Abstract
Tuberculosis of head and neck has been an under diagnosed entity due to large number of smear negative cases, which results in missing out the positive cases, further increasing the burden of TB. The role of cartridge- based nucleic acid amplification test (CBNAAT) with a potential to diagnose TB and rifampicin resistance within 2 h is promising. The study highlights the extended implications of CBNAAT in infectious lesions of head and neck, where the pus or aspirate was subjected to this test, along with other investigations which have been routinely used for detection of extra pulmonary tuberculosis. Twelve patients with infective lesions of head and neck were included in this prospective study, conducted in Department of Otorhinolaryngology, Netaji Subhash Chandra Bose Medical College and hospital, Jabalpur from September 2016 to March 2017. They were investigated for pulmonary and extra pulmonary TB. CBNAAT, microscopy, FNAC and HPR from the site of lesion were done. Nine out of twelve patients were diagnosed positive for Tuberculosis. Microscopy (ZN staining) could detect only two such cases, whereas FNAC showed granulomatous lesion in 3 cases (33.3%). CBNAAT was positive in 77.7% of the total positive cases. Histopathological examination showed 100% results but was feasible only in selected number of cases (4 in this study). CBNAAT provides a promising role in early diagnosis of TB in head and neck. Its high sensitivity and less time taking procedure makes it an excellent tool for timely diagnosis of such cases.
Keywords: CBNAAT, Extra-pulmonary TB, Head and neck TB, GeneXpert MTB/RIF
Introduction
The global burden of tuberculosis (TB) is enormous. More than 9 million new M tuberculosis (MTB) cases and 1.7 million deaths occur annually worldwide [1]. Most of them occur in resource-limited settings. The incidence of TB in underdeveloped countries is increasing, and this is thought to be because of associated poor hygiene conditions and the greater prevalence of AIDS.
Tuberculosis chiefly affects the pulmonary system but it can also involve extrapulmonary sites including the head and neck region. Head and neck manifestations of tuberculosis (TB) are caused by the hematogenous or lymphatic spread of the bacteria to affect the larynx, oropharynx, maxillofacial structures, ear, mastoid, and cervical spine. Other cases of TB of the head and neck are from self-inoculation of open lesions of the aero–digestive tract with infected sputum.
The global priorities for TB care and control are to improve case-detection and to detect cases earlier, including cases of smear- negative disease which are often associated with co-infection with the human immunodeficiency virus (HIV), extra pulmonary TB (where the number of organisms in lesions is generally small) [2, 3] and to enhance the capacity to diagnose multidrug-resistant tuberculosis (MDR-TB) [1, 4].
These cases are often misdiagnosed due to the limitations of conventional diagnostic techniques. Alarming increases in such cases, documented transmission, and rapid morbidity and mortality in these patients have highlighted the urgent need for rapid diagnostic methods.
No single diagnostic test currently satisfies all the demands of “rapid”, “affordable”, and “easy”. The World Health Organization (WHO) has endorsed the use of commercially available liquid culture systems and molecular line probe assays (LPAs) to rapidly detect MDR-TB; however, due to the tests’ complexity and cost, and need for sophisticated laboratory infrastructure and trained personnel, uptake has been limited in many resource-constrained settings [1, 4, 5].
The development of the Xpert® MTB/RIF assay for the GeneXpert platform was completed in 2009 and is considered an important breakthrough in the fight against TB. For the first time, a molecular test is simple and robust enough to be introduced and used outside conventional laboratory settings. Xpert MTB/RIF detects M. tuberculosis as well as mutation that confer rifampicin resistance using three specific primers and have unique molecular probes to ensure a high degree of specificity. The assay provides results directly from sample in less than 2 h. It remains the only self-contained cartridge based fully automated DNA testing platform that can accurately detect both TB and resistance to rifampicin in less than 2 h, and it is the only mature technology among a new generation of automated molecular diagnostic platforms [5].
