Abstract
Pancreatic islet dysfunction, including impaired insulin secretion in β cells and dysregulated glucagon secretion in α cells, is the chief pathology of diabetes. In β cells, oxidative stress, evoked by chronic hyperglycemia, was found to induce dysfunction of a critical transcription factor, PDX1, caused by its nucleocytoplasmic translocation via interactions with the insulin and JNK signaling pathways and another transcription factor, FOXO1. The significance of α-cell insulin signaling in the physiological and pathological regulation of α-cell biology was demonstrated in α-cell-specific insulin receptor knockout mice, which exhibited dysregulated glucagon secretion. Moreover, a high-glucose load directly induced excessive glucagon secretion in a glucagon-secreting cell line and isolated islets, together with impairment of insulin signaling. These findings indicate that disordered insulin signaling is central to the pathophysiology of islet dysfunction in both α and β cells. On the other hand, certain beneficial effects of GLP-1 on dysfunctional α and β cells indicate that it has therapeutic potential for diabetes patients who exhibit insulin resistance in islets. These studies, involving basic medical research approaches, have—at least in part—clarified the molecular mechanisms underlying α- and β-cell dysfunction in diabetes, and offer important clues that should aid the development of future therapeutic approaches to the disease.
Keywords: Islets, Insulin, Glucagon, Oxidative stress, Insulin signaling, Incretin
Introduction
Pancreatic islet dysfunction has been widely accepted as the chief etiology of both type 1 and type 2 diabetes mellitus, together with systemic insulin resistance. It is therefore essential to clarify the underlying mechanisms of islet dysfunction to understand the pathophysiology of metabolic disorders in diabetes as well as to aid the development of future therapeutic approaches. Pancreatic islets control glucose homeostasis through two main glucoregulatory hormones, namely insulin and glucagon, secreted by the pancreatic β and α cells, respectively. During hyperglycemia, such as in the postprandial state, insulin secretion is increased while glucagon secretion is suppressed, leading to a lowering of the blood glucose level via enhanced hepatic and adipose tissue glucose uptake and suppressed hepatic glucose output. In contrast, during hypoglycemia, such as that during starvation, glucagon secretion is promoted while insulin secretion is reduced; the blood glucose level is found to be elevated by the effects of glucagon, including an enhanced hepatic glucose output and glucose production through the breakdown of lipids and proteins, thereby providing critical energy for the central nervous system. Thus, the balance between glucagon and insulin determines systemic glucose homeostasis. However, in the diabetic state, insufficient insulin secretion and production in β cells together with dysregulated glucagon secretion by α cells appear to significantly affect glycemic disorders [1]. Impairment of increased insulin secretion leads to postprandial hyperglycemia through the incomplete suppression of hepatic glucose output. In addition, abnormally elevated glucagon levels due to a lack of suppression further worsen hyperglycemia via excessive hepatic glucose output. On the other hand, a defective response of glucagon to hypoglycemia frequently increases the incidence of hypoglycemia, together with the excessive insulin effect induced by various insulin-increasing therapies [2]. Therefore, dysregulation of both insulin and glucagon secretion forms the basis for the two primary disorders in diabetes, namely hyperglycemia and hypoglycemia. These observations prompted us to explore the molecular mechanisms underlying α- and β-cell dysfunction in diabetes to facilitate the development of an overall therapeutic approach to diabetes.
Molecular mechanisms underlying pancreatic β-cell dysfunction
First, we focused on the possible mechanisms underlying the pancreatic β-cell dysfunction in diabetes. In β cells, under the chronic hyperglycemic condition associated with diabetes, both insulin secretion and biosynthesis are impaired [3]. In addition, the decrease in β-cell mass due to increased apoptosis, impaired proliferation, and possibly dedifferentiation also plays an important part in the pathophysiology. As a common factor involved in both these dysfunctions, we focused on a β-cell specific transcription factor, PDX1 (pancreatic and duodenal homeobox 1), which is important in the pancreas and β-cell development and differentiation, together with the controlling functions of mature β cells, including the expression of insulin [4]. On the other hand, oxidative stress that is induced by excessive glucose has been revealed to be implicated in the progression of β-cell dysfunction in type 2 diabetes [5–12]. Under diabetic conditions, the levels of reactive oxygen species (ROS) increase in many tissues and organs, including β cells, through the activation of the mitochondrial electron transport chain [13] or the acceleration of glycation reactions [5, 7], which causes various forms of tissue damage in patients with diabetes [14]. Interestingly, while oxidative stress has been shown to induce PDX1 dysfunction [7], antioxidant treatment in animal models for type 2 diabetes has been found to retrieve PDX1 expression in β cells [8–10].
