Abstract
Purpose
To determine the efficacy and safety of sitagliptin when used with some therapeutic drugs to treat elderly patients.
Methods
Sitagliptin (50 mg/day) was added to the pre-existing therapy for type 2 diabetes. Changes in the glycated hemoglobin (HbA1c) level after 3 months of treatment were compared with the baseline, and exploratory analysis was performed. These analyses were conducted as subanalyses of the JAMP study, which was an open-label observational study.
Results
For patients who were ≥65 years of age, the change in HbA1c level from baseline ranged from −0.50 to −0.87% at 3 months after starting treatment. There was no significant difference in the change in HbA1c level between the patients treated with different concomitant drugs. No significant difference in HbA1c variations at 3 and 12 months from baseline was noted among the three age groups (≥75, 65–74, and <65 years). Multiple regression analysis was performed, and it revealed that patients with higher HbA1c levels at baseline were likely to show decreased HbA1c levels, while those with higher triglyceride (TG) levels were unlikely to show decreased HbA1c levels.
Conclusion
Sitagliptin has the potential to both improve glycemic control and prevent hypoglycemia, and can be considered a potent alternative drug.
Electronic supplementary material
The online version of this article (doi:10.1007/s13340-017-0330-2) contains supplementary material, which is available to authorized users.
Keywords: DPP-4 inhibitor, Prospective observational study, Elderly patients, Sitagliptin, JAMP subanalysis, Type 2 diabetes mellitus
Introduction
The patient survey conducted in 2014 by the Ministry of Health, Labour, and Welfare of Japan showed that there were 3,166,000 patients with diabetes mellitus, which was the highest number reported thus far. The 2011 survey reported that there were 2,700,000 diabetes mellitus patients, meaning there was an increase of 466,000 between 2011 and 2014 [1]. Among people 70 years of age or older, one in four men (22.3%) and one in six women (17.0%) have diabetes mellitus [2], and this number is expected to increase in the future as Japanese society ages.
The problems associated with severe hypoglycemia in elderly people with diabetes mellitus must therefore be addressed [3]. Because patients who are 75 years of age or older often have impaired cognitive and physical function [4–8], their hypoglycemia symptoms can be overlooked, which may lead to a worsening of their condition [9]. Because renal function is also impaired in patients with diabetes mellitus, caution should be exercised, especially when administering multiple drugs simultaneously [6].
Dipeptidylpeptidase-4 (DPP-4) inhibitors, which are incretin-related drugs that include sitagliptin, are being used increasingly frequently. The hypoglycemic action of DPP-4 inhibitors involves the highly selective inhibition of DPP-4, an enzyme that inactivates incretin. Incretin is a gastrointestinal hormone that enhances insulin secretion. Because its mechanism of action is dependent on the blood glucose level, hypoglycemia is less likely to be induced. Outstanding efficacy and safety profiles of sitagliptin, the first DPP-4 inhibitor to be marketed, have been reported in many studies [10–12].
Sitagliptin has also been studied in relation to the treatment of elderly patients with diabetes, and Shankar et al. compared the additive effect of sitagliptin with that of sulfonylurea (SU) in elderly patients treated with diet therapy or metformin. Blood glucose control was similar in both the treatment groups, but fewer incidences of hypoglycemia or weight gain were noted in patients receiving sitagliptin compared with SU [13]. Another study compared the additive effect of sitagliptin or glimepiride in elderly patients, and none of the patients in the sitagliptin group experienced hypoglycemia; however, some patients in the glimepiride group experienced hypoglycemia [14]. Thus, sitagliptin can be used more safely than other drugs, but it also brings a risk of severe hypoglycemia when used with SU drugs in elderly patients, and dose adjustments for SU drugs must be given careful consideration [15, 16]. More data on the effects of the combined use of sitagliptin with medications other than SU drugs to treat elderly patients with diabetes are needed.
When treating patients with diabetes mellitus, glycemic control is often difficult to achieve with monotherapy and requires combinations of multiple drugs. However, there have been no comparative studies of antidiabetic drugs prescribed to elderly diabetic patients in which the patients were divided into as many as seven different pretreatment groups prior to the start of sitagliptin treatment.
We conducted the Januvia Multicenter Prospective Trial in Type 2 Diabetes (JAMP), which included patients with type 2 diabetes mellitus that was poorly controlled by at least 1 month of diet/exercise therapy or/and oral antidiabetic drug therapy. The patients were divided into seven pretreatment groups, and they received sitagliptin for 1 year [17]. As a subanalysis of the JAMP study, we compared the efficacy and safety of sitagliptin among the groups treated with different therapeutic drugs, and we also examined differences in its efficacy between different age groups.
Subjects and method
This open-label, central registration, multicenter, prospective observational study was conducted at the Tokyo Women’s Medical University Hospital and at 69 collaborating institutions in Japan. Patients were enrolled from January 2011 to June 2013 and were followed up until June 2014. This study was approved by the ethics committee at the Tokyo Women’s Medical University (UMIN000019154).
The study involved outpatients with type 2 diabetes mellitus who were 20 years of age or older and whose blood glucose levels were poorly controlled with diet/exercise therapy alone or with that therapy and the administration of antidiabetic drugs for a month or more.
In accordance with the Japan Diabetes Society guidelines that were available at the start of the study, a poorly controlled blood glucose level was defined as glycated hemoglobin (HbA1c) of ≥6.9% or a fasting blood glucose concentration of ≥130 mg/dL. At the start of the study in 2011, HbA1c values were expressed using the Japan Diabetes Society levels, which is the standard system in Japan, they but were changed to National Glycohemoglobin Standardization Program system values at the end of the study, in accordance with the Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus (Revision for International Harmonization), issued by The Japan Diabetes Society [18].
