Abstract
Aim
In addition to atherosclerosis, impairment of blood rheology is an important factor in cardiovascular events. The present study attempted to clarify the relationship between hemorheology and cardiovascular risk factors in patients with type 2 diabetes mellitus assessed by a microchannel method.
Methods
We enrolled 294 patients with type 2 diabetes mellitus (109 males and 185 females; mean age, 69 ± 11 years) with no history of cardiovascular events. Hemorheology was evaluated with a microchannel array flow analyzer, and the relationship between whole-blood passage time (WBPT) and various clinical parameters was examined.
Results
WBPT was significantly correlated with advanced glycation end-product (AGE) levels at the skin (r = 0.49, p < 0.001), serum reactive oxygen metabolite concentrations (oxidative stress markers) (r = 0.25, p < 0.001), the cardio-ankle vascular index (CAVI, arterial function marker) (r = 0.32, p < 0.001), and a number of classical cardiovascular risk factors in an individual (r = 0.45, p < 0.001). Multiple regression analysis revealed that these factors were selected as independent variables for WBPT as a subordinate factor.
Conclusion
Hemorheology is significantly associated with novel cardiovascular risk factors, such as AGEs, in vivo oxidative stress, and CAVI, and clustering of classical cardiovascular risk factors in patients with type 2 diabetes mellitus.
Keywords: Type 2 diabetes mellitus, Hemorheology, Microchannel method, Cardiovascular risk factors
Introduction
Impairment of blood rheology has been reported to be higher in patients with cardiovascular disease [1, 2]. Clinical studies have indicated that hemorheology is closely associated with glycemic control or metabolic abnormalities in patients with diabetes mellitus [3, 4]. Therefore, evaluation of hemorheology as well as an adequate therapy for its improvement is an important factor in diabetic patients. Recently, a commercial device that evaluates hemorheology (Micro Channel array Flow Analyzer [MC-FAN]) using microscopic images has been explored and is being used in clinical settings [5]. MC-FAN is superior to other methods in terms of accuracy of channel dimensions and high reproducibility, and some studies have reported it to be clinically useful for evaluating hemorheology and cardiovascular disease risk factors [6–8].
The importance of type 2 diabetes mellitus as a risk factor for cardiovascular disease is not always explained by high blood glucose levels alone. Other classical cardiovascular risk factors, such as hypertension, dyslipidemia, obesity, and smoking habits, are also important. Furthermore, novel cardiovascular risk factors, such as insulin resistance, inflammation, oxidative stress, advanced glycation end-products (AGEs), and arterial dysfunction, are also known to affect the occurrence of cardiovascular events in type 2 diabetes mellitus [9–11].
Using MC-FAN, the present study was performed to determine specific factors, including novel risk factors for cardiovascular disease that affect hemorheology in patients with type 2 diabetes mellitus and are related to primary cardiovascular events.
Patients and methods
Patients
This cross-sectional study was conducted at the Hitsumoto Medical Clinic in Shimonoseki City from January 2013 to December 2015. The study population consisted of 294 outpatients with type 2 diabetes mellitus undergoing antidiabetic treatment. None of the patients had a history of cardiovascular events, such as ischemic heart disease, stroke, and perivascular disease. There were 109 males and 185 females, with a mean age of 69 ± 11 years. All participants provided informed consent, and the study protocol conformed to the ethical guidelines of the Declaration of Helsinki as reflected in a priori approval by the Local Ethics Committee of the Hitsumoto Medical Clinic (date of approval 7 January 2013; approval no. 2013-01).
Evaluation of hemorheology by MC-FAN
The evaluation of hemorheology was performed by measuring the whole-blood passage time (WBPT) with the MC-FAN HR300 rheometer (MC Healthcare, Tokyo) as previously reported [5]. Briefly, the microchannel passage time for 100 μl of physiologic saline was first measured as a control; following this, the same measurement was performed with 100 μl of heparinized blood obtained from the subjects. WBPT of the subjects was expressed after correction for the passage time of physiologic saline. The microchannel formation had a width, length, and depth of 7, 30, and 4.5 μm, respectively. Examination was performed within 60 min after blood sampling. The inter- and intra-assay coefficients of variation for WBPT were 8 and 5%, respectively.
