Abstract
The identification of the vitamin D receptor in tissues related to testosterone and cortisol production, in conjunction with the observed correlations between vitamin D levels and these hormones in the general population, suggest vitamin D may influence testosterone and cortisol concentrations in athletes. A cross-sectional study design was used to evaluate the association between 25(OH)D and testosterone and cortisol concentrations in young male ice hockey players (n = 50). All athletes were recruited during October from the Sosnowiec area, Poland (50° N). Commercially available ELISA kits were used to determine total serum 25(OH)D, testosterone and cortisol concentrations. Serum 25(OH)D concentration was analyzed as both a continuous and dichotomous variable, binned at the criteria for deficiency (< 20 ng·ml-1), to investigate a threshold effect. Neither continuous (r = 0.18, p = 0.20) nor dichotomous (r = 0.16, p = 0.27) 25(OH)D concentration was significantly correlated with testosterone concentration. A small, inverse correlation (r = -0.30, p = 0.04) was detected between 25(OH)D and cortisol concentrations when analyzed as a dichotomous variable only. Serum 25(OH)D concentration was neither associated with testosterone (p = 0.09) nor cortisol concentrations (p = 0.11) after adjusting for age, fat free mass and fat mass in sequential linear regression. The inability of vitamin D status to independently predict testosterone and cortisol concentrations suggests that any performance-enhancing effects of vitamin D in athletes are unlikely to be mediated primarily through these hormones, at least amongst young male ice-hockey players.
Keywords: 25-Hydroxyvitamin D, Hormones, Athletes, Exercise performance
INTRODUCTION
Serum 25-hydroxyvitamin D (25[OH]D) concentration has been associated with superior aerobic [1] and anaerobic [1, 2] exercise performance in athletes. However, evidence to support a causal relationship between 25(OH)D concentration and exercise performance in athletes is lacking, as the majority of controlled trials report no effect of vitamin D supplementation [3-6]. To date, most interventions in athletes lack statistical power, have few severely vitamin D deficient athletes and/or do not standardize training exposure before and during the intervention. These design issues make it difficult to detect small effects of vitamin D supplementation on exercise performance. Small enhancements in exercise performance (e.g., 1-2% improvement) may be practically meaningful for high-level athletes [7]; however, conducting interventions to detect these small effects in athletes may not be practical for several reasons, including that large sample sizes would be required to detect differences between treatment groups. Given the methodological issues with previous trials and the practical challenges of conducting interventions with elite athletes, it remains unclear whether vitamin D supplementation is a worthwhile strategy to positively influence athletic performance.
Despite the equivocal results from randomized trials in athletes, several plausible biological mechanisms exist to support a mechanistic link between vitamin D status and physical performance in athletes. Traditionally, the identification of the vitamin D receptor (VDR) in cardiac and skeletal muscle has primarily been cited to support the plausibility of the vitamin D system influencing exercise performance [8]. It appears poor vitamin D status may reduce muscle function due to altered kinetics of the active hormone, 25-hydroxyvitamin D, leading to disruptions in calcium and phosphate control, protein synthesis and phospholipid metabolism [9]. However, the exact mechanism of action remains unclear. The expression of the VDR in other tissues has led to the investigation of alternative mechanisms [10]. The VDR is located in the Leydig cells of the testes [11], the major site of testosterone production in men, and in the paraventricular nuclei within the hypothalamus [12], which are involved in regulatory control of cortisol synthesis in the adrenal gland [13]. In addition, the VDR and glucocorticoid receptor are located adjacent to each other in cells and the results from in vitro studies indicate that vitamin D may increase the effectiveness of cortisol signaling [14], which may reduce systemic concentrations needed to achieve function. Thus, the vitamin D system may influence exercise performance over time by modifying testosterone and cortisol levels.
Testosterone and cortisol production are involved with the remodeling of skeletal muscle. Testosterone is an anabolic steroid hormone associated with muscle protein accretion [15] and increased exercise performance [16, 17]. Cortisol is a glucocorticoid known for its catabolic and anti-inflammatory functions, and chronic elevation of this hormone may be detrimental to exercise performance [18]. In the sport science literature, tracking changes in testosterone and cortisol and their ratio (testosterone-to-cortisol) have been used to monitor athletes and evaluate the relative predominance of anabolic or catabolic metabolism during training [19-24]. In theory, demanding exercise training with inadequate recovery may lead to disturbances in hormonal balance, which may negatively impact skeletal muscle regeneration and compromise function [22]. With that said, studies in athletes assessing changes in hormones with respect to exercise training and performance outcomes have yielded conflicting results [19-25]. Never-the-less, disturbance of testosterone levels [10], or hormonal balance, leading to reduced protein synthesis seems to be a plausible mechanism by which poor vitamin D status may negatively influence exercise performance in athletes.
