Abstract
Currently, many studies draw attention to novel secretory factors, such as adipokines or myokines, derived from the tissues that were not originally recognized as endocrine organs. The liver may contribute to the onset of various kinds of pathologies of type 2 diabetes by way of the production of secretory proteins “hepatokines.” Using the comprehensive gene expression analyses in human livers, we have rediscovered selenoprotein P and LECT2 as hepatokines involved in the onset of dysregulated glucose metabolism. Overproduction of selenoprotein P, previously reported as a transport protein of selenium, induces insulin resistance and hyperglycemia in type 2 diabetic condition. Selenoprotein P also contributes to vascular complications of type 2 diabetes directly by inducing VEGF resistance in vascular endothelial cells. Notably, selenoprotein P impairs health-promoting effects of exercise by inhibiting ROS/AMPK/PGC-1α pathway in the skeletal muscle through its receptor LRP1. Overproduction of LECT2, previously reported as a neutrophil chemotactic protein, links obesity to insulin resistance in the skeletal muscle. Further studies would develop novel diagnostic or therapeutic procedures targeting hepatokines to combat over-nutrition-related diseases such as type 2 diabetes.
Keywords: Hepatokines, Selenoprotein P, LECT2, Insulin resistance
Introduction
Currently, many studies draw attention to novel secretory factors, such as adipokines or myokines, derived from tissues that were not previously recognized as endocrine organs. The liver plays a central role in glucose homeostasis mainly via glucose release and glycogen storage, but the liver is also the site for the production of various secretory proteins. For example, patients with fulminant hepatitis or hepatic failure receive replacement therapy with liver-derived secretory proteins such as albumin in clinical settings. In addition, most of clinicians diagnose various kinds of diseases by measuring blood levels of liver-derived secretory factors such as transaminases or coagulation factors. In fact, our previous reports using comprehensive gene expression analyses have revealed that genes encoding secretory proteins are abundantly expressed in the livers of humans [1]. Because the human liver releases numerous kinds of secretory factors, the identification and functional analysis of liver-secreted factors may not be adequate.
Based on these findings, we hypothesized that, analogous to adipose tissues, the liver may contribute to the development of various kinds of pathologies observed in type 2 diabetes, through the production of secretory proteins, termed “hepatokines”. Since about 15 years before, we have identified key hepatokines involved in the pathologies of type 2 diabetes and have shed light on the previously unknown pathophysiological significance of hepatokine overproduction in type 2 diabetes.
Comprehensive gene expression analysis in livers from people with type 2 diabetes
We investigated comprehensive gene expression profiles in the liver of patients with type 2 diabetes and control subjects with normal glucose tolerance (NGT) using the serial analysis of gene expression (SAGE) technique and DNA chip analysis [1]. Samples for SAGE were obtained from 5 patients with type 2 diabetes and 5 subjects with NGT, all of whom had undergone surgical treatments for malignant tumors such as gastric cancer, gall bladder cancer, and colon cancer. Samples for DNA chip analysis were obtained from 9 patients with type 2 diabetes and 9 subjects with NGT who were admitted to Kanazawa University Hospital from April 2000 to March 2003. Hepatic tissues were obtained with percutaneous needle liver biopsy.
In SAGE analysis, the gene ontology cellular components of all identified transcripts were gathered and reclassified into the 9 representative groups designated by the Gene Ontology consortium. The top class for the type 2 diabetes library was “mitochondria.” On the other hand, the top class for the NGT library was “extracellular,” including genes encoding secretary proteins such as apolipoprotein C-I, apolipoprotein C-II, and albumin. These results raise the possibility that the liver also functions as an endocrine organ that releases various kinds of secretory proteins.
In DNA chip analysis, we computed the mean centroid of oxidative phosphorylation (OXPHOS) genes. In our analysis, the mean centroid of OXPHOS genes in the liver was significantly correlated with fasting plasma glucose levels. In addition, the mean centroid of OXPHOS genes had a tendency to correlate negatively with markers of insulin resistance such as metabolic clearance rate as measured by glucose clamp experiments. Multivariate linear regression models using fasting glucose level as the dependent variable showed that the mean centroid of OXPHOS genes was a predictor of fasting plasma glucose levels, independent of age, body mass index, insulin resistance, and fasting insulin levels. These results suggest that upregulation of OXPHOS genes in the liver might contribute to hyperglycemia and insulin resistance in people with type 2 diabetes.
Identification of selenoprotein P as a hepatokine that induces insulin resistance in type 2 diabetes
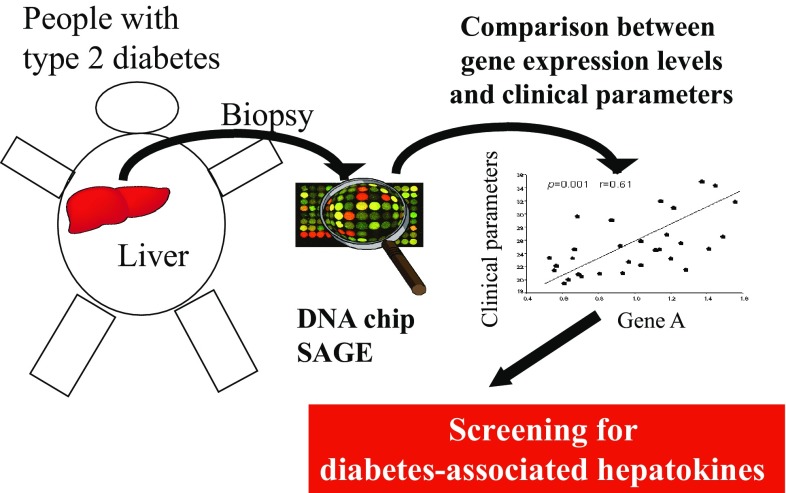
To identify key hepatic secretory proteins involved in insulin resistance, we used the data of comprehensive analyses of gene expression profiles in human livers (Fig. 1). First, using SAGE data library, we identified 117 genes encoding putative secretory proteins with expression levels in people with type 2 diabetes, 1.5-fold or greater higher than those in normal subjects. Next, we used the data of DNA chip analysis to identify genes whose hepatic expression was significantly correlated with insulin resistance. As a result, we found that a negative correlation between hepatic SEPP1 mRNA levels and the metabolic clearance rate of glucose, indicating that elevated hepatic SEPP1 mRNA levels were positively associated with the severity of insulin resistance [2].
Fig. 1.
Screening for diabetes-associated hepatokines using comprehensive gene expression analyses of the livers from people with type 2 diabetes. SAGE serial analysis of gene expression
Selenoprotein P (SeP encoded by the SEPP1 gene) is a secretory protein primarily produced by the liver [3]. SeP contains 10 selenocysteine residues, and functions as a selenium supply protein [4]. SeP exerts anti-oxidative effects by acting directly as an anti-oxidative enzyme and by supplying selenium to target cells [3–5]. However, the role of SeP in the regulation of glucose metabolism and insulin sensitivity was not established. Furthermore, the clinical significance of SeP in human diseases was not well defined, although studies of SeP knockout mice showed SeP deficiency to be associated with neurological injury and low fertility [6, 7].