Evaluation of cardiovascular risk factors
The degree of obesity was estimated from the body mass index, which was calculated as the weight in kilograms divided by the square of the height in meters; obesity was defined as a body mass index ≥25 kg/m2. Current smoking was defined as smoking at least one cigarette per day during the previous 28 days. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or the use of antihypertensive medication. Dyslipidemia was defined as a low-density lipoprotein cholesterol level ≥140 mg/dl, a high-density lipoprotein cholesterol level ≤40 mg/dl, a triglyceride level ≥150 mg/dl, or the use of antidyslipidemic medication. The number of risk factors was defined as the number of classical cardiovascular risk factors, such as hypertension, dyslipidemia, smoking, or obesity, in an individual. Blood cell counts, plasma glucose concentration, plasma insulin concentration, hemoglobin A1c (HbA1c) level, serum lipid concentration, estimated glomerular filtration rate (eGFR), serum high-sensitivity C-reactive protein (hs-CRP) concentration, and derivatives of reactive oxygen metabolites (d-ROMs) were measured. Blood samples were collected from the antecubital vein in the morning after 12 h of fasting. Glucose and insulin concentrations were measured by the glucose oxidase method and an enzyme immunoassay, respectively. To measure insulin resistance, HOMA-IR was calculated as follows [12]: HOMA-IR = fasting glucose concentration (mg/dl) × fasting insulin concentration (µg/ml)/405. HbA1c levels were expressed according to the National Glycohemoglobin Standardization Program. Total cholesterol and triglyceride concentrations were measured by standard enzymatic methods. High- and low-density lipoprotein cholesterol concentrations were measured using selective inhibition and Friedewald’s formula, respectively [13]. Subjects with serum triglyceride concentrations ≥400 mg/dl were excluded. eGFR was calculated with the adjusted Modification of Diet in Renal Disease Study equation, proposed by the working group of the Japanese Chronic Kidney Disease Initiative [14]. hs-CRP concentration was measured by high-sensitivity, latex-enhanced immunonephelometrics. The hydroperoxide level, as an oxidative stress marker, was measured in vivo by the d-ROMs test using a commercial device (Diacron, Grosseto, Italy) [15].
Measurement of AGE
AGE levels were quantified by skin AGE levels, which were based on skin autofluorescence measured with a commercial instrument (AGE ReaderTM; DiagnOptics, Groningen, The Netherlands) as previously described [16, 17]. Briefly, autofluorescence was defined as the average light intensity per nanometer in the range between 300 and 420 nm. Autofluorescence levels, expressed in arbitrary units, were measured on the volar side of the lower arm, approximately 10–15 cm below the elbow fold, with the patient in a seated position. The validity and reliability of autofluorescence levels measured by this method have been established in a Japanese population [17].
Evaluation of arterial function
The cardio-ankle vascular index (CAVI) was measured as an arterial function marker using a VaSera CAVI instrument (Fukuda Denshi, Tokyo, Japan), according to previously described methods [18]. Briefly, the brachial and ankle pulse waves were determined using inflatable cuffs with the pressure maintained between 30 and 50 mmHg to ensure that the cuff pressure had a minimal effect on the systemic hemodynamics. Blood and pulse pressures were simultaneously determined, and the measurements were obtained with the subject in a supine position. CAVI was measured after the subject had rested for 10 min in a quiet room. The average coefficient of variation of CAVI has been shown to be less than 5%, which is small enough for clinical use and indicates that CAVI measurement has good reproducibility.
Statistical analysis
A commercially available statistical software program (StatView-J 5.0; Hulinks, Tokyo, Japan) was used for all statistical analyses. Continuous variables were expressed as mean ± standard deviation. The statistical significance of differences in the mean values was calculated by analysis of variance. The simple regression analysis was estimated by Spearman’s rank correlation analysis. Multivariate analysis was performed using multiple regression analysis. A p value <0.05 was considered to indicate statistical significance.