Serum 25(OH)D concentration has been positively associated with resting testosterone concentrations in the general population [26-28], while limited reports exist examining the relation between 25(OH)D and cortisol concentrations [29]. We are aware of only one study in athletes evaluating the association between 25(OH)D, testosterone and cortisol concentrations. In 45 soccer players, 25(OH)D concentration exhibited a positive association with testosterone concentration and an inverse association with cortisol concentration [30]. Replication of these findings in athletes is needed to confirm the relationship, especially in athletes participating in other sports [30]. Examining the association between 25(OH)D concentration and these hormones may provide tentative evidence for an alternative mechanism by which vitamin D may enhance exercise performance in athletes. This information would aid in determining the plausibility of vitamin D influencing exercise performance through the actions of cortisol and testosterone and may inform future interventions supplementing vitamin D in athletes as a means to manipulate hormonal balance. Establishing mechanisms of action in athletes are especially important given the aforementioned constraints of conducting vitamin D and exercise performance interventions in this population. Therefore, the purpose of this study was to assess the cross-sectional association between 25(OH)D and testosterone and cortisol concentrations in young male athletes of the same sport. We hypothesized there would be a positive association between 25(OH)D and testosterone concentrations and a negative association between 25(OH)D and cortisol concentrations.
MATERIALS AND METHODS
Participants
Fifty male ice hockey players from the Private Athletic High School of the Polish Ice Hockey Federation participated in our study. The anthropometric and demographic data of the participants are presented in Table 1. All athletes were Caucasian and of Polish nationality. The type, intensity and volume of exercise training was similar for all athletes. During the competitive season, athletes participated in five training sessions (120-180 min each) and 1-2 matches per week. Athletes ate at the same school canteen. Ethical approval for this study was provided by the local Ethical Committee. Prior to the study written informed consent was obtained from participants or their parents for cases where the participant was under 18 years of age. The study was conducted according to the Declaration of Helsinki.
TABLE 1.
Descriptive information for the athletes (n = 50).
| Mean (SD) | Min-Max | |
|---|---|---|
| Age (years) | 17.2 (0.9) | 15.6-18.7 |
| Height (cm) | 180.6 (6.6) | 165.5-194.0 |
| Body mass (kg) | 75.6 (10.8) | 52.4-100.8 |
| BMI (kg·m-2) | 23.1 (2.7) | 17.2-28.4 |
| Percent body fat (%) | 12.9 (3.8) | 3.5-20.8 |
| Fat free mass (kg) | 65.6 (7.5) | 47.1-82.9 |
| Fat Mass (kg) | 10.0 (4.1) | 2.0-19.4 |
| Vitamin D (ng·ml-1) | 30.3 (14.9) | 12.5-91.4 |
| Testosterone (nmol·L-1) | 19.2 (4.4) | 9.5-28.8 |
| Cortisol (nmol·L-1) | 493 (62) | 366-630 |
Study Design
A cross-sectional study design was used to evaluate the strength of association between 25(OH)D and testosterone and cortisol concentrations in young male ice hockey players. All athletes were recruited during October from the Sosnowiec area, Poland (50°N). The blood collection and anthropometric measurements took place in the morning (between 8 and 9 a.m.), after overnight fasting and a minimum of 12 hours after the last training session.
Blood and Anthropometric Measurements
After blood collection, all blood samples were centrifuged for 10 minutes at 3500 rpm. Serum samples were stored frozen at -20ºC until the analysis.
Total serum 25(OH)D concentration was measured using commercially available ELISA kits (DiA Source, Belgium) according to the manufacturer’s protocol, with the average intra-assay coefficient of variation below 4.0%. The 25OH Vitamin D Total ELISA (DiA Source, Belgium) has the Certificate of Proficiency by the Vitamin D External Quality Assessment Scheme (DEQAS) Advisory Panel.