The patients were divided into the following seven groups based on the pretreatment received before sitagliptin administration: (1) diet/exercise therapy only; (2) low-dose glimepiride (0.5–1 mg); (3) medium-dose glimepiride (1.5–2 mg); (4) biguanide; (5) thiazolidine; (6) alpha-glucosidase inhibitor; and (7) combined therapy with two or more of the drugs described above.
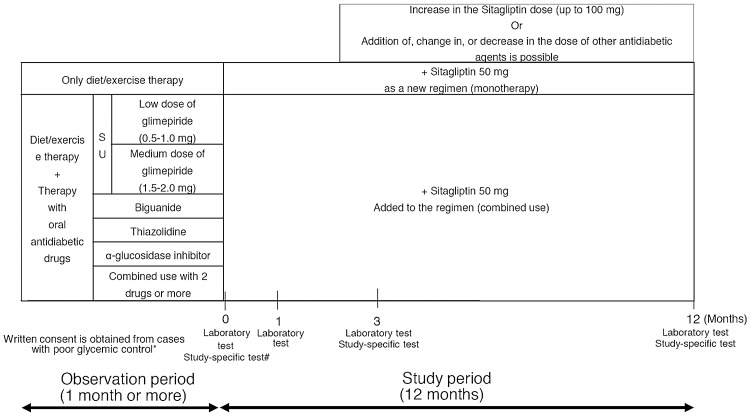
Following the observation period, sitagliptin (50 mg once per day) was administered daily. After the third month of therapy, the dose was increased, changed, or the sitagliptin treatment was discontinued, and other antidiabetic drugs were prescribed on an as-needed basis. The patients were observed for 1 year under these study conditions (Fig. 1).
Fig. 1.
Study design. *Criteria for poor glycemic control: HbA1c of ≥6.9% or fasting blood glucose of ≥130 mg/dL. #Study-specific test (arbitrary): GA, 1.5 AG, C-peptide, proinsulin/insulin ratio
The primary endpoint was the change in HbA1c levels in elderly patients (≥65 years old). Data after 3 months was compared with the baseline and between each of the groups. The secondary endpoint was the change in HbA1c levels at 3 and 12 months from the baseline in elderly patients stratified by age (≥75, 65–74, and <65 years). The safety in patients who were ≥65 years of age was assessed by analyzing the laboratory test results and adverse events at 3 and 12 months from the baseline which were considered to be related to sitagliptin. Adverse events and hypoglycemia were diagnosed by the primary doctor or based on the patient’s explanation of the symptoms.
In addition, multiple regression analysis was performed on the factors that affected the change in HbA1c level at 3 months from baseline.
The JMP software package version 12.1.0 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis processing. The measured values were compared within groups using a paired t test, variations between groups were compared using analysis of variance (ANOVA), and the patients’ backgrounds were compared using the chi-squared test. The factors influencing the decrease in HbA1c levels were assessed using single and multiple regression analyses; parameters with p < 0.20 in the single regression analysis were assessed in the multiple regression analysis. The significance threshold in each two-sided test was p < 0.05. Continuous variables were presented as the mean ± standard deviation and the number of patients (%). Before participating in the study, all patients received a written explanation of the study and provided written informed consent.
Results
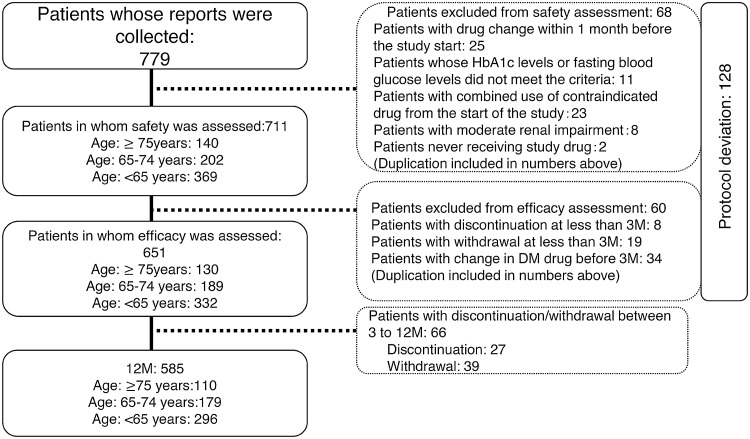
Of the 779 patients enrolled in this study, the safety and efficacy of the treatment were evaluated in 711 (369 were ≥75 years of age, 202 were 65–74 years of age, and 140 were <65 years of age) and 651 (130, 189, and 332, respectively) patients (see Fig. 2).
Fig. 2.
Patient flow
The characteristics of the patients stratified by age (≥75, 65–74, and < 65 years) are described in Table 1. The elderly patients had a smaller abdominal circumference and a longer duration of diabetes, and there were lower numbers of men, smokers, and alcohol drinkers compared with the younger patients.
Table 1.