In December 2010, WHO recommended the use of the Xpert MTB/RIF assay. The WHO’s policy statement was issued in early 2011 and supported by a rapid implementation document, which provided the technical “how-to” and operational considerations for rolling out the use of the assay; the implementation document also provided a simple checklist of prerequisites necessary for implementation along with key action points. An unprecedented uptake of this new technology followed the release of WHO’s policy. By the end of December 2013, more than 2000 GeneXpert instruments and more than 5 million Xpert MTB/RIF cartridges had been procured in the public sector in 98 countries eligible for concessional prices [5].
This study highlights the implications of CBNAAT in infectious lesions of head and neck, where the pus or aspirate is subjected to this test, along with other investigations which have been routinely used for detection of extra pulmonary tuberculosis.
Materials and Methods
This is a prospective observational study.
Twelve patients with age group ranging from 10 to 60 years, who visited the dept of ENT due to suppurative and non suppurative infective lesions of head and neck from September 2016 to March 2017 (6 months) were enrolled in the study.
Inclusion criteria
Abscess in head and neck region
Lesions with active purulent/serous discharge (with at least 1 mL of pus collected at a time)
Cervical lymphadenopathy
Exclusion criteria
Discharge of less than 1 mL
Head and neck malignancy
Neck secondaries
Assessment of each patient was done based on the following.
Detailed History and clinical examination was done. Demographic data including age, sex, socio-economic statutes, rural or urban background was obtained. Past history of Tuberculosis/contact with a TB patient was noted.
Routine blood investigations were done.
Radiological assessment of the lesion along with chest X ray (PA view) was done for all the patients. X ray was done for patients with neck swelling. CT scan/MRI was done for few selected patients subjected to their requirement and availability of resources.
Sputum, if present—ZN staining
Fine needle Aspiration was done from the lesion and was sent for cytology. Purulent aspirate was further subjected to microscopy for acid fast bacilli analysis.
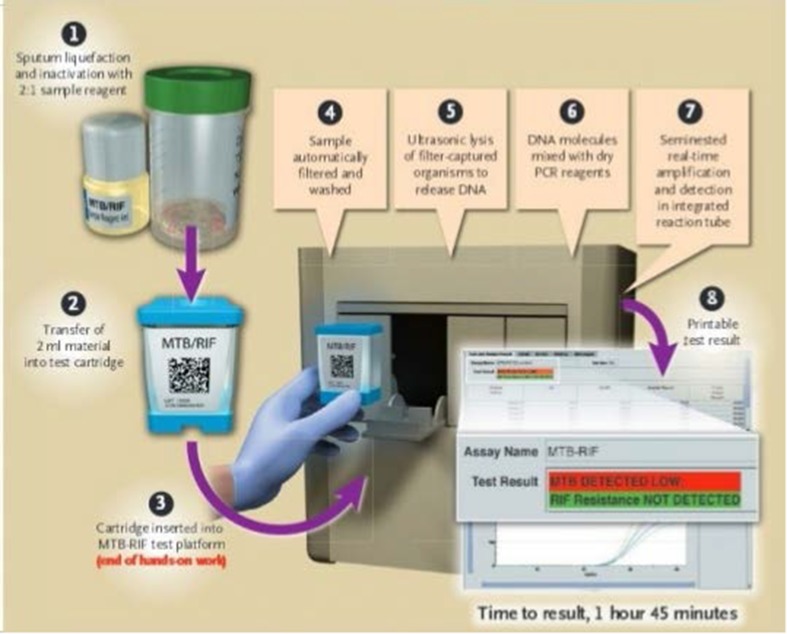
The aspirate was also analyzed by CBNAAT on Xpert-MTB/RIF manufactured by Cepheid endorsed by WHO (2010). It was performed according to the manufacturer’s instructions [CEPHEID, Sunnyvale, CA, USA].