Commercially available ELISA kits (DRG, Germany) were used to determine total cortisol and testosterone concentrations in serum. All samples were assayed in duplicates and the coefficients of variation of the intra-assays were less than 6% for cortisol and testosterone; moreover, the reference material (BIO-RAD, USA) was attached to each analytical run. The hormonal analyses were carried out in the laboratory of the Department of Biochemistry with an implemented quality system (with accreditation of the Polish Centre for Accreditation no. AB946).
Body mass (BM) and body composition were measured using a Tanita Body Composition Analyser MC-420 (Japan) based on the method of bioelectrical impedance (BIA). BIA has been demonstrated to be a valid predictor of body composition in athletes, with prediction error similar to anthropometric techniques and good reliability [31].
Statistical Analysis
Data were evaluated for normal distributions using the Kolmogorov-Smirnov test and evaluation of the histogram for 25(OH)D concentration, each of which indicated a positive skew. The distribution of the 25(OH)D concentration data was improved with a logarithmic transformation. Serum 25(OH)D concentration was also binned at the criteria for deficiency (< 20 ng·ml-1) to investigate a threshold effect. Means ± standard deviations were used to present descriptive characteristics. Associations between continuous and dichotomous variables were evaluated using Pearson correlational coefficients. The associations between 25(OH)D concentration and testosterone and cortisol were evaluated using sequential linear regression (SLR) to control for potential covariates. Correlations between variables and theory were used to identify potential covariates for use in the control step in SLR. Previous literature has reported associations between maturation [32], body composition [33] and hormone status. Age, fat-free mass (FFM) and fat mass (FM) were used in step one for each SLR model, with 25(OH)D entered in step two as either a continuous or dichotomous variable. Multivariate normality was inspected using residual plots from the SLR analysis. Two-sided p-values and an alpha of 0.05 were used to determine statistical significance. All analyses were performed using SPSS version 20.0 (IBM, Armonk, NY). The design of this study allowed for the detection of moderate correlations (0.38) with 80% power.
RESULTS
Descriptive information for the 50 ice hockey players is presented in Table 1. Only 38% (n = 19) of athletes had sufficient 25(OH)D concentration (≥ 30 ng·mL-1 ) [34]. Forty percent (n = 20) of athletes had insufficient 25(OH)D concentration (< 30 ng·mL-1), while 22% (n = 11) were deficient (< 20 ng·mL-1) [34]. Testosterone and cortisol concentrations for the athletes are shown within Table 1.
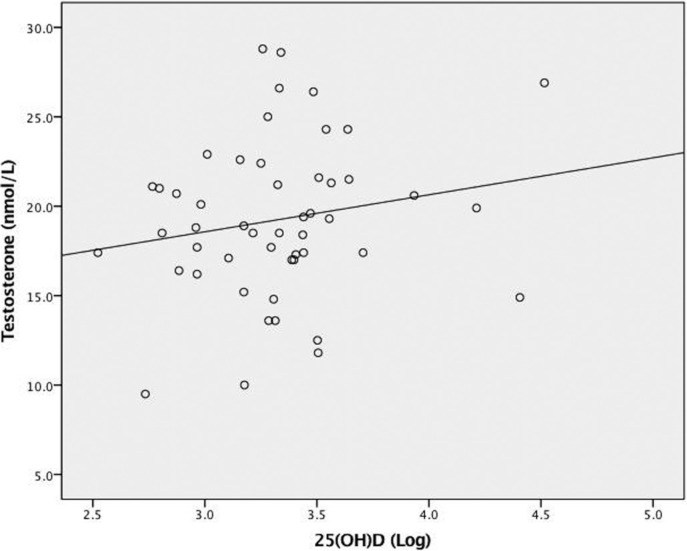
Correlations between testosterone and 25(OH)D concentrations and potential covariates are presented in Table 2. Figure 1 depicts the relationship between 25(OH)D and testosterone concentrations. Neither continuous (r = 0.18, p = 0.20) nor dichotomous (r = 0.16, p = 0.27) 25(OH)D concentration was correlated with testosterone concentration. There was a statistically significant bivariate correlation (r = 0.33, p = 0.019) between age and testosterone concentration. SLR was used to investigate potential suppression of the bivariate relationship between testosterone and 25(OH)D concentrations due to age and to control for body composition (Table 3). Serum 25(OH)D concentration was not a significant predictor of testosterone concentration after controlling for age, FFM and FM (R 2 = 0.17, Finc(1,45) = 2.99, p = 0.09). Analyzing 25(OH)D concentration as a dichotomous variable did not substantially change its relationship to testosterone.
TABLE 2.