Comparison of patient characteristics between the elderly and non-elderly patients
| ≥75 years (n = 130) | 65–74 years (n = 189) | <65 years (n = 332) | p | ||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD or % | n | Mean ± SD or % | n | Mean ± SD or % | ||
| Age | 130 | 80.0 ± 4.7 | 189 | 69.2 ± 2.9 | 332 | 54.5 ± 7.4 | 0.000* |
| Height | 127 | 156.2 ± 9.6 | 187 | 161.0 ± 8.6 | 328 | 166.2 ± 8.8 | 0.000* |
| Waist circumference | 80 | 85.7 ± 10.5 | 120 | 87.3 ± 9.6 | 193 | 89.9 ± 12.0 | 0.009* |
| Duration of diabetes | 111 | 121.4 ± 94.6 | 181 | 114.1 ± 78.4 | 308 | 95.4 ± 74.4 | 0.003* |
| Sex (male) | 67 | 51.5 | 117 | 61.9 | 250 | 75.3 | 0.000* |
| Smoking | 13 | 10.3 | 34 | 18.6 | 96 | 29.8 | 0.000* |
| Alcohol consumption | 39 | 31.7 | 82 | 45.1% | 180 | 56.1 | 0.000* |
| Retinopathy | 8 | 6.2 | 8 | 4.2 | 32 | 9.6 | 0.064 |
| Arteriosclerosis obliterans | 6 | 4.6 | 21 | 11.1 | 28 | 8.4 | 0.122 |
| Stroke | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0.288 |
| Myocardial infarction | 7 | 5.4 | 5 | 2.6 | 6 | 1.8 | 0.108 |
| Angina pectoris | 8 | 6.2 | 8 | 4.2 | 11 | 3.3 | 0.387 |
| Cardiac failure | 5 | 3.8 | 2 | 1.1 | 4 | 1.2 | 0.102 |
| Atrial fibrillation | 4 | 3.1 | 5 | 2.6 | 7 | 2.1 | 0.817 |
| Diet/exercise therapy | 49 | 37.7 | 48 | 25.4 | 92 | 27.7 | 0.044* |
| Low dose of glimepiride | 18 | 13.8 | 25 | 13.2 | 29 | 8.7 | 0.153 |
| Medium dose of glimepiride | 9 | 6.9 | 21 | 11.1 | 20 | 6.0 | 0.104 |
| BG | 9 | 6.9 | 25 | 13.2 | 65 | 19.6 | 0.002* |
| TZD | 7 | 5.4 | 12 | 6.3 | 19 | 5.7 | 0.929 |
| α-GI | 5 | 3.8 | 3 | 1.6 | 10 | 3.0 | 0.446 |
| Multidrug therapy | 33 | 25.4 | 55 | 29.1 | 97 | 29.2 | 0.692 |
| Antihypertensive drug | 81 | 62.3 | 106 | 56.1 | 144 | 43.4 | 0.000* |
| Antihyperlipidemic drug | 62 | 47.7 | 94 | 49.7 | 156 | 47.0 | 0.832 |
| Antihyperuricemic drug | 6 | 4.6 | 9 | 4.8 | 16 | 4.8 | 0.996 |
| Antithrombogenic drug | 22 | 16.9 | 44 | 23.3 | 52 | 15.7 | 0.088 |
* p < 0.05
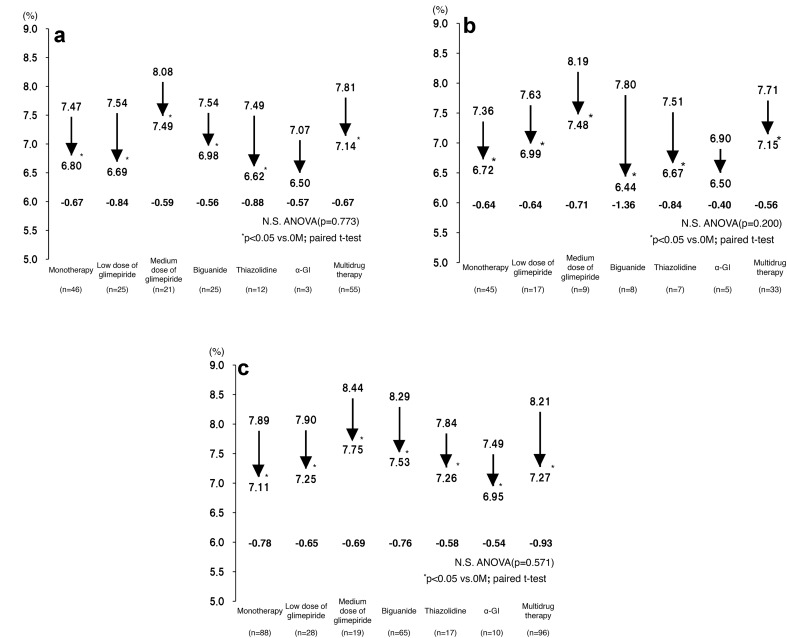
The changes in the patients’ HbA1c levels were assessed after they had been classified into three age groups (≥75, 65–74, and <65 years). There was no significant difference in the change in HbA1c level between the patients treated with different concomitant drugs, except for the alpha-glucosidase inhibitor groups, which contained ≤5 patients (Fig. 3a–c).
Fig. 3.
a ΔHbA1c level at 3 months after the start of therapy according to concomitant drug type (age ≥ 75 years). b ΔHbA1c level at 3 months after the start of therapy according to concomitant drug type (age 65–74 years). c ΔHbA1c level at 3 months after the start of therapy according to concomitant drug type (age < 65 years)
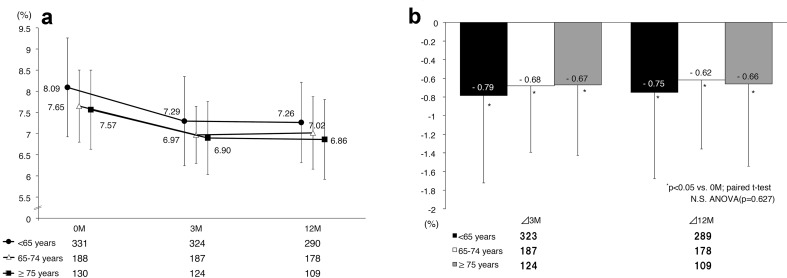
We found significant decreases (p < 0.05) in all three age groups at 3 months after the start of therapy, with the effect lasting until 12 months after starting therapy (Fig. 4a). No significant differences in HbA1c variations at 3 and 12 months from baseline were noted among the three age groups (Fig. 4b).