According to standard operating procedure the sampling reagent (containing NAOH and isopropanol) was added at 2:1 ratio to the sample and kept for 15 min at room temperature with intermittent shaking. 3 mL of this treated sample was transferred to the cartridge and the cartridge was inserted in the module of CBNAAT machine. An automatic process completed the remaining assay steps and the results were displayed on the monitor attached to Gene Xpert after 1 h and 50 min. The sample was diluted with three times the reagent, incubated at room temperature and loaded into the cartridge for automated analysis with results in 100 min. Detection of mycobacteria and rifampicin resistance was carried-out in the same setting.
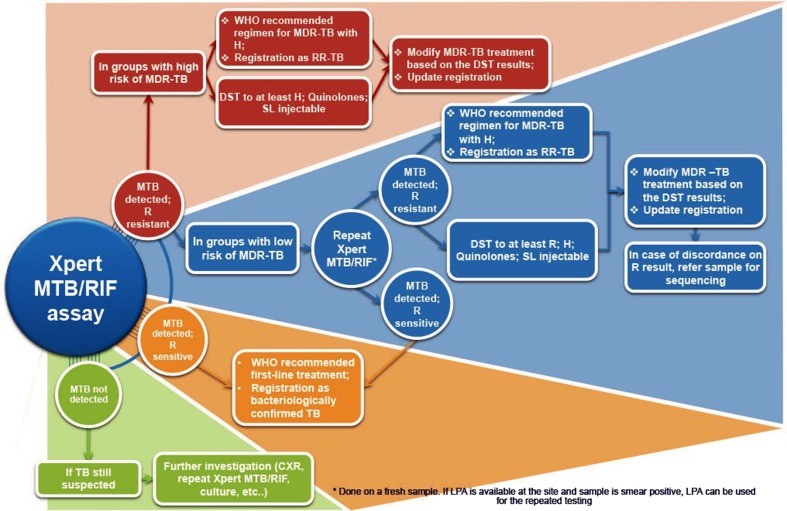
Rifampicin resistant samples were further analysed by LPA. The three steps for LPA test included DNA extraction, multiplex polymerase chain reaction (PCR) amplification and reverse hybridisation (Figs. 1 and 2).
Culture on solid media (Lowenstein–Jensen).
Fig. 1.
Procedure CBNAAT on Xpert-MTB/RIF
Fig. 2.
Algorithm of interpretetion of Xpert MTB/RIF assay
CBNAAT and culture of the sample, were done free of cost at the microbiology laboratory, Indian Council of Medical Research.
Pus for culture and sensitivity.
Ultrasonography of neck
Histopathological report, if required.
As per guidelines according to Definitions and reporting framework for tuberculosis—2013 revision, Geneva, World Health Organization, 2013. Patients detected positive for TB were registered and ATT was started, and response was noted.
Results
Out of 12 patients included in the study 6 were males and 6 were females. Tuberculosis was detected in 9 patients out of which 66% were males and rest were females.
All the patients belonged to lower socio economic strata of the society, and the majority were from rural background (Table 1).
Table 1.
Demographic details of patients
| Demographic characters | Frequency | Percentage |
|---|---|---|
| Male | 6 | 50 |
| Female | 6 | 50 |
| Rural | 10 | 83.3 |
| Urban | 2 | 16.6 |
Six patients presented with cervical lymphadenopathy, whereas four presented with neck abscess with frank purulent discharge, which later required incision and drainage. Two patients presented with post auricular abscess out of which, one was a post operated case of myringoplasty (Table 2).
Table 2.
Symptomatology
| Symptoms | Frequency | Percentage |
|---|---|---|
| Cervical lymphadenopathy | 6 | 50 |
| Neck abscess | 4 | 33.3 |
| Post auricular abscess | 2 | 16.6 |
Two of the twelve cases were non healing and recurrent lesions, already subjected to multiple course of antibiotics with no definite relief (Fig. 3).
Fig. 3.
Distribution of TB positive patients according to presenting complaints (LNpathy, lymphadenopathy; PAS, post aural swelling)
Out of the 12 patients, 9 were diagnosed with tuberculosis through various relevant investigation (Table 3).