Correlations between potential predictor variables and testosterone and cortisol concentrations.
| Testosterone r | Cortisol r | |
|---|---|---|
| Age (years) | 0.332a | 0.082 |
| Body mass (kg) | 0.052 | 0.224 |
| BMI (kg·m-2) | 0.010 | 0.240b |
| Percent body fat (%) | 0.018 | 0.222 |
| Fat free mass (kg) | 0.068 | 0.186 |
| Fat Mass (kg) | 0.012 | 0.253b |
| 25(OH)D concentration (log) | 0.184 | -0.210 |
| 25(OH)D concentration (binned) | 0.159 | -0.296a |
Note. n= 50.
indicates statistically significant at p < 0.05.
indicates trending at p < 0.10.
FIG. 1.
The relationship between 25(OH)D and testosterone concentrations (n = 50). R2 linear = 0.03, p = 0.20.
TABLE 3.
Sequential linear regression with (log of) 25(OH)D concentration predicting testosterone concentration
| Predictors | Regression Coefficient | Standard Error | Standardized Regression Coefficient | p-value | ΔR2 (adjusted) |
|---|---|---|---|---|---|
| Step 1a | 0.057 | ||||
| Age | 2.026 | 0.775 | 0.401 | 0.012 | |
| FFM | -0.085 | 0.132 | -0.145 | 0.521 | |
| FM | 0.189 | 0.236 | 0.174 | 0.429 | |
| Step 2b | 0.039 | ||||
| 25(OH)D | 2.747 | 1.588 | 0.244 | 0.090 |
Note. Vitamin D status for the athletes was log-transformed. n = 50.
Predictors: Age, FFM, and FM.
Predictors: Age, FFM, FM and 25(OH)D.
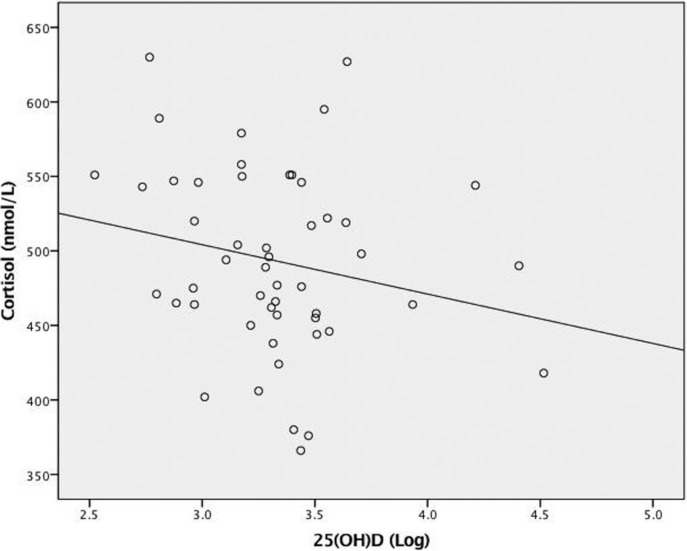
Correlations between cortisol and 25(OH)D concentrations and potential covariates are also within Table 2. Figure 2 depicts the relationship between 25(OH)D and cortisol concentrations. A small, inverse correlation (r = -0.30, p = 0.04) was detected between 25(OH)D and cortisol concentrations when analyzed as a dichotomous variable only. FM was trending toward a statically significant association with 25(OH)D (r = -0.24, p = 0.09; data not shown) and cortisol concentrations (r = 0.25, p = 0.08). In SLR (Table 4), 25(OH)D concentration (dichotomous) was not a significant predictor of cortisol concentration after adjusting for age, FFM and FM (R 2 = 0.13, Finc(1,45) =2.75, p = 0.11). Analyzing 25(OH)D concentration as a continuous variable did not significantly impact the results.
FIG. 2.
The relationship between 25(OH)D and cortisol concentrations (n = 50). R2 linear = 0.04, p = 0.14.
TABLE 4.
Sequential linear regression with (binned) 25(OH)D concentration predicting cortisol concentration.
| Predictors | Regression Coefficient | Standard Error | Standardized Regression Coefficient | p-value | ΔR2 (adjusted) |
|---|---|---|---|---|---|
| Step 1a | 0.012 | ||||
| Age | 8.745 | 11.115 | 0.123 | 0.436 | |
| FFM | -1.085 | 1.926 | -0.131 | 0.576 | |
| FM | 3.938 | 3.349 | 0.259 | 0.246 | |
| Step 2b | 0.036 | ||||
| 25(OH)D | -37.008 | 22.337 | 0.251 | 0.105 |
Note. Vitamin D status for the athletes was binned at deficiency (<20.0 ng·mL-1). n = 50.