To characterize the role of SeP in the development of insulin resistance, we measured serum SeP levels in human samples, using enzyme-linked immunosorbent assays (ELISA). Consistent with elevated hepatic SEPP1 mRNA levels, we found a significant positive correlation between serum SeP levels and both fasting plasma glucose and hemoglobin A1c levels. In addition, serum levels of SeP were significantly elevated in people with type 2 diabetes compared with normal subjects. Similar to data derived from clinical specimens, in rodent models of type 2 diabetes, including OLETF rats and KKAy mice, hepatic Sepp1 mRNA and serum SeP levels were elevated. These results suggest that production of SeP is increased in both rodents and humans with type 2 diabetic condition.
To clarify the pathophysiology contributing to the hepatic expression of SeP in type 2 diabetes, we investigated the effects of nutrients on Sepp1 mRNA expression in cultured hepatocytes. We found that the addition of glucose or palmitate upregulated Sepp1 expression, whereas insulin downregulated it. Consistent with the negative regulation of Sepp1 by insulin in hepatocytes, Sepp1 mRNA levels were elevated in the livers of fasting C57BL6J mice, compared with those that had been fed.
Because it is technically very difficult to produce cell culture or animal model in which SeP is over-expressed, we purified SeP from human plasma using chromatographic methods. At first, we expected that treatment with SeP promotes insulin signal cascade via anti-oxidative actions. However, unexpectedly, treatment of primary hepatocytes with purified SeP induced a reduction in insulin-stimulated phosphorylation of insulin receptor (IR), and Akt. Moreover, SeP increased phosphorylation of IRS1 at Ser307, the downregulator of tyrosine phosphorylation of IRS. Similar effects of SeP were also observed in C2C12 myocytes. Consistent with the results of insulin signaling, treatment with SeP upregulated mRNA expression of Pck1 and G6pc, key gluconeogenic enzymes, and increased glucose release in the insulin-treated hepatocytes. These in vitro experiments indicate that SeP impairs insulin signal transduction and dysregulates cellular glucose metabolism.
To examine the physiological effects of SeP in vivo, we treated female C57BL/6J mice with purified human SeP. Injection of purified human SeP resulted in serum levels of 0.5–1.5 μg/mL. These concentrations correspond to the incremental change of SeP serum concentrations in people with normal glucose tolerance to those with type 2 diabetes. Glucose and insulin tolerance tests revealed that SeP-induced glucose intolerance and insulin resistance. Western blot analysis showed a reduction in insulin-induced serine phosphorylation of Akt in both liver and skeletal muscle of SeP-injected mice. Hyperinsulinemic–euglycemic clamp studies showed that SeP increased endogenous glucose production and decreased peripheral glucose disposal. These data indicate that SeP impairs insulin signaling in the liver and skeletal muscle and induces glucose intolerance in vivo.
We confirmed the effects of lowered SeP using Sepp1 knockout mice. Gucose loading test revealed that SeP knockout mice showed improved glucose tolerance. Insulin loading test revealed that SeP knockout mice showed lower blood glucose levels 60 min after insulin injection. Insulin signaling, including phosphorylation of Akt and insulin receptor, was enhanced in the liver and skeletal muscle of SeP knockout mice. These results suggest that suppression of SeP production improves glucose metabolism and insulin sensitivity in vivo.
Adenosine monophosphate-activated protein kinase (AMPK) is a serine/threonine kinase that phosphorylates a variety of energy-associated enzymes and functions as a metabolic regulator that promotes insulin sensitivity [8]. We found that SeP treatment reduced phosphorylation of AMPKα and ACC in both H4IIEC hepatocytes and mouse liver. In contrast, Sepp1-deficient mice exhibited increased phosphorylation of AMPKα and ACC in the liver. To determine whether AMPK pathways were involved in the action of SeP, we infected H4IIEC hepatocytes with constitutively active (CA) AMPK. When CA-AMPK was over-expressed, SeP was unable to impair insulin-stimulated Akt phosphorylation. In addition, co-administration of 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR), a known activator of AMPK, rescued cells from the inhibitory effects of SeP on insulin signaling. These results suggest that reduced phosphorylation of AMPK mediates, at least in part, the inhibitory effects of SeP on insulin signal transduction.
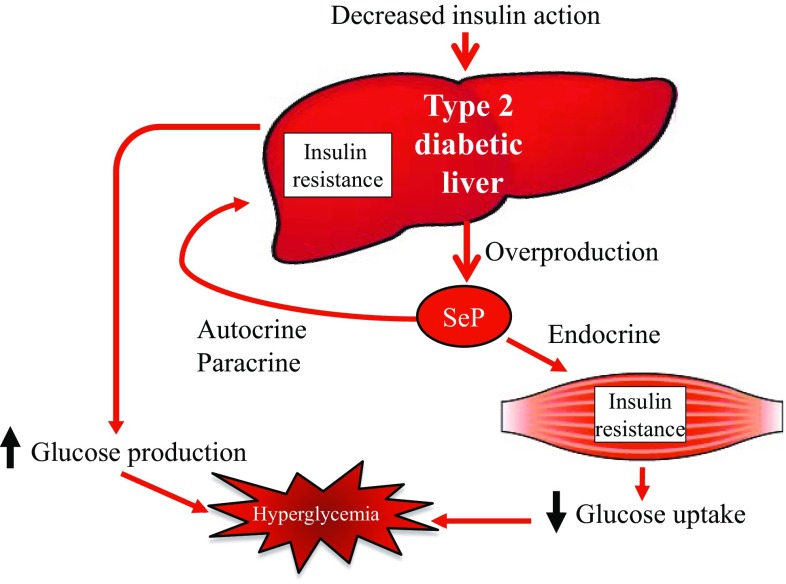
This study reveals that hepatic overproduction of SeP contributes to the development of hyperglycemia in type 2 diabetes by inducing insulin resistance in the liver and skeletal muscle (Fig. 2) [2]. This study sheds light on a previously underexplored function of the liver that is similar to adipose tissue; the liver may participate in the pathogenesis of insulin resistance through hormone secretion. Overproduction of SeP in the liver may be a novel therapeutic target to treat insulin resistance and hyperglycemia in type 2 diabetes.
Fig. 2.
Overproduction of selenoprotein P (SeP) contributes to the onset of hyperglycemia in type 2 diabetes by inducing insulin resistance in the liver and skeletal muscle
Metformin suppressed selenoprotein P production in hepatocytes via AMPK phosphorylation
Metformin is widely used as an anti-diabetic drug internationally. Adenosine monophosphate-activated protein kinase (AMPK) mediates mainly the glucose-lowering actions of metformin, including the suppression of hepatic gluconeogenesis [8]. In contrast, several reports indicate that the oral administration of metformin in human increases insulin sensitivity in skeletal muscle, increases serum adiponectin, and improves aortic arteriosclerosis [9–11]. To date, however, the molecular mechanisms underlying the systemic actions of metformin are not fully understood.