Table 3.
Cases diagnosed with TB
| Frequency | Percentage | |
|---|---|---|
| Total cases | 12 | 100 |
| Positive for TB | 9 | 75 |
| Negative for TB | 3 | 25 |
Out of twelve patients subjected to CBNAAT, 58.3% were found to be positive for tuberculosis. Out of these, only 16.6% were positive for tuberculosis through microscopic analysis of acid fast bacilli (ZN staining).
When subjected to cytology, three samples showed granulomatous disease whereas two showed chronic nonspecific inflammation.
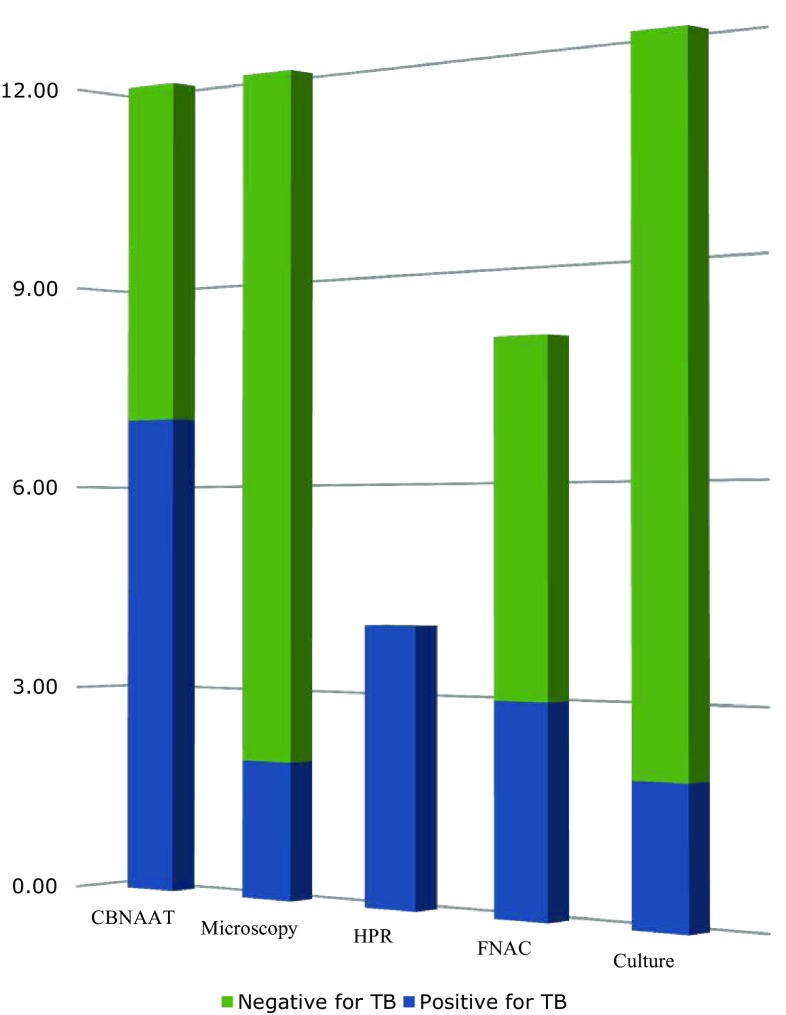
Histopathological examination was done in four cases of cervical lymphadenopathy, subjected to requirement. All of them showed caseating necrosis which was suggestive of Tuberculosis (Table 4 and Fig. 4).
Table 4.
Diagnosis of TB as per various investigations
| Results | Frequency | Percentage |
|---|---|---|
| Total positive cases | 9 | 100 |
| CBNAAT positive TB cases | 7 | 77.7 |
| Microscopically confirmed TB | 2 | 22.2 |
| Histopathologically proven TB | 4 | 44.4 |
| FNAC proven tubercular granulomatous lesion | 3 | 33.3 |
| Culture proven TB | 2 | 22.2 |
Fig. 4.