Predictors: Age, FFM, and FM.
Predictors: Age, FFM, FM and 25(OH)D.
DISCUSSION
The primary aim of this study was to evaluate the cross-sectional associations between 25(OH)D and testosterone and cortisol concentrations in young male ice hockey players. Vitamin D status was analyzed as both continuous and dichotomous variables to investigate a threshold effect at the criteria for deficiency (< 20.0 ng·ml-1). Bivariate correlations between variables indicated that the relationship between 25(OH)D and cortisol concentrations was improved when analyzed as a dichotomous variable (r = -0.30, p = 0.04 vs. r = -0.22, p = 0.12), whereas the correlation for testosterone concentration was not enhanced (r = 0.16, p = 0.27 vs. r = 0.18, p = 0.20). The main finding of this study was that 25(OH)D concentration was neither associated with testosterone (p = 0.09) nor cortisol concentrations (p = 0.11) after adjusting for age and body composition.
Our failure to detect a statistically significant association between 25(OH)D and testosterone concentrations is not in agreement with the majority of previous literature. Cross-sectional studies, primarily conducted in aging populations, have consistently reported a positive association between 25(OH)D and testosterone concentrations in men [26-28]. One intervention found a small increase in testosterone concentration with vitamin D supplementation [35], though this finding has not been confirmed in other intervention trials [36, 37]. In terms of studies with athletes, a recent observational investigation in soccer players detected a weak, positive association between 25(OH)D and testosterone concentrations along with similar seasonal variation [30]. In contrast to many of these prior observational investigations, we did not detect an association between 25(OH)D and testosterone concentrations in young ice hockey players. It is likely that the association between 25(OH)D and testosterone concentrations is weak and larger cohorts are needed to detect such small correlations. Furthermore, it is also possible that severe vitamin D deficiency (< 10 ng·mL-1) is required to substantially reduce testosterone concentrations in men. Observational evidence indicates that the relationship between 25(OH)D and testosterone concentrations is not linear, but rather the association is strongest in individuals with deficient 25(OH)D concentrations [28]. However, neither sample size nor the prevalence of vitamin D deficiency explain the differing findings in our study and that in Lombardi et al. [30]. While neither study reported athletes with severe deficiency, the prevalence of deficiency (< 20 ng·mL-1) and insufficiency (< 30 ng·mL-1) was greater in our slightly larger cohort of athletes. It should be noted that Lombardi et al. [30] reported a bivariate correlation in athletes competing on different teams and did not control for confounding variables, which tend to augment bivariate relationships.
Similar to the investigation in soccer players [30], we detected a bivariate association between 25(OH)D and cortisol concentrations. However, this association was no longer significant after adjusting for body composition and age. It is not known if the association between 25(OH)D and cortisol concentrations reported in Lombardi et al. [30] would persist after statistical adjustment for confounding, specifically adjustment for body composition and training volume. Evidence suggests adipose tissue and exercise training influence cortisol, testosterone and 25(OH)D concentrations [33, 38-40].
Serum 25(OH)D concentration has been associated with numerous aspects of physical performance in observational studies of athletes [1, 2]. In non-athletes and athletes, 25(OH)D concentration has demonstrated positive associations with resting testosterone levels [26-28, 30]. An inverse association between 25(OH)D and cortisol concentrations has also been reported in athletes [30]. Based on these links, it is plausible that some of the purported benefit of vitamin D on physical performance is mediated through favorable changes in anabolic and catabolic hormones. The inability of 25(OH)D concentration to independently predict testosterone and cortisol concentrations in our athletes suggests that any performance-enhancing effects of vitamin D are unlikely to be mediated, at least primarily, through these hormones amongst young male ice-hockey players. Instead, actions through the VDR in cardiac and skeletal muscle, or other tissues, may represent more plausible mechanisms. Future studies incorporating larger cohorts and measurement of relevant confounding variables are needed to elucidate the relationship between 25(OH)D and testosterone and cortisol concentrations in athletes.