We examined the effects of metformin on SeP mRNA in H4IIEC hepatocytes. Metformin suppressed Sepp1 mRNA expression in a concentration- and time-dependent manner, similar to G6pc and Pck1 that encode gluconeogenic enzymes, respectively. The human SEPP1 promoter region was cloned to a luciferase reporter vector as reported previously [21]. Similar to the mRNA results, metformin suppressed SEPP1 promoter activity in a concentration- and time-dependent manner, suggesting that it directly decreases SEPP1 transcriptional activity in H4IIEC3 hepatocytes.
The action of metformin on Sepp1 was also examined in mice. Following the fasting for 4 h, C57BL/6J mice were administrated 300 mg/kg of metformin. Gene expression of Sepp1 in the liver was significantly decreased by metformin. These results indicate that metformin suppresses gene expression for Sepp1 in the liver of mice, as well as in the cultured hepatocytes.
Metformin is known to exert anti-diabetic effects by activating AMPK pathways [31]. Hence, to determine whether AMPK pathways are involved in the metformin-induced suppression of SEPP1 promoter activity, cells were treated with compound C, a representative AMPK inhibitor. Metformin-induced phosphorylation of AMPK and acetyl-CoA carboxylase (ACC) was canceled by the co-administration of compound C in H4IIEC3 hepatocytes. Co-administration of compound C partly rescued the cells from the inhibitory effects of metformin on the SEPP1 promoter. In contrast, treatment with AICAR, a known activator of AMPK, decreased SEPP1 promoter activity similar to metformin. Transfection with constitutive active-AMPK suppressed Sepp1 and G6pc mRNA expression, while transfection with dominant-negative AMPK enhanced Sepp1 and G6pc mRNA expression. These results suggest that metformin decreases SEPP1 promoter activity, at least partly, by activating AMPK.
To determine the nature of the metformin-response element in the SEPP1 promoter region, several deletion mutants of the SEPP1 promoter were constructed. Because the early reports indicate that AMPK directly phosphorylates FoxO3a and regulates its transcriptional activity [16], this investigation focused on the two putative FoxO-binding sites. To determine the critical FoxO-binding site for metformin-induced SEPP1 suppression, we constructed luciferase vectors which deleted either of two putative FoxO-binding sites. Luciferase assay revealed that a putative FoxO-binding site was essential for metformin-induced SEPP1 suppression. The interaction of FoxO proteins with DNA sequences in the SEPP1 promoter was examined using a ChIP assay. The ChIP assay indicates that treatment with metformin decreased the binding of FoxO3a to SEPP1 promoter, whereas it increased the binding of FoxO1. These results suggest that FoxO3a, but not FoxO1, is associated with the metformin-induced suppression of SEPP1 expression.
To elucidate the mechanism by which metformin inactivates FoxO3a, FoxO3a protein levels in hepatocytes treated with metformin were examined. Metformin treatment neither alters mRNA levels of Foxo3a nor protein levels of FoxO3a. However, FoxO3a was phosphorylated by treatment with metformin. FoxO3a protein levels were decreased by metformin treatment in the nuclear fraction. These results suggest that metformin inactivates FoxO3a by phosphorylating and decreasing nucleus FoxO3a protein levels.
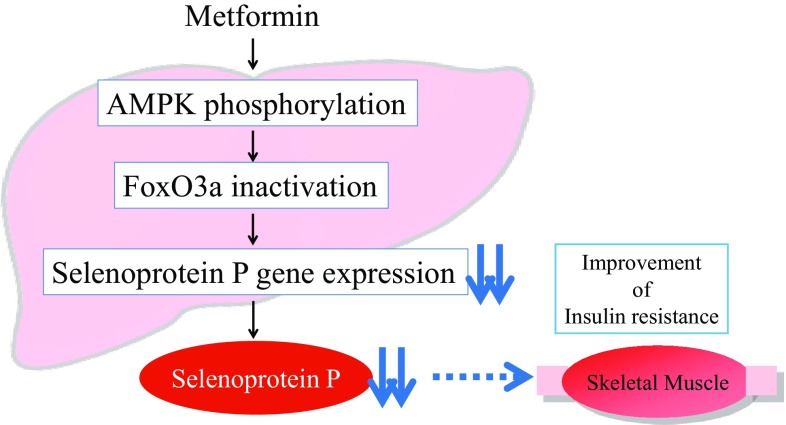
This report demonstrates that metformin suppresses production of the insulin resistance-inducing hepatokine SeP by activating AMPK and subsequently inactivating FoxO3a in H4IIEC3 hepatocytes. Our study provides a novel mechanism of action for metformin involving improvement of systemic insulin sensitivity via the regulation of SeP production (Fig. 3) [9], and suggests that AMPK/FoxO3a pathway in the liver may be a therapeutic target to the development of new-anti-diabetic drugs.
Fig. 3.
Metformin suppresses production of selenoprotein P via AMPK/FoxO3a pathway in the hepatocytes, leading to the improvement of insulin resistance in the skeletal muscle. AMPK AMP-activated protein kinase, FoxO3a forkhead box O3a
Hepatokine selenoprotein P impairs angiogenesis by inducing VEGF resistance in vascular endothelial cells
Angiogenesis is a physiological process involving the growth of new blood vessels from pre-existing vascular structures and the subsequent formation of vascular network. A number of abnormalities associated with angiogenesis were reported in people with type 2 diabetes [10], and impaired angiogenesis is linked to the development of various vascular complications in diabetes mellitus. Patients with type 2 diabetes show poor development of coronary collateral vessels on coronary angiography [11]. Moreover, people with diabetes have significantly lower capillary densities in areas of myocardial infarction [12]. These reports suggest that the angiogenic response to infarction and/or ischemia is inhibited at the levels of capillaries and small arterioles in type 2 diabetes. Inadequate vascular formation could attenuate perfusion recovery in response to ischemia, thereby partially accounting for the poor clinical outcomes in type 2 diabetic patients with coronary heart disease or peripheral artery disease [13, 14]. In addition, insufficient angiogenesis is involved in abnormal wound healing and the development of skin ulcers in diabetes [15].
Vascular endothelial growth factor (VEGF) is a mediator of angiogenesis under physiological and pathophysiological conditions. VEGF binds and phosphorylates the receptors, leading to the activation of a variety of signaling cascades such as MAPK. Angiogenic gene therapy using plasmids encoding VEGF has been attempted in patients with coronary or peripheral artery diseases [16]. However, diabetes mellitus people often show a poor response to therapeutic angiogenesis [17]. Therefore, VEGF resistance, a defect of VEGF-related signal transduction, has been postulated as a molecular basis for the dysregulated angiogenesis in diabetes mellitus [10, 18]. However, the molecular mechanisms underlying VEGF resistance in diabetes mellitus were not fully understood.