Fig. 4.
a HbA1c level; b changes in HbA1c
The factors influencing the changes in the HbA1c levels were analyzed in patients who were ≥65 years old. The factors with p > 0.20 in the single regression analysis included smoking, TG levels, concomitant use of agents to correct insulin resistance, and antihypertensive drugs. Multiple regression analysis using the above factors was performed, and it revealed that patients with higher HbA1c levels at baseline were likely to show decreased HbA1c levels, while those with higher TG levels were unlikely to show decreased HbA1c levels (Table 2).
Table 2.
Analysis of factors affecting HbA1c variations at 3 months after the start of therapy (age ≥ 65 years)
| Variables | Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Partial regression coefficient | Standard partial regression coefficient | Lower limit | Upper limit | p value* | Partial regression coefficient | Standard partial regression coefficient | Lower limit | Upper limit | p value* | |
| Age | −0.001 | −0.007 | −0.014 | 0.012 | 0.907 | |||||
| Sex (male) | −0.048 | −0.033 | −0.213 | 0.117 | 0.567 | |||||
| Duration of diabetes | 0.000 | 0.012 | −0.001 | 0.001 | 0.837 | |||||
| HbA1c | −0.476 | −0.572 | −0.552 | −0.399 | 0.000* | −0.481 | −0.581 | −0.561 | −0.401 | 0.000* |
| Systolic blood pressure | 0.001 | 0.016 | −0.005 | 0.006 | 0.777 | |||||
| Smoking | −0.153 | −0.077 | −0.376 | 0.071 | 0.180 | −0.080 | −0.042 | −0.266 | 0.105 | 0.393 |
| Alcohol consumption | 0.078 | 0.052 | −0.093 | 0.249 | 0.370 | |||||
| Group with diet/exercise therapy | 0.033 | 0.020 | −0.147 | 0.212 | 0.721 | |||||
| Group with SU (excluding multidrug therapy) | −0.036 | −0.021 | −0.229 | 0.158 | 0.717 | |||||
| Insulin resistance improving drug (excluding multidrug therapy) | −0.143 | −0.073 | −0.361 | 0.074 | 0.196 | −0.173 | −0.093 | −0.349 | 0.004 | 0.055 |
| BMI | −0.014 | −0.063 | −0.038 | 0.011 | 0.273 | |||||
| Waist circumference | −0.003 | −0.040 | −0.013 | 0.007 | 0.573 | |||||
| Triglyceride | 0.001 | 0.095 | 0.000 | 0.002 | 0.104 | 0.001 | 0.128 | 0.000 | 0.002 | 0.010* |
| LDL-C | −0.001 | −0.026 | −0.004 | 0.002 | 0.661 | |||||
| eGFR | −0.001 | −0.030 | −0.006 | 0.004 | 0.614 | |||||
| Antihypertensive drug | 0.108 | 0.073 | −0.057 | 0.274 | 0.198 | −0.056 | −0.038 | −0.196 | 0.085 | 0.437 |
| Antihyperlipidemic drug | 0.036 | 0.025 | −0.126 | 0.199 | 0.660 | |||||
Factors with p < 0.2 in the single regression analysis were extracted, and multiple regression analysis was performed using these factors
Patients with higher baseline HbA1c levels were likely to show large decreases in HbA1c levels, while patients with higher triglyceride levels were unlikely to do so
* p < 0.05
Variations in the laboratory results for patients who were ≥65 years old were measured at 3 and 12 months from baseline. We found no significant variations in the pulse rate, body weight, body mass index (BMI), aspartate aminotransferase (AST), alanine aminotransferase (ALT), urine albumin-to-creatinine ratio, or platelet count. Significant variations were found in the systolic and diastolic blood pressure, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), γ-glutamyltransferase (γ-GTP), and estimated glomerular filtration rate (eGFR), although these variations were not large (Table 3).
Table 3.