Results
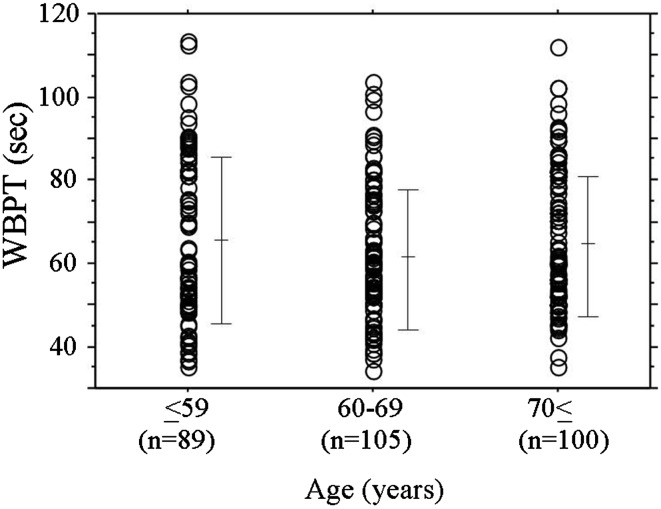
Baseline clinical characteristics are shown in Table 1, and the dot graph of WBPT according to age is shown in Fig. 1. The mean value of WBPT was 61.3 ± 18.1 s, ranging from 31.2 to 113.5 s. There was no correlation between age and WBPT. Correlations between WBPT and various clinical parameters are shown in Table 2. There were significant correlations between WBPT and obesity, body mass index, current smoking, hypertension, white blood cell count, red blood cell count, hematocrit, HbA1c, skin autofluorescence, serum triglyceride concentration, eGFR, hs-CRP, d-ROMs test, CAVI, and number of risk factors. Fasting blood glucose levels and HOMA-IR were not significantly correlated with WBPT.
Table 1.
Baseline clinical characteristics
| n (male/female) | 294 (109/185) |
| Age (years) | 64 ± 11 |
| Obesity, n (%) | 135 (46) |
| Body mass index | 24.3 ± 3.9 |
| Current smoking, n (%) | 86 (29) |
| Hypertension, n (%) | 197 (67) |
| Systolic blood pressure (mmHg) | 140 ± 18 |
| Diastolic blood pressure (mmHg) | 84 ± 11 |
| White blood cell (/μl) | 6590 ± 1400 |
| Red blood cell (104/μl) | 428 ± 44 |
| Hematocrit (%) | 38.5 ± 4.1 |
| Platelet (104/μl) | 22.0 ± 6.2 |
| Fasting blood glucose (mg/dl) | 133 ± 26 |
| Immunoreactive insulin (μg/ml) | 8 ± 5 |
| HbA1c (%) | 7.0 ± 0.9 |
| HOMA-IR | 2.6 ± 1.7 |
| Skin autofluoresence (AU) | 2.6 ± 0.5 |
| Dyslipidemia, n (%) | 212 (72) |
| Total cholesterol (mg/dl) | 222 ± 40 |
| LDL cholesterol (mg/dl) | 144 ± 39 |
| Triglyceride (mg/dl) | 148 ± 64 |
| HDL cholesterol (mg/dl) | 48 ± 15 |
| eGFR (ml/min/1.73 m2) | 61 ± 21 |
| log-hs-CRP (mg/l) | −1.1 ± 0.5 |
| d-ROMs test (U.Carr) | 339 ± 92 |
| CAVI | 9.3 ± 1.3 |
| Number of risk factors | 2.1 ± 1.0 |
| WBPT (s) | 61.3 ± 18.1 |
| Medication | |
| Sulfonylurea, n (%) | 154 (52) |
| DPP-4 inhibitor, n (%) | 102 (35) |
| Insulin, n (%) | 20 (7) |
| Statin, n (%) | 142 (48) |
| RAS inhibitor, n (%) | 122 (41) |
| Eicosapentaenoic acid, n (%) | 12 (4) |
Continuous values are mean ± SD
HbA1c hemoglobin A1c, HOMA-IR homeostasis assessment insulin resistance, LDL low-density lipoprotein, HDL high-density lipoprotein, eGFR estimated glomerular filtration rate, hs-CRP high sensitivity C reactive protein, d-ROMs derivatives of reactive oxygen metabolites, CAVI cardio-ankle vascular index, Risk factor obesity, current smoker, hypertensin, dyslipidemia, WBPT whole-blood passage time, DPP dipeptidyl peptidase, RAS renin-angiotensin system
Fig. 1.