Strengths of this study are that the athletes had similar training regimens and dietary options, statistical adjustment for body composition, and simultaneous assessment of both testosterone and cortisol. Training volume is an important modifier of cortisol and testosterone concentrations [39] and physical activity levels are associated with 25(OH)D concentration [38]. Thus, the relative homogeneity of training within this sample likely reduces any potential impact exercise volume may have on the associations between 25(OH)D and cortisol and testosterone concentrations. With regards to diet, effects on testosterone are most likely mediated through long-term changes in body composition [33]. Given the similar nutritional options available to the athletes, and the statistical adjustment for body composition, it is unlikely that unmeasured dietary variables significantly influenced the association between 25(OH)D and testosterone concentrations. Lastly, the concurrent measurement of cortisol and testosterone may provide a better overall picture of the anabolic/catabolic hormonal environment than testosterone alone [41]. Importantly, the majority of previous studies examining the association between 25(OH)D and testosterone concentrations have failed to incorporate cortisol measurements [26-28].
Although this investigation had strengths, several important limitations need to be acknowledged. While this population of young male ice-hockey players fell within a relatively narrow age range (15.6-18.7 years), maturation differences could confound the associations between 25(OH)D and hormone concentrations, particularly for testosterone, as it is known to increase substantially throughout adolescence [32]. Age was used as a proxy measure for maturation and entered in SLR as a control variable to help minimize this problem, but residual confounding remains a possibility given the limited accuracy of age for determining physical maturation. The use of BIA to assess body composition is another limitation. The prediction error associated with this technique is higher than that of DEXA and hydrostatic weighing. The reduced accuracy of BIA may have diminished our ability to detect statistically significant associations between body composition and other variables. It may also have impacted our ability to adjust for body composition in SLR. Future investigations should use DEXA or hydrostatic weighing for the assessment of body composition. Furthermore, we only assessed the association between 25(OH)D and hormone concentrations during October and it is possible that the strength of association is modified by season due to changing 25(OH)D concentrations. This study’s modest sample size of 50 is a limitation, only allowing for the detection of moderate correlations (0.38) with 80% statistical power. A sample size of greater than 120 would be required to detect correlations less than 0.25. With that said, it seems unlikely that such small associations in a cross-sectional, observational study would translate to meaningful causal effects in randomized trials.
CONCLUSIONS
In the present study, no statistically significant associations were found between 25(OH)D and testosterone concentrations. While there was a small, statistically significant inverse correlation between dichotomous 25(OH)D status and cortisol concentration, this association was not significant after adjusting for body composition and age. Our data suggest that any performance-enhancing effects of vitamin D are unlikely to be primarily mediated through these hormones in young hockey athletes. Genomic and nongenomic signaling through the VDR at cardiac and skeletal muscle seem to be more plausible mechanisms. With that said, monitoring vitamin D status remains a critical strategy to identify athletes that are deficient and that would benefit from supplemental vitamin D intake for other reasons.
Acknowledgements
The study was financed by the Fund for the Development of Physical Culture from the Ministry of Sport and Tourism, Republic of Poland and Institute of Sport – National Research Institute.
REFERENCES
- 1.Koundourakis NE, Androulakis NE, Malliaraki N, Margioris AN. Vitamin D and Exercise Performance in Professional Soccer Players. PLoS ONE. 2014;9(7):e101659. doi: 10.1371/journal.pone.0101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzgerald JS, Peterson BJ, Warpeha JM, Johnson SC, Ingraham SJ. Association Between Vitamin D Status and Maximal- Intensity Exercise Performance in Junior and Collegiate Hockey Players. J Strength Cond Res. 2015;29(9):2513–21. doi: 10.1519/JSC.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 3.Dubnov-Raz G, Livne N, Raz R, Cohen AH, Constantini NW. Vitamin D Supplementation and Physical Performance in Adolescent Swimmers. Int J Sport Nutr Exerc Metab. 2015;25(4):317–25. doi: 10.1123/ijsnem.2014-0180. [DOI] [PubMed] [Google Scholar]