SeP has heparin-binding properties [19] and is associated with endothelial cells in rat tissues [20], suggesting that SeP exerts some actions on vascular endothelial cells. We hypothesized that the liver-derived secretory protein SeP contributes to angiogenesis-associated vascular complications in type 2 diabetes. To assess the direct action of SeP on vascular endothelial cells, we treated HUVECs with purified human SeP protein. VEGF-induced proliferation of HUVECs was significantly suppressed by treatment with 10 μg/mL of SeP. Co-administration of buthionine sulfoximine (BSO), an inhibitor of glutathione synthesis, partly rescued the suppressive effect of SeP. Next, we examined the effects of SeP on VEGF-induced migration in HUVECs. VEGF promoted the migration of HUVECs into a polycarbonate filters. This migration was inhibited by the addition of SeP in a concentration-dependent manner. In the absence of VEGF, treatment with SeP did not affect the migration of HUVECs, suggesting that SeP modulates VEGF-dependent migration of endothelial cells. We further examined the effects of SeP on tubule formation in HUVECs. HUVECs cultured on Matrigel containing VEGF showed morphological tubule formation. SeP inhibited tubule formation of HUVECs in a concentration-dependent manner. These in vitro results indicate that SeP impairs VEGF-dependent angiogenesis of vascular endothelial cells.
Next, we determined whether SeP affects VEGF signal transduction in endothelial cells. Pretreatment with SeP reduced VEGF-stimulated phosphorylation of VEGFR2 and ERK1/2 in HUVECs. Co-administration of BSO partially abolished the inhibitory effect of SeP on VEGF signaling. These results indicate that SeP, at physiological concentrations, impairs VEGF signal transduction in vascular endothelial cells.
To clarify the mechanism by which an anti-oxidative protein SeP impairs VEGF signaling, we assessed the action of SeP on the acute generation of reactive oxygen species (ROS) stimulated by VEGF. VEGF-induced ROS burst is reported to be required for the subsequent VEGF signal transduction [21]. Stimulation with 50 ng/mL of VEGF for 5 min significantly increased cellular levels of ROS in HUVEC. Pretreatment with SeP suppressed intracellular levels of ROS with VEGF stimulation. These results suggest that SeP-induced VEGF resistance is associated with the reduction of ROS burst stimulated by VEGF.
Next, to clarify whether hepatic overexpression of SeP affects angiogenesis-related disorder in vivo, we used a hydrodynamic injection method to generate mice that overexpress human SEPP1 mRNA in the liver. We created excisional wounds in the dorsal skin of the mice and quantified the rate of wound healing. Wound closure was significantly impaired in the mice overexpressing SEPP1. In contrast, Sepp1−/− mice showed an improvement of the wound closure. These results indicate that the hepatokine SeP delays the wound healing of the skin in mice.
To determine whether attenuation of SeP expression enhances angiogenesis in vivo, we generated hindlimb ischemia in SeP heterozygous knockout mice. 5 days after femoral artery ligation, SeP heterozygous knockout mice showed a significant increase of blood flow compared with wild-type mice. Histological examination showed increased vessel density in the hindlimb musculature as determined by immunostaining with anti-CD31 antibody.
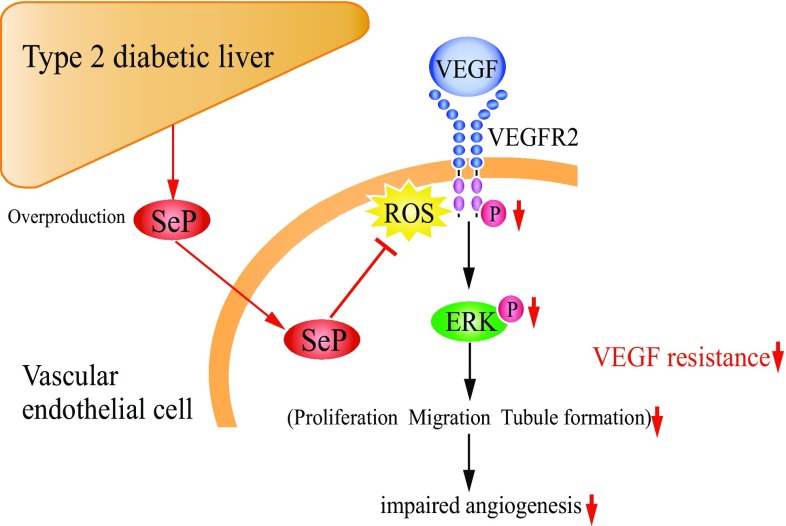
These results indicate that the liver-derived secretory protein SeP impairs angiogenesis by inducing VEGF resistance in the vascular endothelial cells (Fig. 4) [22]. Hepatic overproduction of SeP may contribute to the onset of impaired angiogenesis in type 2 diabetes. Overproduction of SeP in the liver may be a novel therapeutic target for the treatment of VEGF resistance, as well as insulin resistance, in people with type 2 diabetes.
Fig. 4.
Overproduction of selenoprotein P (SeP) contributes to the onset of impaired angiogenesis in type 2 diabetes by inducing vascular endothelial growth factor (VEGF) resistance in the vascular endothelial cells. ROS reactive oxygen species, ERK extracellular signal-regulated kinase
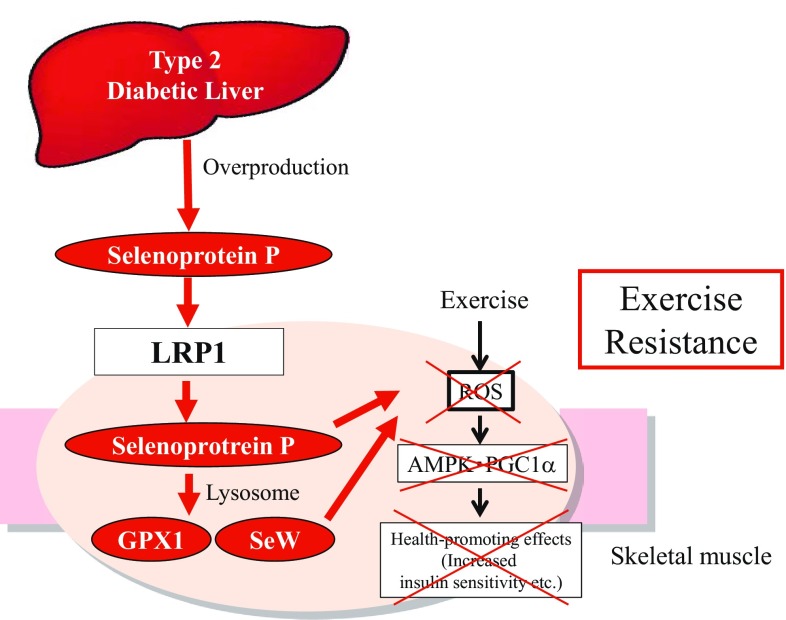
Hepatokine selenoprotein P and its receptor LRP1 impair health-promoting effects of exercise training
Physical exercise has health-promoting effects in humans [23]. However, not all people derive equal improvement of metabolic health from exercise. In particular, some people show complete non-responsiveness to exercise training in terms of aerobic endurance improvement [24]. Similarly, 20% of patients with type 2 diabetes show a poor hypoglycemic effect to regular exercise therapy [25]. These findings suggest that some people are suffering from so-called “exercise resistance” and derive limited benefit from the physical exercise.