Variations in laboratory results for patients ≥ 65 years old
| 0 months | 3 months | 12 months | Change at 3 months | Change at 12 months | |
|---|---|---|---|---|---|
| SBP | 132.1 ± 15.4 (n = 316) | 129.6 ± 14.6 (n = 311) | 131.1 ± 14 (n = 284) | −2.6 ± 16.1 (n = 311)* | −0.4 ± 16.1 (n = 283) |
| DBP | 73.4 ± 10.4 (n = 316) | 72.1 ± 9.9 (n = 311) | 73.2 ± 10.7 (n = 284) | −1.4 ± 10.1 (n = 311)* | −0.3 ± 10.1 (n = 283) |
| Pulse rate | 75.3 ± 11.6 (n = 259) | 74.8 ± 11.1 (n = 252) | 75.7 ± 11.9 (n = 229) | −0.5 ± 9.8 (n = 247) | 0.7 ± 10.1 (n = 226) |
| Body weight | 61.1 ± 10.9 (n = 310) | 61.3 ± 10.9 (n = 293) | 61.2 ± 10.6 (n = 271) | −0.1 ± 1.7 (n = 292) | −0.3 ± 2.4 (n = 270) |
| BMI | 24.1 ± 3.4 (n = 307) | 24.2 ± 3.4 (n = 290) | 24.1 ± 3.4 (n = 270) | 0 ± 0.7 (n = 289) | −0.1 ± 1 (n = 269) |
| HbA1c | 7.62 ± 0.89 (n = 318) | 6.94 ± 0.75 (n = 311) | 6.96 ± 0.89 (n = 287) | −0.68 ± 0.73 (n = 311)* | −0.63 ± 0.8 (n = 287)* |
| TG | 136.5 ± 89.6 (n = 297) | 129.8 ± 74.5 (n = 289) | 125.6 ± 64.8 (n = 269) | −7 ± 80 (n = 280) | −10 ± 81.9 (n = 260)* |
| HDL-C | 55.9 ± 14.7 (n = 288) | 54.3 ± 15 (n = 282) | 54.2 ± 15.4 (n = 265) | −1.4 ± 8.1 (n = 274)* | −1.4 ± 7.9 (n = 256)* |
| LDL-C | 110.2 ± 28.2 (n = 294) | 106.8 ± 26.5 (n = 283) | 109.7 ± 29.2 (n = 267) | −2.6 ± 19.1 (n = 273)* | −0.3 ± 23.5 (n = 256) |
| AST | 24.4 ± 10.4 (n = 297) | 24.3 ± 10.7 (n = 287) | 24.5 ± 10.5 (n = 267) | 0 ± 7.6 (n = 278) | 0.4 ± 9 (n = 258) |
| ALT | 23.7 ± 14.8 (n = 297) | 22.6 ± 14.7 (n = 286) | 23 ± 15.5 (n = 266) | −1.1 ± 9.8 (n = 277) | −0.5 ± 11.8 (n = 257) |
| γ-GTP | 41.5 ± 57.7 (n = 293) | 38 ± 46.1 (n = 283) | 39.3 ± 47.1 (n = 267) | −3 ± 21.5 (n = 272)* | −2.8 ± 31.8 (n = 255) |
| UACR | 55.9 ± 168.6 (n = 137) | 51.9 ± 137.1 (n = 125) | 99.2 ± 383.4 (n = 147) | −19.7 ± 117.2 (n = 108) | 38.1 ± 249.1 (n = 115) |
| eGFR | 72.4 ± 16.5 (n = 269) | 69.5 ± 15.8 (n = 260) | 68.5 ± 15.5 (n = 270) | −2.7 ± 8.1 (n = 253)* | −3.8 ± 9.5 (n = 262)* |
| Platelets | 21.1 ± 5.7 (n = 284) | 20.9 ± 5.2 (n = 275) | 21.2 ± 5.8 (n = 258) | −0.3 ± 3.1 (n = 266) | 0.1 ± 3.4 (n = 249) |
* p < 0.05
In addition, among the 319 patients who were ≥65 years old and in whom the treatment safety was assessed, hypoglycemia was noted in only one patient receiving sitagliptin monotherapy. Another adverse event that was considered to have a causal relationship with sitagliptin was anemia, which was found in just one patient receiving sitagliptin monotherapy.
Discussion
The study compared the additive effect of sitagliptin in reducing HbA1c in patients who were classified into three age groups (≥75, 65–74, and <65 years) and were divided into seven groups based on the pretreatment drug. We observed a significant reduction in HbA1c levels in all age groups and all seven pretreatment groups except for the alpha-glucosidase inhibitor groups, which had ≤5 patients, but there was no difference compared with each pretreatment group.
Our results suggest that monotherapy with sitagliptin in patients during the early stages can lead to improvements in glycemic control. Additional administration of sitagliptin in patients who are already being treated for diabetes mellitus and who have poor glycemic control can also have a significant hypoglycemic effect regardless of the kind of drug that is used concomitantly. Results of comparing the patients classified into three age groups (≥75, 65–74, and <65 years) indicated that there was no significant difference in efficacy between the age groups, which suggests that sitagliptin can improve blood glucose levels in patients of any age.
The safety assessment showed that among the 319 patients who were ≥65 years old, only one patient (0.3%) receiving monotherapy with sitagliptin was suspected of having hypoglycemia. Barzilia et al. compared treatments with sitagliptin and placebo for 24 weeks in 206 elderly patients who were ≥65 years old, and reported no hypoglycemia in the group receiving sitagliptin; thus, sitagliptin demonstrated no safety concerns [19]. The results of our study are consistent with those of previous studies that demonstrated the safety of sitagliptin. Thus, sitagliptin is unlikely to cause hypoglycemia in elderly patients with diabetes mellitus.
A high dose of SU drugs incurs a risk of hypoglycemia. However, sitagliptin acts in a blood glucose level-dependent manner, and is unlikely to cause hypoglycemia. A previous study involving elderly patients reported that, despite increasing the dose of sitagliptin to 100 mg, the incidence of hypoglycemia was lower in the sitagliptin group compared with patients who were not taking sitagliptin [20]. Our study also confirmed that there was no increase in the incidence of hypoglycemia among the 31 patients whose sitagliptin dose was increased to 100 mg. Thus, sitagliptin was considered to be safe even at high doses (Table S1 in the Electronic supplementary material, ESM).
Elderly people often have multiple comorbidities and physical function is likely to worsen in this age group [21]. Therefore, severe hypoglycemia can be easily induced. Severe hypoglycemia impairs cognitive function and is associated with a risk of cardiovascular events [22]. Based on this background, the glycemic control target for elderly patients with diabetes was prepared by the Joint Committee of the Japan Diabetes Society and the Japan Geriatrics Society in 2015 to improve therapeutic outcomes [23]. The committee emphasized that the glycemic control target should be defined individually after careful consideration of the patient’s medical history and factors affecting their health status, such as age, cognitive and physical function, comorbid conditions, risk of severe hypoglycemia, and life expectancy. It also stressed that, if severe hypoglycemia is a concern, a safer treatment must be performed by setting a lower target. Based on this background, sitagliptin was administered only once per day, thus allowing good compliance with a lower risk of hypoglycemia, even in elderly patients.