- 4.Close GL, Leckey J, Patterson M, Bradley W, Owens DJ, Fraser WD, et al. The effects of vitamin D(3) supplementation on serum total 25[OH]D concentration and physical performance: a randomised dose-response study. Br J Sports Med. 2013;47(11):692–6. doi: 10.1136/bjsports-2012-091735. [DOI] [PubMed] [Google Scholar]
- 5.Todd JJ, McSorley EM, Pourshahidi LK, Madigan SM, Laird E, Healy M, et al. Vitamin D3 supplementation using an oral spray solution resolves deficiency but has no effect on VO2 max in Gaelic footballers: results from a randomised, double-blind, placebo-controlled trial. Eur J Nutr. 2016;56(4):1–11. doi: 10.1007/s00394-016-1202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jastrzebska M, Kaczmarczyk M, Jastrzebski Z. Effect of Vitamin D Supplementation on Training Adaptation in Well-Trained Soccer Players. J Strength Cond Res. 2016;30(9):2648–55. doi: 10.1519/JSC.0000000000001337. [DOI] [PubMed] [Google Scholar]
- 7.Currell K, Jeukendrup AE. Validity, reliability and sensitivity of measures of sporting performance. Sports Med. 2008;38(4):297–316. doi: 10.2165/00007256-200838040-00003. [DOI] [PubMed] [Google Scholar]
- 8.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491S–9S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 9.Lanteri P, Lombardi G, Colombini A, Banfi G. Vitamin D in exercise: Physiologic and analytical concerns. Cli Chim Acta. 2013;415:45–53. doi: 10.1016/j.cca.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Dahlquist DT, Dieter BP, Koehle MS. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J Int Soc Sports Nutr. 2015;12(33):1–12. doi: 10.1186/s12970-015-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen MB, Nielsen JE, Jorgensen A, Rajpert-De Meyts E, Kristensen DM, Jorgensen N, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25(5):1303–11. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- 12.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic–neurohypophysial system regulates the hypothalamic–pituitary–adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25(3):132–49. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Kalra N, Ishmael F. Cross-talk between vitamin D, estrogen and corticosteroids in glucocorticoid resistant asthma. OA inflammation. 2014;2(1):2. [Google Scholar]
- 15.Griggs RC, Kingston W, Jozefowicz RF, Herr BE, Forbes G, Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol. 1989;66(1):498–503. doi: 10.1152/jappl.1989.66.1.498. [DOI] [PubMed] [Google Scholar]
- 16.Rogerson S, Weatherby RP, Deakin GB, Meir RA, Coutts RA, Zhou S, et al. The Effect of Short-Term use of Testosterone Enanthate on Muscular Strength and Power in Healthy Young Men. J Strength Cond Res. 2007;21(2):354–61. doi: 10.1519/R-18385.1. [DOI] [PubMed] [Google Scholar]
- 17.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281(6):E1172–81. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 18.Hackney AC, Waltz E. Hormonal adaptation and the stress of exercise training: the role of glucocorticoids. TSS. 2013;4(20):165–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman JR, Epstein S, Yarom Y, Zigel L, Einbinder M. Hormonal and Biochemical Changes in Elite Basketball Players During a 4-Week Training Camp. J Strength Cond Res. 1999;13(3):280–5. [Google Scholar]
- 20.Banfi G, Dolci A. Free testosterone/cortisol ratio in soccer: usefulness of a categorization of values. J Sports Med Phys Fitness. 2006;46(4):611. [PubMed] [Google Scholar]
- 21.Argus CK, Gill ND, Keogh JW, Hopkins WG, Beaven CM. Changes in strength, power, and steroid hormones during a professional rugby union competition. J Strength Cond Res. 2009;23(5):1583–92. doi: 10.1519/JSC.0b013e3181a392d9. [DOI] [PubMed] [Google Scholar]
- 22.Coutts A, Reaburn P, Piva T, Murphy A. Changes in selected biochemical, muscular strength, power, and endurance measures during deliberate overreaching and tapering in rugby league players. Int J Sports Med. 2007;28(2):116–24. doi: 10.1055/s-2006-924145. [DOI] [PubMed] [Google Scholar]