The dot graph of WBPT according to age. Bar expressed mean ± SD. WBPT whole-blood passage time
Table 2.
Relationship between WBPT and various clinical parameters
| r | p value | |
|---|---|---|
| Sex (female = 0, male = 1) | 0.10 | 0.10 |
| Age | 0.06 | 0.23 |
| Obesity (no = 0, yes = 1) | 0.31 | <0.001 |
| Body mass index | 0.12 | <0.05 |
| Current smoking (no = 0, yes = 1) | 0.25 | <0.001 |
| Hypertension (no = 0, yes = 1) | 0.24 | <0.001 |
| Systolic blood pressure | 0.02 | 0.68 |
| Diastolic blood pressure | 0.04 | 0.45 |
| White blood cell | 0.16 | <0.01 |
| Red blood cell | 0.17 | <0.01 |
| Hematocrit | 0.20 | <0.001 |
| Platelet | 0.09 | 0.13 |
| Fasting blood glucose | 0.06 | 0.24 |
| Immunoreactive insulin | 0.11 | 0.05 |
| HbA1c | 0.12 | <0.05 |
| HOMA-IR | 0.10 | 0.06 |
| Skin autofluoresence | 0.49 | <0.001 |
| Dyslipidemia | 0.10 | 0.09 |
| Total cholesterol | −0.08 | 0.19 |
| LDL cholesterol | −0.04 | 0.45 |
| Triglyceride | 0.15 | <0.01 |
| HDL cholesterol | 0.09 | 0.13 |
| eGFR | −0.14 | <0.05 |
| log-hs-CRP | 0.12 | <0.05 |
| d-ROMs test | 0.25 | <0.001 |
| CAVI | 0.32 | <0.001 |
| Number of risk factors | 0.45 | <0.001 |
| Sulfonylurea (no = 0, yes = 1) | 0.04 | 0.46 |
| DPP-4 inhibitor (no = 0, yes = 1) | 0.06 | 0.24 |
| Insulin (no = 0, yes = 1) | 0.07 | 0.23 |
| Statin (no = 0, yes = 1) | −0.09 | 0.12 |
| RAS inhibitor (no = 0, yes = 1) | −0.08 | 0.18 |
| Eicosapentaenoic acid (no = 0, yes = 1) | −0.02 | 0.76 |
r expresses the correlation coefficient between WBPT and clinical parameters. Abbreviations as in Table 1
Multiple regression analysis for WBPT as a subordinate factor was performed with explanatory variables with significance in univariate analysis. Skin autofluorescence, number of risk factors, current smoking, d-ROMs test, CAVI, and hematocrit were selected as independent variables (Table 3).
Table 3.
Multiple regression analysis for WBPT
| Explanatory factor | β | t value | p value |
|---|---|---|---|
| Skin autofluoresence | 0.47 | 9.1 | <0.001 |
| Number of risk factors | 0.25 | 3.6 | <0.001 |
| Current smoking | 0.17 | 3.0 | <0.001 |
| d-ROMs test | 0.15 | 2.5 | <0.01 |
| CAVI | 0.13 | 2.3 | <0.05 |
| Hematocrit | 0.11 | 2.1 | <0.05 |
| Obesity | 0.09 | 1.8 | 0.06 |
| log-hs-CRP | 0.09 | 1.7 | 0.07 |
| White blood cell | 0.06 | 1.3 | 0.21 |
| Hypertension | 0.06 | 1.2 | 0.24 |
| Red blood cell | 0.03 | 0.8 | 0.56 |
| eGFR | −0.01 | −0.3 | 0.87 |
| HbA1c | 0.01 | 0.3 | 0.88 |
| Triglyceride | −0.01 | −0.2 | 0.90 |
R 2 = 0.44, F value = 24.9, p < 0.001, (n = 294). Abbreviations as in Table 1
Discussion
A recent Japanese clinical study using MC-FAN indicated that the mean WBPT values in patients without diabetes mellitus and in healthy control subjects were 39.9 and 37.1 s, respectively [19, 20]. The mean WBPT of the patients in the present study was 61.3 s, indicating that blood rheology was impaired in patients with type 2 diabetes mellitus.