Exercise-induced acute generation of ROS not only induces oxidative damage, but also functions as signaling molecules to mediate beneficial molecular adaptations by exercise [26]. Adenosine monophosphate-dependent protein kinase (AMPK) and peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α, encoded by Ppargc1a), both of which play a major role in exercise-mediated skeletal muscle adaptations [27, 28], are activated by acute generation of ROS [29, 30]. Notably, recent growing evidence suggests that supplementation with antioxidants such as vitamin C abolishes exercise-induced molecular alterations and limits the positive effects of exercise training in humans [31, 32]. Acute generation of ROS may mediate the positive effects of exercise training by activating several molecules including AMPK and PGC-1α in muscle. However, a few reports were available on endogenous anti-oxidative inhibitory factors, as opposed to exogenously administered antioxidants, that reduce exercise-induced ROS generation and impair the effects of exercise.
SeP reduces oxidative stress by acting directly as an anti-oxidative enzyme and by supplying selenium to target cells [3–5]. Based on the previous reports suggesting the involvement of acute generation of ROS in the effects of exercise, we hypothesized that the antioxidant hepatokine SeP causes “exercise resistance” by suppressing exercise-induced ROS in the skeletal muscle [33].
First, we subjected Sepp1-deficient mice to a high-fat diet (HFD) and regular exercise training. SeP deficiency did not alter endurance capacity in the absence of exercise training. However, after exercise training, Sepp1-deficient mice showed higher aerobic exercise capacity in a running endurance test, although all the mice were subjected to the same intensity and duration of exercise training. The glucose-lowering effect of insulin was higher in trained Sepp1-deficient mice. Mitochondrial DNA content was higher in trained Sepp1-deficient mice. These results indicate that deficiency of SeP enhances the responsiveness to regular exercise training in mice.
Next, we obtained skeletal muscle samples after a single bout of exercise for 3 h. Sepp1-deficient mice after acute exercise showed higher AMPK phosphorylation and TBARS levels, a marker of oxidative stress, in the skeletal muscle. After acute exercise, Consistent with the result in AMPK phosphorylation, exercise-induced gene expression of Ppargc1a was also higher in exercised Sepp1-deficient mice. To determine whether ROS elevation mediates the phenotype of Sepp1h-deficient mice, we injected these animals with the anti-oxidant NAC. NAC pretreatment abrogated the elevation of exercise-induced AMPK phosphorylation and TBARS, resulting in a reduction in endurance capacity in exercised Sepp1-deficient mice. These results indicate that SeP deficiency enhances ROS-mediated AMPK phosphorylation in the skeletal muscle during exercise, leading to enhanced exercise responsiveness in mice.
In the experiments to mimic exercise in cultured myotubes, treatment with SeP suppressed H2O2-stimulated AMPK phosphorylation and mitochondrial DNA content. Among selenoproteins containing a selenocysteine residue, production of glutathione peroxidase 1 (GPX1 encoded by Gpx1) and selenoprotein W (SelW encoded by Selpw1), but not of selenoprotein N (SelN), was induced by treatment with SeP. SeP reduced mRNA levels of genes expressed downstream of AMPK, such as Ppargc1a, in the presence of H2O2. Knockdown for AMPKα by small interfering RNA (siRNA) abolished the inhibitory actions of SeP on Ppargc1a expression in C2C12 myotubes. These results indicate that SeP impairs the effects of ROS on AMPK phosphorylation and Ppargc1a gene expression in cultured myotubes.
To identify the functional receptor of SeP in the skeletal muscle, we transfected C2C12 mouse myotubes with siRNA specific to the genes encoding two abundant LDLR family members, because the early reports showed that LDLR family proteins function as SeP receptors in the testis or kidney [34, 35]. Knockdown of Lrp1 (low-density lipoprotein receptor-related protein 1), but not of Ldlr (low-density lipoprotein receptor), reduced SeP-induced Gpx1 mRNA in C2C12 cells. Similarly, the inhibitory effects of SeP on the expression of Ppargc1a were completely abolished by knockdown of Lrp1. Furthermore, knockdown of Lrp1 canceled the inhibitory effect of SeP on H2O2-induced AMPK phosphorylation in C2C12 myotubes. C2C12 cells transfected with siRNA for Lrp1 showed lower specific binding to 75Se-labeled SeP. Finally, C2C12 myotubes with siRNA for Lrp1 exhibited lower SeP uptake compared with cells with control siRNA. These results indicate that LRP1 functions as the primary SeP receptor in cultured mouse myotubes.
To assess the role of LRP1 on exercise resistance induced by SeP in vivo, we generated muscle-specific LRP1 KO (musLRP1KO) mice by crossing mice bearing a conditioning allele for LRP1 with muscle creatine kinase (MCK)-Cre transgenic mice. Western blotting and hyperinsulinemic–euglycemic clamp studies showed that LRP1 deficiency enhanced insulin signaling and subsequent glucose uptake in the skeletal muscle of mice. To determine whether LRP1 mediates the effects of SeP in the skeletal muscle, we treated musLRP1KO mice with SeP. Western blotting revealed that musLRP1KO mice injected with SeP showed lower uptake of SeP in the skeletal muscle. AMPK phosphorylation induced by a single bout of exercise was suppressed by treatment with SeP in the negative control mice but not in musLRP1KO mice. Similarly, SeP-induced suppression of Ppargc1a gene expression during exercise was abolished in musLRP1KO mice. These results indicate that LRP1 functions as an uptake receptor of SeP in the skeletal muscle of mice.
To further examine the effect of LRP1 on exercise responsiveness in the skeletal muscle, we subjected musLRP1KO mice to an HFD and exercise training. Trained musLRP1KO mice showed a higher aerobic exercise capacity and glucose-lowering effect of insulin. These results indicate that LRP1 deficiency in the skeletal muscle enhances the responsiveness to exercise training for exercise capacity and insulin sensitivity in mice.
Finally, to further establish a role for SeP in regulation of exercise responsiveness, the relationship between pre-training plasma SeP concentrations and the effect of exercise training on exercise capacity in humans were assessed. A total of 31 sedentary postmenopausal women without obesity or type 2 diabetes underwent aerobic exercise training at 60–75% of the individually determined maximal heart rates for 8 weeks. Exercise capacity was measured as maximal oxygen consumption () using an ergometer and online computer-assisted circuit spirometry. Exercise training increased but did so without affecting plasma concentrations of SeP. There was no significant correlation between and SeP concentrations in the pre-training condition. However, pre-training plasma SeP concentrations negatively correlated with training-induced increment in (). People in the highest tertile for showed a significant reduction of pre-training plasma SeP concentrations versus those in the lowest tertile. Multivariate regression analysis showed that pre-training SeP concentrations predicted independently of other clinical parameters such as age and body weight. These results indicate that pre-training plasma SeP concentrations predict the ineffectiveness of exercise training in humans.