Although significant decreases in some results were seen in the various laboratory tests performed after the administration of sitagliptin, the variations in the values were small, and therefore no problems with clinical safety are expected. The overall eGFR significantly decreased, and while some patients with a high baseline eGFR showed only a slight decrease, other patients with a low baseline eGFR did not show a further decrease (Table S2 in ESM). As other studies have also reported, patients taking sitagliptin showed neither weight gain [24, 25] nor deterioration in any major laboratory findings after long-term administration [26], and sitagliptin seems to be a safe drug choice.
Our study showed that the hypoglycemic action of sitagliptin continued until 12 months after starting therapy. Although the observation period of our study was 12 months, Ching-Jung et al. reported that the blood glucose-improving effect of sitagliptin in elderly patients with a mean age of 71.3 ± 11.7 years lasted from 6 to 48 months, with no adverse events of hypoglycemia [27]. Sitagliptin may also allow stable glycemic control for a long time in elderly patients.
The multivariable analysis of the factors influencing HbA1c variations in elderly patients suggested that higher HbA1c levels before therapy are associated with greater improvement, while high TG levels suppress improvements in HbA1c levels. Some reports indicate that insulin resistance associated with aging causes abnormalities in glucose and lipid metabolism [28–32], and the same mechanism was considered to have contributed to the results of our study. Although sitagliptin is expected to be effective in many clinical contexts, patients with high TG levels should be treated after careful consideration of the glycemic control effect.
Limitations
There are several limitations to this study. Firstly, the JAMP study was an open-label observational study. Secondly, at the start of this study, drugs such as glinides, insulin, and sodium-glucose cotransporter 2 (SGLT2) inhibitors were not approved for insurance coverage. Thus, no data were available on the combined use of sitagliptin and these drugs, making it impossible to examine their effects in this study.
Conclusion
We studied patients with long-term diabetes who had already been treated with antidiabetic agents and were prescribed sitagliptin in addition to other agents when poor glycemic control was noted. This situation more closely reflects actual clinical practice than studies such as a Japanese dose-ranging study of sitagliptin [33]. Patients receiving sitagliptin achieved good outcomes in these studies, including in situations where its use was combined with other antidiabetic drugs.
Our results have demonstrated that concomitant antidiabetic drugs do not affect the glycemic control effect in elderly patients. No increase in the incidence of hypoglycemia was observed, even after the dose of sitagliptin was increased to 100 mg. Thus, sitagliptin has the potential to both improve glycemic control and prevent hypoglycemia, and it can be considered a potent alternative drug.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our sincere gratitude to Mr. Shogo Shishikura (MSD K.K.) (Teikyo University Graduate School of Public Health, MPH) for scientific advice, including references, when revising the manuscript. We would also like to thank Nouvelle Place Inc. for conducting the data analyses.
Funding
This study was funded by the Japan Diabetes Foundation.
Conflict of interest
Hiroshi Sakura received honoraria from Mitsubishi Tanabe Pharma Corporation and a research grant from Ono Pharmaceutical Co., Ltd. The other authors declare that they have no conflict of interest.
JAMP Study Investigators
Akiko Sato (Maruyama Internal Medicine Clinic); Akira Miyashita (Miyashita Surgery Clinic); Asako Kokubo (Kokubo Clinic); Atsuro Tsuchiya (Tsuchiya Clinic); Dai Hirohara (Hanazono Clinic); Daiji Kogure (Kogure Clinic); Daijo Kasahara (Kasahara Clinic); Hideki Tanaka (Internal Medicine, Seiwa Clinic); Hideki Tanaka (Internal Medicine, Nishiarai Hospital); Hideo Tezuka (Tezuka Clinic); Hiroyuki Kuroki (Internal Medicine, Johsai Hospital); Jun Ogino (Department of Diabetes, Endocrine and Metabolic Diseases, Tokyo Women’s Medical University Yachiyo Medical Center); Kanu Kin (Internal Medicine, Nishiarai Lifestyle-Related Diseases Clinic); Kanu Kin (Internal Medicine, Nishiarai Hospital); Kazuko Muto (Tokyo Women’s Medical University); Kazuo Suzuki (Kenkokan Suzuki Clinic of Internal Medicine); Keiko Iseki (Iseki Clinic); Keita Watanabe (Watanabe Clinic); Kenshi Higami (Higami Hospital); Kenzo Matsumura (Matsumura Gastroenterological Clinic); Kiyotaka Nakajima (Ebisu Clinic); Koki Shin (Shin Clinic); Kuniya Koizumi (Kuniya Clinic); Maki Saneshige (Mugishima Medical Clinic); Makio Sekine (Sekine Clinic); Makoto Yaida (Urban Heights Clinic); Mari Kiuchi (physician, Kanauchi Medical Clinic); Mari Mugishima (Mugishima Medical Clinic); Mari Osawa (Department of Diabetes Mellitus, Institute of Geriatrics, Tokyo Women’s Medical University); Masae Banno (Banno Medical Clinic); Masahiro Yamamoto (Internal Medicine 