High blood glucose concentrations have been reported to cause impaired blood rheology via several pathways [21, 22]. However, the present study found no association between fasting blood glucose levels and WBPT, which is in accordance with the results of other clinical studies using MC-FAN. HbA1c levels showed no significant relationship with WBPT on multivariate analysis, even though the result of single regression analysis was significant in patients with type 2 diabetes mellitus. On the other hand, skin autofluorescence levels, acting as a marker of AGEs in vivo, were selected as the strongest variable for WBPT elevation as a subordinate factor. Estimation of hemorheology by MC-FAN is an in vitro method that uses artificial blood vessels with a lumen width of 7 μm and a depth of 4.5 μm, similar to the dimensions of a capillary. Meerwaldt et al. reported that the level of pentosidine, which is a major component of AGEs, measured at the volar side of the lower arm by skin biopsy was correlated with skin autofluorescence [23]. Capillary vessels are present in the area of skin biopsy. Therefore, a significant relation between skin autofluorescence and WBPT may reflect the accumulation of AGEs in tissues through impairment of blood rheology within the capillaries. On the other hand, some basic studies have reported that AGEs influence hemorheology through mechanisms such as leukocyte-endothelial interaction or platelet aggregation [24, 25]. Furthermore, skin autofluorescence has been reported to be correlated with micro- and macrovascular damage in patients with type 2 diabetes mellitus [11]. Thus, the results of this and previous studies indicate that AGEs and impairment of hemorheology are associated with each other not only on the volar side of the lower arm but also in systemic arterial vessel walls, consequently causing cardiovascular events. Yamagishi et al. reported that AGEs were a marker that is compatible with the theory of “hyperglycemic memory” [26]. Genevieve et al. examined the relation between skin autofluorescence and HbA1c [27]. They measured HbA1c levels every 6 months and reported that the skin autofluorescence level was significantly related to the means of the last five and ten HbA1c values. Thus, the results of this and previous studies indicate that we should perform long-term glucose control to improve the hemorheology.
Some researchers have reported that high hematocrit levels increase the risk of diabetes or diabetic complications [28]. Higher hematocrit levels also decrease blood flow by increasing the whole-blood viscosity, causing skeletal muscle and pancreatic beta cell dysfunction as well as vascular events. In the present study, hematocrit levels were selected as independent variables for WBPT in both controls and patients with type 2 diabetes mellitus. Other reports using MC-FAN have indicated a significant relationship between hematocrit and WBPT [19]. Therefore, hematocrit is an important factor in hemorheology, and it can be evaluated by MC-FAN.
A number of studies have indicated that oxidative stress contributes to the development of diabetic complications [29, 30]. The results of the present study indicate a significant relationship between oxidative stress and WBPT in patients with type 2 diabetes mellitus. Several mechanisms cause impairment of blood rheology via oxidative stress, such as platelet aggregation and elevation of plasma viscosity [31, 32]. In contrast, antidiabetic, antihypertensive, and antihyperlipidemic drugs have been reported to decrease oxidative stress in vivo [33–35]. Some researchers have reported the clinical usefulness of these drugs in improving hemorheology [36–38]. The results of the present cross-sectional study indicate no correlation between medication and WBPT. However, interventional studies are warranted to examine the effectiveness of medications on oxidative stress and WBPT. We expect to discover new applications of antidiabetic, antihypertensive, and antihyperlipidemic drugs for the prevention of cardiovascular disease in patients with type 2 diabetes.