Diagnosis of TB as per various relevant investigations
Sputum analysis for acid fast bacilli was done for all the patients, and was negative in all twelve of them.
There was no significant past history of TB or history of contact with any TB case.
Chest X-ray of two patients showed significant pathological changes—one of which was empyema. Pus was drained from pleural cavity, and was subjected to examination. Pus was negative for tuberculosis through the above mentioned investigations. Pus Culture showed growth of Staphylococcus aureus and was treated accordingly.
Anti TB treatment was started for all the patients who were diagnosed positive for TB, and response was found to be satisfactory based on patients’ clinical improvement.
STATISTICS: The test was compared with the gold standard test for TB- culture. Statistical analysis was done using software STATA 12. Specificity was found to be 100% whereas sensitivity was 28.6%. Small sample size was a limitation in the statistical analysis of the study.
Discussion
Tuberculosis in otorhinolaryngology and head and neck was thought to be rare, but it is the inaccuracy and low sensitivity of the diagnostic techniques which create this misconception. Tuberculosis in head and neck usually goes unrecognized, which without treatment is potentially lethal and remains an epidemiological threat.
In this study, nine cases of head and neck tuberculosis were found in the duration of 6 months. In the literature, most of the articles concerning head and neck tuberculosis are case reports. In a study by Bruzgielewicz et al. [6] in Medical university of Warsaw on an average, three cases of head and neck TB in a year were found. Nalini and Subramaniam [7] from India presented very extensive material of 117 cases from a 4-year period [5]. Authors from the United Kingdom described 23 cases of head and neck tuberculosis also from a 4-year period, but they noted that all patients were immigrants (Asian, African, Afro-Caribbean and Caucasian) [8, 9].
Male preponderance is in sync with the other studies done in past, with regard to tuberculosis in head and neck [10]. It can be due to additional risk factors such as smoking and alcohol abuse, both of which in our country are more prevalent in men. All the patients of TB belonged to the lower socio economic strata of the society. This justifies the high prevalence of TB in presence of bad hygiene and lower standard of living.
Cervical lymphadenopathy (66.6%) was found to be the most common presenting complaint in TB of Head and neck. According to a study by Bruzgielewicz et al.2 cervical lymphadenopathy was found in 35.6% of such patients. Other presenting features included neck abscess in 22.2% and post auricular abscess in 11.1% of patients with TB Head and neck.
Microscopy and AFB analysis of sputum as well as non respiratory samples, has been a standard protocol for detection of tuberculosis. Due to low sensitivity and increased number of smear negative tuberculosis in HIV positive patients, it results in missing out a large number of positive cases [11].
FNAC detected granulomatous lesion in 33.3% of the patients diagnosed with TB. The cytology patterns observed were granulomatous inflammation and necrosis without acid fast bacilli.
Histopathological examination showed 100% sensitivity and specificity, thus is the most reliable and specific investigation, when required to be done.
Culture, the gold standard for TB showed positive results in two cases.
Compared with culture results, the sensitivity and specificity of Xpert MTB/RIF were 28.6 and 100%, respectively.
High specificity (100%) and a good predictive value was in accordance with different studies in the literature [12].
The observed sensitivity of Xpert MTB/RIF of 28.6% for EPTB was also consistent with seven other published studies in which reported sensitivities ranged from 25.0 to 95.1% [12]. The heterogeneity between studies may reflect differences between patient populations, patient selection, type of EPTB, the quality of the samples, differences in sample processing and the diagnostic gold standard used. An important limitation of this study is the small sample size. This can be considered as a lacuna and necessitates further studies of CBNAAT for extra pulmonary TB with larger sample size.
There are only a few studies on CBNAAT from India. A study done in 2011 in Hyderabad showed incremental case detection of 10.8% when CBNAAT was used to diagnose tuberculosis over and above fluorescent microscopy [13, 11].