1, Shimane University Faculty of Medicine); Masatake Hiratsuka (Higashishinagawa Clinic); Masumi Hosoya (Yasui Clinic); Michika Atsuta (Internal Medicine, Nishiarai Lifestyle-Related Diseases Clinic); Mitsutoshi Kato (Kato Clinic of Internal Medicine); Miwa Morita (Internal Medicine 1, Shimane University Faculty of Medicine); Munehiro Miyamae (Johsai Hospital); Mutsumi Iijima (Abe Hospital); Naomi Okuyama (Shinjuku Mitsui Building Clinic); Nobuo Hisano (Mejiro Medical Clinic); Norihiro Tsuchiya (Omotesando Naika Ganka); Rie Wada (Kanauchi Medical Clinic); Rie Wada (Nerimasakuradai Clinic); Ryuji Momose (Momo Medical Clinic); Sachiko Otake (Tokyo Women’s Medical University); Satoko Maruyama (Shinjuku Mitsui Building Clinic); Satoru Takada (Internal Medicine, Social Welfare Corporation, Shineikai Takinogawa Hospital); Shigeki Dan (Ube Internal Medicine and Pediatrics Hospital); Shigeki Nishizawa (Nishizawa Medical Clinic); Shigeo Yamashita (Department of Diabetes and Endocrinology, JR Tokyo General Hospital); Shingo Saneshige (Internal Medicine, Kamiochiai Shin Clinic); Shinichi Teno (Teno Clinic); Shinji Tsuruta (Diabetic Medicine, Itabashi Chuo Medical Center); Shinobu Kumakura (Kumakura Medical Clinic); Sumiko Kijima (Abe Hospital); Takashi Kondo (Kondo Clinic); Takeo Onishi (Internal Medicine, Onishi Clinic); Taku Kudo (Internal Medicine, Social Welfare Corporation, Shineikai Takinogawa Hospital); Tatsushi Sugiura (Internal Medicine, Seiwa Clinic); Toshihiko Ishiguro (Kaname Clinic); Yasue Suzuki (Suzuki Medical Clinic); Yasuhiro Tomita (Nakanobu Clinic); Yasuko Takano (Department of Diabetes, Shiseikai Daini Hospital); Yoshihisa Akimoto (Akimoto Yoshi Medical Clinic); Yoshiko Odanaka (Ito Internal Medicine Pediatrics Clinic); Yoshimasa Tasaka (Tokyo Women’s Medical University); Yoshitaka Aiso (Internal Medicine, Diabetes, Aiso Clinic); Yukiko Inoue (Inoue Medical Clinic); Yukinobu Kobayashi (Kobayashi Clinic).
Ethical approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The ethics committee at the Tokyo Women’s Medical University approved the study (approval number: 2064) on 11 January 2011. Informed consent or a substitute for it was obtained from all patients before they were included in the study.
Footnotes
A complete list of the JAMP (Januvia Multicenter Prospective Trial in Type 2 Diabetes) Study Investigators is provided in the “Compliance with ethical standards” section.
Change history
10/14/2017
Erratum to: Diabetol Int DOI 10.1007/s13340-017-0330-2.
Contributor Information
Noriko Ujihara, Phone: +81-3-3499-1911, Email: ujihara.noriko@twmu.ac.jp.
for the JAMP Study Investigators:
Akiko Sato, Akira Miyashita, Asako Kokubo, Atsuro Tsuchiya, Dai Hirohara, Daiji Kogure, Daijo Kasahara, Hideki Tanaka, Hideki Tanaka, Hideo Tezuka, Hiroyuki Kuroki, Jun Ogino, Kanu Kin, Kanu Kin, Kazuko Muto, Kazuo Suzuki, Keiko Iseki, Keita Watanabe, Kenshi Higami, Kenzo Matsumura, Kiyotaka Nakajima, Koki Shin, Kuniya Koizumi, Maki Saneshige, Makio Sekine, Makoto Yaida, Mari Kiuchi, Mari Mugishima, Mari Osawa, Masae Banno, Masahiro Yamamoto, Masatake Hiratsuka, Masumi Hosoya, Michika Atsuta, Mitsutoshi Kato, Miwa Morita, Munehiro Miyamae, Mutsumi Iijima, Naomi Okuyama, Nobuo Hisano, Norihiro Tsuchiya, Rie Wada, Rie Wada, Ryuji Momose, Sachiko Otake, Satoko Maruyama, Satoru Takada, Shigeki Dan, Shigeki Nishizawa, Shigeo Yamashita, Shingo Saneshige, Shinichi Teno, Shinji Tsuruta, Shinobu Kumakura, Sumiko Kijima, Takashi Kondo, Takeo Onishi, Taku Kudo, Tatsushi Sugiura, Toshihiko Ishiguro, Yasue Suzuki, Yasuhiro Tomita, Yasuko Takano, Yoshihisa Akimoto, Yoshiko Odanaka, Yoshimasa Tasaka, Yoshitaka Aiso, Yukiko Inoue, and Yukinobu Kobayashi
References
- 1.Ministry of Health, Labour and Welfare. Summary of “Patient Survey”. Tokyo: Ministry of Health, Labour and Welfare; 2015. http://www.mhlw.go.jp/english/database/db-hss/sps_2014.html. Accessed 26 Jul 2017
- 2.Ministry of Health, Labour and Welfare. Results of “National Health and Nutrition Survey”. Tokyo: Ministry of Health, Labour and Welfare; 2015. http://www.mhlw.go.jp/stf/houdou/0000106405.html. Accessed 26 Jul 2017
- 3.Seltzer HS. Drug-induced hypoglycemia. A review of 1418 cases. Endocrinol Metab Clin North Am. 1989;18(1):163–183. [PubMed] [Google Scholar]
- 4.Araki A. Low well-being, cognitive impairment and visual impairment associated with functional disabilities in elderly Japanese patients with diabetes mellitus. Geriatr Gerontol Int. 2004;4(1):15–24. doi: 10.1111/j.1447-0594.2003.00108.x. [DOI] [Google Scholar]
- 5.Ma F. Conversion of mild cognitive impairment to dementia among subjects with diabetes: a population-based study of incidence and risk factors with five years of follow-up. J Alzheimers Dis. 2015;43(4):1441–1449. doi: 10.3233/JAD-141566. [DOI] [PubMed] [Google Scholar]
- 6.Solini A. Renal insufficiency and cardiovascular events study group: age, renal dysfunction, cardiovascular disease, and antihyperglycemic treatment in type 2 diabetes mellitus. J Am Geriatr Soc. 2013;61(8):1253–1261. doi: 10.1111/jgs.12381. [DOI] [PubMed] [Google Scholar]