Arterial dysfunction, such as arterial stiffness, and endothelial dysfunction are reported to have important roles in the progression of atherosclerosis. In this context, CAVI is a novel marker of arterial function [18, 39, 40]. Takahashi et al. reported the effects on CAVI of beraprost sodium, a prostaglandin I2 analogue that has a potent vasodilating effect, and compared its effects on CAVI with its effects on brachial ankle pulse wave velocity (baPWV) [39]. They concluded that beraprost sodium did not decrease blood pressure but decreased CAVI, whereas baPWV remained unchanged. CAVI is a parameter for determining arterial stiffness that is relatively independent of blood pressure levels compared with baPWV. There are few reports on the relation between blood rheology and arterial stiffness in patients with diabetes mellitus. Recently, Li et al. reported a relationship between whole-blood fluidity and stiffness of the aortic artery using baPWV in patients with type 2 diabetes mellitus [41]. The results of this study indicated that hemorheology may affect the stiffness of the aortic artery independently of blood pressure levels. Some studies have reported a relationship between endothelial dysfunction and unfavorable blood rheology [20]. On the other hand, Endo et al. reported that CAVI reflected endothelial dysfunction, which is estimated by brachial artery flow-mediated vasodilatation [40]. Thus, a significant relationship between WBPT and CAVI in patients with type 2 diabetes mellitus may be partly explained by endothelial function.
It is well known that smoking habits are one of the most important cardiovascular risk factors worldwide. Recently, Shimada et al. reported a relationship between hemorheology, estimated by MC-FAN, and tobacco consumption or smoking cessation in patients. They reported that WBPT was significantly positively correlated with daily consumption of tobacco in multivariate analysis and that 3 months of smoking cessation significantly reduced WBPT [42]. The results of the present study also indicated that current smoking was an independent factor determining WBPT in patients with type 2 diabetes mellitus. Smoking is also known to affect oxidative stress and AGE formation. Thus, in patients with type 2 diabetes mellitus, smoking is considered to have a stronger influence on hemorheology than do fasting blood glucose and HbA1c levels.
Patients with just one cardiovascular risk factor will not always have a cardiovascular event. Patients with multiple risk factors are most likely to have a problem [43]. In the present study, clustering of classical cardiovascular risk factors, such as hypertension, dyslipidemia, smoking, and obesity, was significantly correlated with WBPT, even though each parameter was weakly correlated with WBPT. MC-FAN is a simple method that uses artificial blood vessels. Estimation of WBPT by MC-FAN is expected to increase the reliability of prediction of cardiovascular disease in patients with type 2 diabetes mellitus.
This study has several limitations. First, the medical treatments for diabetes mellitus, hypertension, and dyslipidemia may have influenced the study results. Second, HOMA-IR has limitations as a marker of insulin resistance, especially in patients with high blood glucose levels. This study included a substantial number of patients with type 2 diabetes mellitus who had high fasting blood glucose levels. Therefore, additional studies using some other accurate marker of insulin resistance, such as a glucose clamp test, are warranted to evaluate the putative relationship between insulin resistance and hemorheology in patients with type 2 diabetes mellitus. Third, several parameters, such as current smoking, hematocrit, d-ROMs test results, and CAVI, were weakly correlated with WBPT, even though these parameters were selected as independent variables for WBPT by multivariate analysis. Finally, data on cardiovascular disease are lacking in this study. Further studies are needed to clarify the relation of WBPT and cardiovascular disease and to examine the significance of WBPT as a predictor of cardiovascular events in patients with type 2 diabetes mellitus.
In conclusion, the present hemorheological study using MC-FAN indicated that novel risk factors for cardiovascular disease, such as AGEs, in vivo oxidative stress, and CAVI, and clustering of classical cardiovascular risk factors are more important in determining the impairment of hemorheology than are fasting blood glucose and HbA1c levels in patients with type 2 diabetes mellitus.
Conflict of interest
None.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients before they were included in the study.