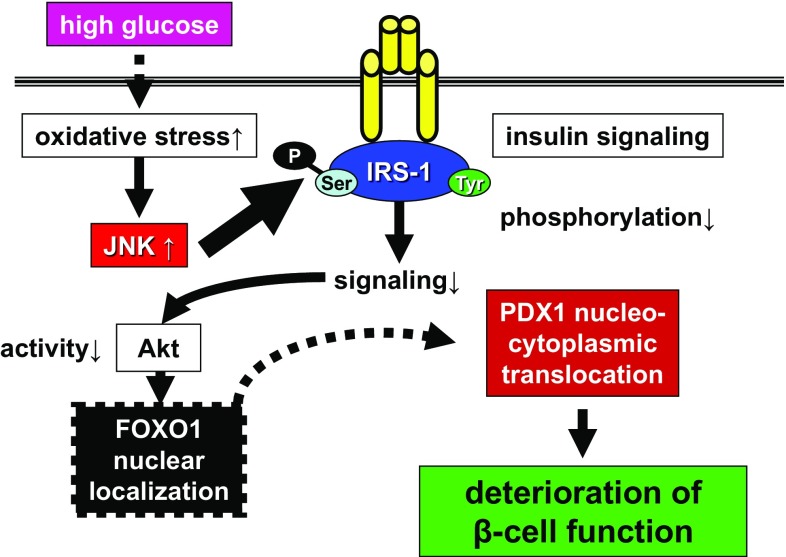
Therefore, we further explored the molecular mechanism of the oxidative stress-induced deterioration of PDX1 function by targeting its intracellular localization [15]. Immunocytochemistry of endogenous PDX1 and exogenously introduced green fluorescent protein (GFP)-tagged PDX1 showed that PDX1 translocated from the nuclei into the cytoplasm of the β-cell line, HIT-T15, in response to the oxidative stress. In contrast, a dominant negative form of a stress-signaling kinase, c-Jun N-terminal kinase (JNK), inhibited the oxidative stress-induced PDX1 translocation, suggesting an essential role for this stress-transmitting pathway. Through deletion studies of GFP-tagged PDX1 fragments, we identified a classical leucine-rich nuclear export signal (NES) in the mouse PDX1 protein. These findings revealed a novel mechanism that negatively regulates PDX1 functions by oxidative stress, leading to greater understanding of β-cell dysfunction in diabetes.
Since oxidative stress and the activation of the JNK pathway induce nucleocytoplasmic translocation of PDX1, we sought to explore the missing link that mediates the phenomenon between the JNK pathway and PDX1. We focused on another important transcription factor, FOXO1 (forkhead box O1), which is reported to counterlocalize PDX1 [16], and found that FOXO1 mediates between the JNK pathway and PDX1 [17]. FOXO1 was found to change its intracellular localization from the cytoplasm to the nucleus in HIT-T15 cells under an oxidative stress load. Oxidative stress impaired insulin signaling activity, represented by the phosphorylation of Akt, which in turn critically controlled the intracellular localization of FOXO1 by direct phosphorylation [18]. The overexpression of JNK also induced the nuclear localization of FOXO1, while the suppression of JNK reduced the oxidative stress-induced nuclear localization of FOXO1. Furthermore, adenovirus-mediated FOXO1 overexpression reduced the nuclear expression of PDX1, whereas siRNA-mediated knockdown of FOXO1 retained the nuclear PDX1 under oxidative stress. Based on these findings, we clarified that the JNK-Akt-FOXO1-PDX1 axis was pertinent to the impairment of PDX1 function, and that this pathway may explain, at least in part, the molecular mechanisms involved in β-cell dysfunction in diabetes (Fig. 1). These data also highlight the pathophysiological significance of the JNK pathway in diabetes and necessitate further studies targeting the signaling [19, 20].
Fig. 1.