Our study demonstrates that overproduction of hepatokine SeP causes exercise resistance by impairing exercise-induced molecular adaptations in the skeletal muscle through its receptor LRP1 (Fig. 5) [33]. Much progress has been made in the development of exercise mimetics that induce adaptations similar to exercise by targeting nuclear receptors and their coregulators [28]. However, to date, a little attention has been paid to intrinsic inhibitory factors that impair the molecular adaptations induced by exercise. The current findings suggest that future screening for small molecule inhibitors of the SeP-LRP1 axis may identify exercise-enhancing drugs to treat physical inactivity-associated diseases such as type 2 diabetes.
Fig. 5.
Overproduction of selenoprotein P impairs the health-promoting effects of exercise by inhibiting ROS (reactive oxygen species)/AMPK (AMP-activated protein kinase)/PGC-1α (peroxisome proliferator-activated receptor γ coactivator-1α) pathway in the skeletal muscle through its receptor LRP1 (low-density lipoprotein receptor-related protein 1). Selenoprotein P suppresses ROS generation by directly acting as an anti-oxidative enzyme and indirectly inducing the other selenoproteins such as glutathione peroxidase 1 (GPX1) and selenoprotein W (SeW)
Identification of LECT2 as a hepatokine that is over-produced in obesity
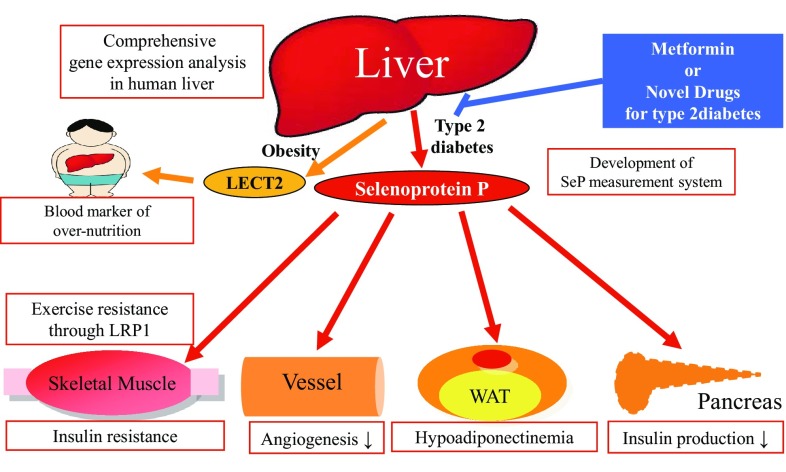
Growing evidence suggests a central role for fatty liver disease in the development of insulin resistance in obesity [36, 37]. Kotronen et al. [38] reported that intrahepatocellular rather than intramyocellular fat associates with hyperinsulinemia independently of obesity in non-diabetic men. Fabbrini et al. [39] have revealed that intrahepatic triglyceride, but not visceral adipose tissue, is a better marker of multi-organ insulin resistance associated with obesity. D’Adamo et al. [40] showed that obese adolescents with high hepatic fat content exhibit lower whole-body insulin sensitivity independently of visceral fat and intramyocellular lipid. These papers indicate a strong correlation between fatty liver and muscle insulin resistance in humans, but it was still unknown whether fatty liver disease directly causes muscle insulin resistance in obesity (Figs. 5, 6).
Fig. 6.
Overproduction of hepatokines contributes to the various kinds of pathologies observed in type 2 diabetes and obesity. Our studies open the door for inhibitor of hepatokine production as novel drugs for type 2 diabetes-associated pathologies. LECT2 leukocyte cell-derived chemotaxin 2, LRP1 low-density lipoprotein receptor-related protein 1, WAT white adipose tissue
To identify hepatokines involved in obesity-induced insulin resistance, we performed liver biopsies in humans and conducted a comprehensive analysis of gene expression profile. As a result, we found a positive correlation between hepatic LECT2 mRNA levels and BMI, indicating that elevated hepatic LECT2 mRNA levels were associated with obesity in humans [41].
Leukocyte cell-derived chemotaxin 2 (LECT2) is a secretory protein identified in the process of screening for a novel neutrophil chemotactic protein [42]. LECT2 (in humans encoded by the LECT2 gene) is expressed preferentially by human adult and fetal liver cells [43]. The early study using Lect2-deficient mice showed that LECT2 negatively regulates the homeostasis of natural killer T cells in the liver [44]. More recently, Anson et al. reported that LECT2 exerts anti-inflammatory and tumor-suppressive actions in β-catenin-induced liver tumorigenesis [45]. To date, however, the role of LECT2 in the development of obesity and insulin resistance induced by over-nutrition was not established.
To confirm the elevation of LECT2 in animal models with obesity, we fed C57BL6J mice with a HFD. HFD increased body weight time-dependently, and tended to increase triglyceride contents in the liver. Gene expression for Lect2 was elevated in the livers of the mice fed HFD, in accordance with the steatosis-associated genes such as Fasn and Srebp1c. Serum concentrations of LECT2 showed a sustained increase since a week after the beginning of HFD. These results indicate that diet-induced obesity increases LECT2 production in the liver of mice.
To determine whether AMPK, a starvation-sensing kinase, regulates LECT2 expression, we transfected H4IIEC hepatocytes with adenoviruses either encoding constitutively active or dominant-negative AMPK. Transfection with constitutively active-AMPK significantly decreased mRNA levels for Lect2 in H4IIEC hepatocytes, similarly to those for G6Pc that encodes key gluconeogenic enzyme glucose-6 phosphatase already-known to be suppressed by AMPK [46]. In contrast, transfection with dominant-negative AMPK increased Lect2 gene expression. These results indicate that AMPK negatively regulates LECT2 production in the cultured hepatocytes.
Next, we examined the role of LECT2 in the development of insulin resistance in mice. We found that gene expression for Lect2 in the liver was overwhelmingly dominant compared with that in the other tissues in mice. This result suggests that the contribution of the other tissues except the liver on the circulating levels of LECT2 is very small or negligible in mice. Hence, we used systemic knockout mice of LECT2 in the following experiments. Body weight, food intake, and resting heat production were unaffected by Lect2 knockout. However, the treadmill running challenge revealed that physical exercise-assessed muscle endurance was significantly higher in Lect2−/− mice. A glucose or insulin loading test revealed that Lect2−/− mice showed lower blood glucose levels after glucose or insulin injection. Lect2−/− mice exhibited an increase in insulin-stimulated Akt phosphorylation in skeletal muscle, but not in the liver or adipose tissue. Consistent with the results of insulin signaling, hyperinsulinemic–euglycemic clamp studies showed that the glucose infusion rate and peripheral glucose disposal were increased, whereas endogenous glucose production was unaffected by Lect2 deletion. These results indicate that an Lect2 deletion increases insulin sensitivity in the skeletal muscle in mice.