References
- 1.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007;115:2119–2127. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo K, Ueda Y, Nishio M, Hirata A, Asai M, Nemoto T, et al. Thrombogenic potential of whole blood is higher in patients with acute coronary syndrome than in patients with stable coronary diseases. Thromb Res. 2011;128:268–273. doi: 10.1016/j.thromres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Demirtunc R, Duman D, Basar M, Bilgi M, Teomete M, Garip T. The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complicat. 2009;23:89–94. doi: 10.1016/j.jdiacomp.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Le Dévéhat C, Vimeux M, Khodabandehlou T. Blood rheology in patients with diabetes mellitus. Clin Hemorheol Microcirc. 2004;30:297–300. [PubMed] [Google Scholar]
- 5.Kikuchi Y, Sato K, Mizuguchi Y. Modified cell flow microchannels in a single-crystal silicon substrate and flow behavior of blood cells. Microvasc Res. 1994;47:126–139. doi: 10.1006/mvre.1994.1008. [DOI] [PubMed] [Google Scholar]
- 6.Kurihara T, Deguchi S, Kato J, Furakawa M, Tsuchiya M, Akimoto M, et al. Impaired blood rheology by remnant-like lipoprotein particles: studies in patients with fatty liver disease. Clin Hemorheol Microcirc. 2001;24:217–225. [PubMed] [Google Scholar]
- 7.Seki K, Sumino H, Nara M, Ishiyama N, Nishino M, Murakami M. Relationships between blood rheology and age, body mass index, blood cell count, fibrinogen, and lipids in healthy subjects. Clin Hemorheol Microcirc. 2006;34:401–410. [PubMed] [Google Scholar]
- 8.Hitsumoto T. Factors affecting impairment of blood rheology in obese subjects. J Cardiol. 2012;60:401–406. doi: 10.1016/j.jjcc.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 10.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, et al. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2-imbalance in insulin-resistant rat aorta. Diabetes. 1999;48:2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- 11.Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, et al. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care. 2006;29:2654–2659. doi: 10.2337/dc05-2173. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Fridewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Hara S, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11:41–50. doi: 10.1007/s10157-006-0453-4. [DOI] [PubMed] [Google Scholar]
- 15.Cearone MR, Belcaro G, Caratelli M, Cornelli U, DeSanctis MT, Incandela L, et al. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–130. [PubMed] [Google Scholar]
- 16.Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, et al. Increased accumulation of skin advanced glycation end-products precedes and correlates with clinical manifestation of diabetic neuropathy. J Am Soc Nephrol. 2005;16:3687–3693. doi: 10.1681/ASN.2005020144. [DOI] [PubMed] [Google Scholar]
- 17.Nomoto K, Yagi M, Arita S, Hamada U, Yonei Y. A survey of fluorescence derived from advanced glycation end products in the skin of Japanese: differences with age and measurement location. Anti Aging Med. 2012;9:119–124. [Google Scholar]
- 18.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI) J Atheroscler Thromb. 2006;13:101–107. doi: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida K, Kimura T, Aoki T, Tsunekawa K, Araki O, Shoho Y, et al. Fasting serum insulin levels and insulin resistance are associated with blood rheology in Japanese young adults without diabetes. J Int Med Res. 2016;44:496–507. doi: 10.1177/0300060515627561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagi H, Sumino H, Aoki T, Tsunekawa K, Araki O, Kimura T, et al. Impaired blood rheology is associated with endothelial dysfunction in patients with coronary risk factors. Clin Hemorheol Microcirc. 2016;62:139–150. doi: 10.3233/CH-151960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bareford D, Jennings PE, Stone PC, Baar S, Barnett AH, Stuart J. Effects of hyperglycaemia and sorbitol accumulation on erythrocyte deformability in diabetes mellitus. J Clin Pathol. 1986;39:722–727. doi: 10.1136/jcp.39.7.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demirtunc R, Duman D, Basar M, Bilgi M, Teomete M, Garip T. The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complicat. 2009;23:89–94. doi: 10.1016/j.jdiacomp.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 24.Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, et al. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101:1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa Y, Suehiro A, Higasa S, Namba M, Kakishita E. Enhancing effect of advanced glycation end products on serotonin-induced platelet aggregation in patients with diabetes mellitus. Thromb Res. 2002;107:319–323. doi: 10.1016/S0049-3848(02)00348-1. [DOI] [PubMed] [Google Scholar]