An important and remarkable finding in this study was the absence of pulmonary Tuberculosis in any of the proven cases of TB head and neck. This necessitates the need of always keeping tuberculosis in mind in every case of infectious lesion even in complete absence of any symptom or investigation suggestive of pulmonary TB.
Conclusion
Missing out a large number of undiagnosed cases of Tuberculosis remains to be a global concern. The development of the Xpert® MTB/RIF assay for the GeneXpert platform is considered as an important breakthrough in the fight against TB. CBNAAT provides a robust and a promising role in early diagnosis of TB in head and neck as well as other cases of smear negative TB such as TB-HIV and MDR TB. Its high specificity and less time taking procedure makes it an excellent tool for timely diagnosis of such cases.
Conflict of interest
The authors have no conflict of interest. The CBNAAT test was done free of cost at the ICMR microbiology laboratory (endorsed by WHO-2010), Jabalpur with the permission of the Director of ICMR, Jabalpur; with no conflict of interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Helb D, et al. Rapid detection of mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hopewell PC, Small PM. Tuberculosis and nontuberculous mycobacterial infections. In: Stein JH, editor. Internal medicine. 4. St Louis: Mosby; 1994. pp. 2193–2212. [Google Scholar]
- 3.Das DK. Fine-needle aspiration cytology in the diagnosis of tuberculous lesions. Lab Med. 2000;31(11):625–632. doi: 10.1309/UJ0B-VDWV-U0LE-E0QQ. [DOI] [Google Scholar]
- 4.World Health Organization . Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 5.World Health Organization (2014) Xpert MTB/RIF implementation manual: technical and operational ‘how-to’; practical considerations. ISBN: 978 92 4 150670 0 (NLM classi cation: WF 310) World Health Organization
- 6.Bruzgielewicz A, Rzepakowska A, Osuch-Wójcikewicz E, et al. Tuberculosis of the head and neck—epidemiological and clinical presentation. Arch Med Sci. 2014;10(6):1160–1166. doi: 10.5114/aoms.2013.34637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalini B, Subramaniam V. Tuberculosis in ear, nose and throat practice: its presentation and diagnosis. Am J Otolaryngol. 2006;27:39–45. doi: 10.1016/j.amjoto.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization (2013) Definitions and reporting framework for tuberculosis—2013 revision. World Health Organization, Geneva (WHO/HTM/TB/2013.2). available at http://
- 9.Penfold CN, Revington PJ. A review of 23 patients with tuberculosis of head and neck. Br J Oral Maxillof Surg. 1996;34:508–510. doi: 10.1016/S0266-4356(96)90246-6. [DOI] [PubMed] [Google Scholar]
- 10.Sanjay A, Satyadeo C, Satyendra M, Atul K (2016) To study the usefulness of CBNAAT (cartridge based nuclear acid amplification test) in BAL (bronchoalveolar lavage) samples in the diagnosis of smear-negative/non sputum producing patients with suspected tuberculosis. J Evol Med Dent Sci/eISSN- 2278-4802, pISSN- 2278-4748/Vol. 5/Issue 01
- 11.Dewan R, Anuradha S, Khanna A, et al. Role of cartridge-based nucleic acid amplification test (CBNAAT) for early diagnosis of pulmonary tuberculosis in HIV. JIACM. 2015;16(2):114–117. [Google Scholar]
- 12.Lawn SD, Zumla AI. Diagnosis of extrapulmonary tuberculosis using the Xpert® MTB/RIF assay. Expert Rev Anti Infect Ther. 2012;10(6):631–635. doi: 10.1586/eri.12.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sowjanya DS, Behera G, Ramana Reddy VV (2014) CBNAAT: a novel diagnostic tool for rapid and specific detection of mycobacterium tuberculosis in pulmonary sample. Int J Health Res Modern Integr Med Sci (IJHRMIMS), ISSN 2394-8612 (P), ISSN 2394-8620 (O)