To examine the effect of LECT2 on insulin signaling in vitro, we transfected C2C12 myocytes with a plasmid expression vector encoding mouse LECT2. LECT2 transfection suppressed myotube differentiation in C2C12 cells. The cells transfected with the LECT2 vector showed a decrease in insulin-stimulated Akt phosphorylation and an increase in basal c-Jun-N-terminal kinase (JNK) phosphorylation. To further confirm the acute action of LECT2 on insulin signaling, we treated C2C12 myotubes with recombinant LECT2 protein. Treatment with LECT2 protein for 3 h decreased insulin-stimulated Akt phosphorylation. In addition, treatment with LECT2 protein for 30–60 min increased JNK phosphorylation transiently in C2C12 myotubes. To determine whether JNK pathway mediates LECT2-induced insulin resistance, we transfected C2C12 myoblasts with siRNAs specific for JNK1 and JNK2. Because knockdown of JNK is known to alter the myotube differentiation in C2C12 myotubes [47], we used undifferentiated C2C12 myoblasts to purely assess the action of LECT2 on insulin signal transduction. Double knockdown of JNK1 and JNK2 rescued the cells from the inhibitory effects of LECT2 on insulin signaling. These in vitro experiments indicate that, at nearly physiological concentrations, LECT2 impairs insulin signal transduction by activating JNK in the cultured myotubes.
Our research reveals that the overproduction of hepatokine LECT2 contributes to the development of muscle insulin resistance in obesity [41]. The current study demonstrates a previously unrecognized role for LECT2 in glucose metabolism and suggests that LECT2 is a strong candidate to explain a mechanism by which the fatty liver leads to whole-body insulin resistance in obesity.
Conclusions and future perspectives
We have rediscovered two liver-derived secretory factors, SeP and LECT2, as hepatokines that induce insulin resistance and hyperglycemia (Fig. 6). After the publication of our reports on the hepatokines, many researchers have paid much attention to liver-derived secretory factors that play a major role in the regulation of glucose metabolism [48, 49]. Notably, our research reveals that abnormal production of hepatokines contributes to the onset of various kinds of systemic pathologies observed in type 2 diabetes, and suggests that hepatokines may be promising targets to treat or diagnose the pathologies in over-nutrition-associated diseases such as type 2 diabetes. Actually, high-throughput screening for low molecular inhibitors of hepatokine production is now ongoing. In addition, neutralizing antibodies against SeP was reported to improve insulin resistance in animal models with diabetes [50]. For the diagnosis of hepatokines-related pathologies, the rapid measurement system of blood concentrations of human SeP has been already established [51]. We strongly expect that further studies would develop novel diagnostic or therapeutic procedures targeting hepatokines to combat over-nutrition-related diseases such as type 2 diabetes.
Acknowledgements
Parts of this review were presented by the author as the Lilly Award Lecture at the 61th Annual Meeting of the Japan Diabetes Society, Tokyo, Japan. The author sincerely thanks Profs. Toshinari Takamura and Shuichi Kaneko (Kanazawa University) for their support and mentoring, and also colleagues in the Department of Endocrinology and Metabolism, Kanazawa University Graduate School of Medical Sciences for their support. The author would also like to thank Dr. Masato Kasuga (PRESTO, Japan Science and Technology Agency) for his support and helpful advice.
Funding
This work was supported by the following grants: JSPS KAKENHI Grant Numbers 23791022, 25461334, and 16K09740; the Mochida Memorial Foundation for Medical and Pharmaceutical Research; the Takeda Science Foundation; JST Adaptable and Seamless Technology transfer Program (A-STEP) Grant Numbers AS2311400F and 15im0302407.
Conflict of interest
Hirofumi Misu received research grant from Eli Lilly, Takeda Pharmaceutical Company, Novartis International AG, and Merck & Co., Inc.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study. All experimental protocols were approved by the Ethics Committees of Kanazawa University (Approval no. 2009-067, December 1 2009, Approval no. 2011-049, October 12 2011, and Approval no. 2014-002, September 30 2014).
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Misu H, Takamura T, Matsuzawa N, et al. Genes involved in oxidative phosphorylation are coordinately upregulated with fasting hyperglycaemia in livers of patients with type 2 diabetes. Diabetologia. 2007;2:268–277. doi: 10.1007/s00125-006-0489-8. [DOI] [PubMed] [Google Scholar]
- 2.Misu H, Takamura T, Takayama H, et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;5:483–495. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Burk RF, Hill KE. Selenoprotein P-expression, functions, and roles in mammals. Biochem Biophys Acta. 2009;11:1441–1447. doi: 10.1016/j.bbagen.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito Y, Takahashi K. Characterization of selenoprotein P as a selenium supply protein. Eur J Biochem. 2002;22:5746–5751. doi: 10.1046/j.1432-1033.2002.03298.x. [DOI] [PubMed] [Google Scholar]
- 5.Arteel GE, Mostert V, Oubrahim H, et al. Protection by selenoprotein P in human plasma against peroxynitrite-mediated oxidation and nitration. Biol Chem. 1998;8–9:1201–1205. [PubMed] [Google Scholar]
- 6.Hill KE, Zhou J, McMahan WJ, et al. Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem. 2003;16:13640–13646. doi: 10.1074/jbc.M300755200. [DOI] [PubMed] [Google Scholar]
- 7.Schomburg L, Schweizer U, Holtmann B, et al. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J. 2003;370(Pt 2):397–402. doi: 10.1042/bj20021853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruderman NB, Carling D, Prentki M, et al. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;7:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takayama H, Misu H, Iwama H, et al. Metformin suppresses expression of the selenoprotein P gene via an AMP-activated kinase (AMPK)/FoxO3a pathway in H4IIEC3 hepatocytes. J Biol Chem. 2014;1:335–345. doi: 10.1074/jbc.M113.479386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons M. Angiogenesis, arteriogenesis, and diabetes: paradigm reassessed? J Am Coll Cardiol. 2005;5:835–837. doi: 10.1016/j.jacc.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Abaci A, Oguzhan A, Kahraman S, et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;17:2239–2242. doi: 10.1161/01.CIR.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 12.Yarom R, Zirkin H, Stammler G, et al. Human coronary microvessels in diabetes and ischaemia. Morphometric study of autopsy material. J Pathol. 1992;3:265–270. doi: 10.1002/path.1711660308. [DOI] [PubMed] [Google Scholar]
