symptom by the local application of ginger extract nanoparticles: a preliminary report with short term follow-up. J Med Assoc Thai. 2015;98(9):871–877. [PubMed] [Google Scholar]
- 86.Rondanelli M., Riva A., Morazzoni P., et al. The effect and safety of highly standardized Ginger (Zingiber officinale) and Echinacea (Echinacea Angustifolia) extract supplementation on inflammation and chronic pain in NSAIDs poor responders. A pilot study in subjects with knee arthrosis. Nat Prod Res. 2017;31(11):1309–1313. doi: 10.1080/14786419.2016.1236097. [DOI] [PubMed] [Google Scholar]
- 87.Cabrera C., Artacho R., Giménez R. Beneficial effects of green tea–a review. J Am Coll Nutr. 2006;25(2):79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 88.Singh R., Akhtar N., Haqqi T.M. Green tea polyphenol epigallocatechin-3-gallate: inflammation and arthritis. Life Sci. 2010;86(25–26):907–918. doi: 10.1016/j.lfs.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rasheed Z., Anbazhagan A.N., Akhtar N., et al. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res Ther. 2009;11(3):R71. doi: 10.1186/ar2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmed S., Wang N., Lalonde M., et al. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J Pharmacol Exp Therapeut. 2004;308(2):767–773. doi: 10.1124/jpet.103.059220. [DOI] [PubMed] [Google Scholar]
- 91.Katiyar S.K., Raman C. Green tea: a new option for the prevention or control of osteoarthritis. Arthritis Res Ther. 2011;13(4) doi: 10.1186/ar3428. 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rasheed Z., Rasheed N., Al-Shaya O. Epigallocatechin-3-O-gallate modulates global micro RNA expression in interleukin-1β-stimulated human osteoarthritis chondrocytes: potential role of EGCG on negative co-regulation of microRNA-140-3p and ADAMTS5. Eur J Nutr. 2017 Jan 21 doi: 10.1007/s00394-016-1375-x. [DOI] [PubMed] [Google Scholar]
- 93.Hashempur M.H., Sadrneshin S., Mosavat S.H., et al. Green tea (Camellia sinensis) for patients with knee osteoarthritis: a randomized open-label active-controlled clinical trial. Clin Nutr. 2016 Dec 18 doi: 10.1016/j.clnu.2016.12.004. S0261–5614(16)31345-0. [DOI] [PubMed] [Google Scholar]
- 94.Schwager J., Hoeller U., Wolfram S., et al. Rose hip and its constituent galactolipids confer cartilage protection by modulating cytokine, and chemokine expression. BMC Compl Alternative Med. 2011;11(article 105) doi: 10.1186/1472-6882-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwager J., Richard N., Schoop R., et al. A novel rose hip preparation with enhanced anti-inflammatory and chondroprotective effects. Mediat Inflamm. 2014;2014:105710. doi: 10.1155/2014/105710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kharazmi A., Winther K. Rose hip inhibits chemotaxis and chemiluminescence of human peripheral blood neutrophils in vitro and reduces certain inflammatory parameters in vivo. Inflammopharmacology. 1999;7(4):377–386. doi: 10.1007/s10787-999-0031-y. [DOI] [PubMed] [Google Scholar]
- 97.Jäger A.K., Petersen K.N., Thomasen G., et al. Isolation of linoleic and alpha-linolenic acids as COX-1 and -2 inhibitors in the rose hip. Phytother Res. 2008;22(7):982–984. doi: 10.1002/ptr.2446. [DOI] [PubMed] [Google Scholar]
- 98.Saaby L., Jäger A.K., Moesby L., et al. Isolation of immunomodulatory triterpene acids from a standardized rose hip powder (Rosa canina L.) Phytother Res. 2011;25(2):195–201. doi: 10.1002/ptr.3241. [DOI] [PubMed] [Google Scholar]
- 99.Winther K., Apel K., Thamsborg G. A powder made from seeds and shells of a rose-hip subspecies (Rosa canina) reduces symptoms of knee and hip osteoarthritis: a randomized, double-blind, placebo-controlled clinical trial. Scand J Rheumatol. 2005;34(4):302–308. doi: 10.1080/03009740510018624. [DOI] [PubMed] [Google Scholar]
- 100.Rossnagel K., Roll S., Willich S.N. The clinical effectiveness of rosehip powder in patients with osteoarthritis. A systematic review. MMW - Fortschritte Med. 2007;149(27–28 Suppl):51–56. [PubMed] [Google Scholar]
- 101.Christensen R., Bartels E.M., Altman R.D., et al. Does the hip powder of Rosa canina (rosehip) reduce pain in osteoarthritis patients?–a Meta-Analysis of randomized controlled trials. Osteoarthritis Cartilage. 2008;16(9):965–972. doi: 10.1016/j.joca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 102.Chrubasik C., Roufogalis B.D., Müller-Ladner U., et al. A systematic review on the Rosa canina effect and efficacy profiles. Phytother Res. 2008;22(6):725–733. doi: 10.1002/ptr.2400. [DOI] [PubMed] [Google Scholar]
- 103.Chrubasik-Hausmann S., Chrubasik C., Neumann E., et al. A pilot study on the effectiveness of a rose hip shell powder in patients suffering from chronic musculoskeletal pain. Phytother Res. 2014;28(11):1720–1726. doi: 10.1002/ptr.5192. [DOI] [PubMed] [Google Scholar]