- 7.Huang ES. Rates of complications and mortality in older diabetes patients: the diabetes and aging study. JAMA Intern Med. 2014;174(2):251–258. doi: 10.1001/jamainternmed.2013.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lind M. The relationship between glycaemic control and heart failure in 83,021 patients with type 2 diabetes. Diabetologia. 2012;55(11):2946–2953. doi: 10.1007/s00125-012-2681-3. [DOI] [PubMed] [Google Scholar]
- 9.Bremer JP. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32(8):1513–7. [DOI] [PMC free article] [PubMed]
- 10.Aschner P. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 11.Charbonnel B. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29(12):2638–2643. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstock J. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28(10):1556–1568. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Shankar R. A comparison of glycaemic effects of sitagliptin and sulfonylureas in elderly patients with type 2 diabetes mellitus. Int J Clin Pract. 2015;69(6):626–631. doi: 10.1111/ijcp.12607. [DOI] [PubMed] [Google Scholar]
- 14.Hartley P. Efficacy and tolerability of sitagliptin compared with glimepiride in elderly patients with type 2 diabetes mellitus and inadequate glycemic control: a randomized, double-blind, non-inferiority trial. Drugs Aging. 2015;32(6):469–76. [DOI] [PubMed]
- 15.Umezawa S. Two-year assessment of the efficacy and safety of sitagliptin in elderly patients with type 2 diabetes: post hoc analysis of the ASSET-K study. BMC Endocr Disord. 2015;15(34):1–8. doi: 10.1186/s12902-015-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermansen K. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9(5):733–745. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 17.Sakura H. Effect of sitagliptin on blood glucose control in patients with type 2 diabetes mellitus who are treatment naive or poorly responsive to existing antidiabetic drugs: the JAMP study. BMC Endocr Disord. 2016;16(1):70. [DOI] [PMC free article] [PubMed]
- 18.Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M, Ueki K. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus (revision for international harmonization of HbA1c in Japan). J Jpn Diabetes Soc. 2012;55(7):485–504.
- 19.Barzilia N. Efficacy and tolerability of sitagliptin monotherapy in elderly patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011;27(5):1049–1058. doi: 10.1185/03007995.2011.568059. [DOI] [PubMed] [Google Scholar]
- 20.Round E. Safety of sitagliptin in elderly patients with type 2 diabetes: a pooled analysis of 25 clinical studies. Drugs Aging. 2014;31(3):203–214. doi: 10.1007/s40266-014-0155-7. [DOI] [PubMed] [Google Scholar]
- 21.Nelson EA. Aged heterogeneity: fact or fiction? The fate of diversity in gerontological research. Gerontologist. 1992;32(1):17–23. doi: 10.1093/geront/32.1.17. [DOI] [PubMed] [Google Scholar]
- 22.Goto A. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. doi: 10.1136/bmj.f4533. [DOI] [PubMed] [Google Scholar]
- 23.The Japan Diabetes Society. Diabetes in the elderly (including dementia). In: Guideline for the treatment for diabetes in Japan 2016. Tokyo: Nankodo; 2016. p. 411–48.
- 24.Scott R. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61(1):171–180. doi: 10.1111/j.1742-1241.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 25.Nonaka K. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79(2):291–298. doi: 10.1016/j.diabres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Tada Y. Long-term efficacy and safety of sitagliptin in elderly patients with type 2 diabetes mellitus. Intern Med. 2016;55(10):1275–1278. doi: 10.2169/internalmedicine.55.6011. [DOI] [PubMed] [Google Scholar]
- 27.Ching-Jung H. The durability of sitagliptin in elderly patients with type 2 diabetes. Clin Interv Aging. 2014;7(9):1905–1911. doi: 10.2147/CIA.S72396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris MI. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults: the Third National Health and Examination Survey. Diabetes Care. 1998;21(4):518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 29.Suh DC. Comorbid conditions and glycemic control in elderly patients with type 2 diabetes mellitus, 1988 to 1994 to 1999 to 2004. J Am Geriatr Soc. 2008;56(3):484–492. doi: 10.1111/j.1532-5415.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- 30.Bosu R. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52(7):1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 31.Sakurai T. Age-associated increase in abdominal obesity and insulin resistance, and usefulness of AHA/NHLBI definition of metabolic syndrome for predicting cardiovascular disease in Japanese elderly with type 2 diabetes mellitus. Gerontology. 2010;56:141–149. doi: 10.1159/000246970. [DOI] [PubMed] [Google Scholar]
- 32.Zhuravlyova LV. The factors of the progression of metabolic disorders in the pancreas in patients with associated clinical variants of the chronic pancreatitis and type 2 diabetes mellitus. Lik Sprava. 2015;2015(5–6):46–51. [PubMed] [Google Scholar]
- 33.Iwamoto Y. Dose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J. 2010;57(5):383–394. doi: 10.1507/endocrj.K09E-272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.