- 13.Al-Delaimy WK, Merchant AT, Rimm EB, et al. Effect of type 2 diabetes and its duration on the risk of peripheral arterial disease among men. Am J Med. 2004;4:236–240. doi: 10.1016/j.amjmed.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 14.Hueb W, Gersh BJ, Costa F, et al. Impact of diabetes on five-year outcomes of patients with multivessel coronary artery disease. Ann Thorac Surg. 2007;1:93–99. doi: 10.1016/j.athoracsur.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 15.Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;6:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boodhwani M, Sellke FW. Therapeutic angiogenesis in diabetes and hypercholesterolemia: influence of oxidative stress. Antioxid Redox Signal. 2009;8:1945–1959. doi: 10.1089/ars.2009.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes—a review. Diabet Med. 2010;1:4–14. doi: 10.1111/j.1464-5491.2009.02866.x. [DOI] [PubMed] [Google Scholar]
- 18.Waltenberger J. VEGF resistance as a molecular basis to explain the angiogenesis paradox in diabetes mellitus. Biochem Soc Trans. 2009;37(Pt 6):1167–1170. doi: 10.1042/BST0371167. [DOI] [PubMed] [Google Scholar]
- 19.Arteel GE, Franken S, Kappler J, et al. Binding of selenoprotein P to heparin: characterization with surface plasmon resonance. Biol Chem. 2000;3:265–268. doi: 10.1515/BC.2000.034. [DOI] [PubMed] [Google Scholar]
- 20.Burk RF, Hill KE, Boeglin ME, et al. Selenoprotein P associates with endothelial cells in rat tissues. Histochem Cell Biol. 1997;1:11–15. doi: 10.1007/s004180050141. [DOI] [PubMed] [Google Scholar]
- 21.Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2007;6:731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 22.Ishikura K, Misu H, Kumazaki M, et al. Selenoprotein P as a diabetes-associated hepatokine that impairs angiogenesis by inducing VEGF resistance in vascular endothelial cells. Diabetologia. 2014;9:1968–1976. doi: 10.1007/s00125-014-3306-9. [DOI] [PubMed] [Google Scholar]
- 23.Bishop-Bailey D. Mechanisms governing the health and performance benefits of exercise. Br J Pharmacol. 2013;6:1153–1166. doi: 10.1111/bph.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchard C, An P, Rice T, et al. Familial aggregation of VO2max response to exercise training: results from the HERITAGE Family Study. J Appl Physiol. 1999;3:1003–1008. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 25.Stephens NA, Sparks LM. Resistance to the beneficial effects of exercise in type 2 diabetes: are some individuals programmed to fail? J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2014-2545. [DOI] [PubMed] [Google Scholar]
- 26.Niess AM, Simon P. Response and adaptation of skeletal muscle to exercise–the role of reactive oxygen species. Front Biosci. 2007;12:4826–4838. doi: 10.2741/2431. [DOI] [PubMed] [Google Scholar]
- 27.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;7203:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;3:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardaci S, Filomeni G, Ciriolo MR. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J Cell Sci. 2012;125(Pt 9):2115–2125. doi: 10.1242/jcs.095216. [DOI] [PubMed] [Google Scholar]
- 30.Kang C, O’Moore KM, Dickman JR, et al. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med. 2009;10:1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;21:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Cabrera MC, Domenech E, Romagnoli M, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;1:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 33.Misu H, Takayama H, Saito Y, et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat Med. 2017;4:508–516. doi: 10.1038/nm.4295. [DOI] [PubMed] [Google Scholar]
- 34.Olson GE, Winfrey VP, Nagdas SK, et al. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J Biol Chem. 2007;16:12290–12297. doi: 10.1074/jbc.M611403200. [DOI] [PubMed] [Google Scholar]
- 35.Olson GE, Winfrey VP, Hill KE, et al. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J Biol Chem. 2008;11:6854–6860. doi: 10.1074/jbc.M709945200. [DOI] [PubMed] [Google Scholar]
- 36.Takamura T, Misu H, Ota T, et al. Fatty liver as a consequence and cause of insulin resistance: lessons from type 2 diabetic liver. Endocr J. 2012;9:745–763. doi: 10.1507/endocrj.EJ12-0228. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh PS, Hsieh YJ. Impact of liver diseases on the development of type 2 diabetes mellitus. World J Gastroenterol. 2011;48:5240–5245. doi: 10.3748/wjg.v17.i48.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotronen A, Seppala-Lindroos A, Bergholm R, et al. Tissue specificity of insulin resistance in humans: fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia. 2008;1:130–138. doi: 10.1007/s00125-007-0867-x. [DOI] [PubMed] [Google Scholar]
- 39.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;36:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Adamo E, Cali AM, Weiss R, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;8:1817–1822. doi: 10.2337/dc10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lan F, Misu H, Chikamoto K, et al. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes. 2014;5:1649–1664. doi: 10.2337/db13-0728. [DOI] [PubMed] [Google Scholar]
- 42.Yamagoe S, Yamakawa Y, Matsuo Y, et al. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol Lett. 1996;1:9–13. doi: 10.1016/0165-2478(96)02572-2. [DOI] [PubMed] [Google Scholar]
- 43.Yamagoe S, Mizuno S, Suzuki K. Molecular cloning of human and bovine LECT2 having a neutrophil chemotactic activity and its specific expression in the liver. Biochim Biophys Acta. 1998;1:105–113. doi: 10.1016/S0167-4781(97)00181-4. [DOI] [PubMed] [Google Scholar]
- 44.Saito T, Okumura A, Watanabe H, et al. Increase in hepatic NKT cells in leukocyte cell-derived chemotaxin 2-deficient mice contributes to severe concanavalin A-induced hepatitis. J Immunol. 2004;1:579–585. doi: 10.4049/jimmunol.173.1.579. [DOI] [PubMed] [Google Scholar]
- 45.Anson M, Crain-Denoyelle AM, Baud V, et al. Oncogenic beta-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest. 2012;2:586–599. doi: 10.1172/JCI43937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viollet B, Guigas B, Leclerc J, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol. 2009;1:81–98. doi: 10.1111/j.1748-1716.2009.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alter J, Rozentzweig D, Bengal E. Inhibition of myoblast differentiation by tumor necrosis factor alpha is mediated by c-Jun N-terminal kinase 1 and leukemia inhibitory factor. J Biol Chem. 2008;34:23224–23234. doi: 10.1074/jbc.M801379200. [DOI] [PubMed] [Google Scholar]
- 48.Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;3:144–152. doi: 10.1038/nrendo.2012.258. [DOI] [PubMed] [Google Scholar]
- 49.Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;9:509–520. doi: 10.1038/nrendo.2017.56. [DOI] [PubMed] [Google Scholar]
- 50.Mita Y, Nakayama K, Inari S, et al. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat Commun. 2017;1:1658. doi: 10.1038/s41467-017-01863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka M, Saito Y, Misu H, et al. Development of a sol particle homogeneous immunoassay for measuring full-length selenoprotein P in human serum. J Clin Lab Anal. 2016;2:114–122. doi: 10.1002/jcla.21824. [DOI] [PMC free article] [PubMed] [Google Scholar]