- 26.Yamagishi S, Nakamura K, Imaizumi T. Advanced glycation end products (AGEs) and diabetic vascular complications. Curr Diabetes Rev. 2005;1:93–106. doi: 10.2174/1573399052952631. [DOI] [PubMed] [Google Scholar]
- 27.Genevieve M, Vivot A, Gonzalez C, Raffaitin C, Barberger-Gateau P, Gin H, et al. Skin autofluorescence is associated with past glycaemic control and complications in type 1 diabetes mellitus. Diabetes Metab. 2013;39:349–354. doi: 10.1016/j.diabet.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Facchini FS, Carantoni M, Jeppesen J, Reaven GM. Hematocrit and hemoglobin are independently related to insulin resistance and compensatory hyperinsulinemia in healthy, non-obese men and women. Metabolism. 1998;47:831–835. doi: 10.1016/S0026-0495(98)90121-4. [DOI] [PubMed] [Google Scholar]
- 29.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. J Int Med Res Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 31.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28:354–362. doi: 10.1093/eurheartj/ehl441. [DOI] [PubMed] [Google Scholar]
- 32.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alam MA, Chowdhury MR, Jain P, Sagor MA, Reza HM. DPP-4 inhibitor sitagliptin prevents inflammation and oxidative stress of heart and kidney in two kidney and one clip (2K1C) rats. Diabetol Metab Syndr. 2015;7:107. doi: 10.1186/s13098-015-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lodovici M, Bigagli E, Tarantini F, Di Serio C, Raimondi L. Losartan reduces oxidative damage to renal DNA and conserves plasma antioxidant capacity in diabetic rats. Exp Biol Med (Maywood). 2015;240:1500–1504. doi: 10.1177/1535370215570826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endo K, Miyashita Y, Sasaki H, Ebisuno M, Ohira M, Saiki A, et al. Probucol and atorvastatin decrease urinary 8-hydroxy-2′-deoxyguanosine in patients with diabetes and hypercholesterolemia. J Atheroscler Thromb. 2006;13:68–75. doi: 10.5551/jat.13.68. [DOI] [PubMed] [Google Scholar]
- 36.Ott C, Raff U, Schmidt S, Kistner I, Friedrich S, Bramlage P, Harazny JM, et al. Effects of saxagliptin on early microvascular changes in patients with type 2 diabetes. Cardiovasc Diabetol. 2014;13:19. doi: 10.1186/1475-2840-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada K, Hirayama T, Hasegawa Y. Antiplatelet effect of losartan and telmisartan in patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2007;16:225–231. doi: 10.1016/j.jstrokecerebrovasdis.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 38.van der Loo B, Spring S, Koppensteiner R. High-dose atorvastatin treatment in patients with peripheral arterial disease: effects on platelet aggregation, blood rheology and plasma homocysteine. Clin Hemorheol Microcirc. 2011;47:241–251. doi: 10.3233/CH-2011-1386. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi M, Shiba T, Hirano K, Hitsumoto T, Shirai K. Acute decrease of cardio-ankle vascular index with the administration of beraprost sodium. J Atheroscler Thromb. 2012;19:479–484. doi: 10.5551/jat.9266. [DOI] [PubMed] [Google Scholar]
- 40.Endo K, Saiki A, Ohira M, Miyashita Y, Shirai K. Cardio-ankle vascular index may reflect endothelial function in type 2 diabetes. Int J Clin Pract. 2011;65:1200–1201. doi: 10.1111/j.1742-1241.2011.02741.x. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Tian XX, Liu T, Wang RT. Association between whole blood viscosity and arterial stiffness in patients with type 2 diabetes mellitus. Endocrine. 2015;49:148–154. doi: 10.1007/s12020-014-0451-3. [DOI] [PubMed] [Google Scholar]
- 42.Shimada S, Hasegawa K, Wada H, Terashima S, Satoh-Asahara N, Yamakage H, et al. High blood viscosity is closely associated with cigarette smoking and markedly reduced by smoking cessation. Circ J. 2011;75:185–189. doi: 10.1253/circj.CJ-10-0335. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura Y, Yamamoto T, Okamura T, Kadowaki T, Hayakawa T, Kita Y, et al. Combined cardiovascular risk factors and outcome: NIPPON DATA80, 1980–1994. Circ J. 2006;70:960–964. doi: 10.1253/circj.70.960. [DOI] [PubMed] [Google Scholar]