- 23.Schelling X, Calleja-Gonzalez J, Torres-Ronda L, Terrados N. Using testosterone and cortisol as biomarker for training individualization in elite basketball: a 4-year follow-up study. J Strength Cond Res. 2015;29(2):368–78. doi: 10.1519/JSC.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 24.Martinez AC, Seco Calvo J, Tur Mari JA, Abecia Inchaurregui LC, Orella EE, Biescas AP. Testosterone and cortisol changes in professional basketball players through a season competition. J Strength Cond Res. 2010;24(4):1102–8. doi: 10.1519/JSC.0b013e3181ce2423. [DOI] [PubMed] [Google Scholar]
- 25.Bonato M, La Torre A, Saresella M, Marventano I, Merati G, Vitale JA. Salivary cortisol concentration after high-intensity interval exercise: Time of day and chronotype effect. Chronobiol Int. 2017:1–10. doi: 10.1080/07420528.2017.1311336.. [DOI] [PubMed] [Google Scholar]
- 26.Anic GM, Albanes D, Rohrmann S, Kanarek N, Nelson WG, Bradwin G, et al. Association between serum 25-hydroxyvitamin D and serum sex steroid hormones among men in NHANES. Clin Endocrinol. 2016;85(2):258–66. doi: 10.1111/cen.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehr E, Pilz S, Boehm BO, Marz W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol. 2010;73(2):243–8. doi: 10.1111/j.1365-2265.2009.03777.x. [DOI] [PubMed] [Google Scholar]
- 28.Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin Endocrinol. 2012;77(1):106–12. doi: 10.1111/j.1365-2265.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally JD, Doherty DR, Lawson ML, Al-Dirbashi OY, Chakraborty P, Ramsay T, et al. The relationship between vitamin D status and adrenal insufficiency in critically ill children. J Clin Endocrinol Metab. 2013;98(5):E877–81. doi: 10.1210/jc.2013-1126. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi G, Vitale JA, Logoluso S, Logoluso G, Cocco N, Cocco G, et al. Circannual rhythm of plasmatic vitamin D levels and the association with markers of psychophysical stress in a cohort of Italian professional soccer players. Chronobiol Int. 2017;34(4):471–9. doi: 10.1080/07420528.2017.1297820. [DOI] [PubMed] [Google Scholar]
- 31.Graves JE, Kanaley JA, Garzarella L, Pollock ML. Anthropometry and body composition measurement. In: Maud PJ, Foster C, editors. Physiological assessment of human fitness. Champaign, IL: Human Kinetics; 2006. pp. 185–225. [Google Scholar]
- 32.Andersson A, Juul A, Petersen JH, Müller J, Groome NP, Skakkebæk NE. Serum Inhibin B in Healthy Pubertal and Adolescent Boys: Relation to Age, Stage of Puberty, and Follicle-Stimulating Hormone, Luteinizing Hormone, Testosterone, and Estradiol Levels 1. J Clin Endocrinol Metab. 1997;82(12):3976–81. doi: 10.1210/jcem.82.12.4449. [DOI] [PubMed] [Google Scholar]
- 33.Morisset AS, Blouin K, Tchernof A. Impact of diet and adiposity on circulating levels of sex hormone-binding globulin and androgens. Nutr Rev. 2008;66(9):506–16. doi: 10.1111/j.1753-4887.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 34.Hollis BW. Assessment and interpretation of circulating 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in the clinical environment. Endocrinol Metab Clin North Am. 2010;39(2):271–86. doi: 10.1016/j.ecl.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011;43(3):223–5. doi: 10.1055/s-0030-1269854. [DOI] [PubMed] [Google Scholar]
- 36.Heijboer AC, Oosterwerff M, Schroten NF, Eekhoff EM, Chel VG, Boer RA, et al. Vitamin D supplementation and testosterone concentrations in male human subjects. Clin Endocrinol. 2015;83(1):105–10. doi: 10.1111/cen.12711. [DOI] [PubMed] [Google Scholar]
- 37.Scholten SD, Sergeev IN, Song Q, Birger CB. Effects of vitamin D and quercetin, alone and in combination, on cardiorespiratory fitness and muscle function in physically active male adults. Open Access J Sports Med. 2015;6:229–39. doi: 10.2147/OAJSM.S83159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brock K, Huang W, Fraser D, Ke L, Tseng M, Stolzenberg-Solomon R, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol. 2010;121(1):462–6. doi: 10.1016/j.jsbmb.2010.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostrowski KJ, Wilson GJ, Weatherby R, Murphy PW, Lyttle AD. The Effect of Weight Training Volume on Hormonal Output and Muscular Size and Function. J Strength Cond Res. 1997;11(3):148–54. [Google Scholar]
- 40.Fitzgerald JS, Peterson BJ, Wilson PB, Rhodes GS, Ingraham SJ. Vitamin D status is associated with adiposity in male ice hockey players. Med Sci Sports Exerc. 2015;47(3):655–61. doi: 10.1249/MSS.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 41.Papacosta E, Nassis GP. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J Sci Med Sport. 2011;14(5):424–34. doi: 10.1016/j.jsams.2011.03.004. [DOI] [PubMed] [Google Scholar]