Molecular model underlying impaired PDX1 function in β cells under diabetes. Oxidative stress induced by chronic hyperglycemia upregulates JNK signaling in β cells. JNK impairs insulin signaling through the increase in IRS-1 serine phosphorylation and decrease in functioning tyrosine phosphorylation. Impaired Akt activity fails to phosphorylate FOXO1, leading to its nuclear localization. Nuclear-localized FOXO1 induces the nucleocytoplasmic translocation of PDX1, which eventually results in the deterioration of PDX1 function and β-cell function. IRS insulin receptor substrate
Significance of glucagon in energy homeostasis and its regulation in secretion
Recent advances highlight the significance of glucagon and the pancreatic endocrine α cells in the pathophysiology of diabetes, as well as in the maintenance of islet attributes [2]. Glucagon, which is secreted by the pancreatic α cells, acts predominantly in the liver to promote hepatic glycogenolysis and gluconeogenesis, and simultaneously inhibits glycolysis and glycogenesis [21, 22]. Thus, glucagon that is released in response to hypoglycemia works as a counter-regulatory hormone to restore the appropriate blood glucose level. Since insulin suppresses hepatic output while enhancing hepatic glucose uptake and glycogenesis, the balance between these two hormones in the hepatocyte determines the hepatic glucose metabolism. In addition to its action on liver, glucagon affects several metabolic organs, including adipose tissue, that together favor the maintenance of systemic energy homeostasis. Taken together, these findings indicate that the essential functions of glucagon are not limited to the counteraction of hypoglycemia; glucagon serves to provide energy to the whole body, including to the central nervous system and skeletal muscles, by mobilizing glucose from energy storage tissues, liver, and adipose tissues in demanding situations such as starvation, exercise, and crisis.
The secretion of glucagon from α cells is tightly regulated by a large number of factors, demonstrating the physiological importance of glucagon. In general, it is believed that the secretion of glucagon is stimulated in response to hypoglycemia and suppressed by hyperglycemia in vivo [23]. However, whether α cells can directly sense the extracellular glucose concentration in order to promptly respond by inducing glucagon secretion remains unclear [24–28]. Therefore, the regulation of glucagon secretion is not determined by blood glucose levels alone; additional control by other factors, including the neural and endocrine systems and intraislet interactions, is likely. Several lines of evidence indicate a significant contribution of nutrient-independent mechanisms to the regulation of glucagon secretion. The central nervous system is reported to sense the ambient glucose level via the hypothalamus [29–31] and to modulate the secretion of the islet hormones via the autonomic nervous system [32, 33]. The incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are known to affect glucagon secretion [23]. GLP-1 suppresses glucagon secretion while GIP enhances glucagon secretion in hypoglycemia despite stimulating insulin secretion from the β cells in a manner similar to that of GLP-1 [34–36].
Intraislet regulation of glucagon secretion by insulin
In addition to glucose, various regulatory mechanisms for glucagon secretion have been uncovered. Among these, intraislet regulation by the secretory products from the neighboring β cells has been suggested to play a critical role in the physiological tuning of glucagon secretion from α cells. This concept is supported, at least in rodents, by the anatomical characteristics of the pancreatic islets, wherein intercellular crosstalk between different islet cell types is predicted to occur through the intraislet auto-/paracrine effects of their secretions. In particular, as α cells are closely aligned with β cells [37], α cells are putative direct targets of the secretory products, such as insulin, from β cells. Indeed, various β-cell secretions including insulin [38–41], GABA [42, 43], and zinc ions [44, 45] have been shown to affect glucagon secretion from α cells, mainly through suppression.
Insulin, the major secretory product of β cells, has been proposed to be one of the intraislet paracrine factors that can modulate the secretion of glucagon from neighboring α cells. Furthermore, various components of the insulin signaling pathway, such as the insulin receptor, are abundantly expressed in α cells, suggesting an important role for insulin signaling in α cells [26, 46, 47]. Historically, exogenous insulin has been reported to suppress glucagon secretion in vivo [38, 40, 48], whereby a direct effect of insulin on the modulation of glucagon secretion has been suggested. In addition, insulin is reported to stimulate glucagon secretion through a “switch-off” mechanism. During hypoglycemia, a decrease in intraislet insulin concentration may act as a trigger for glucagon secretion when the α cells sense a decrease in the ambient insulin level. This is supported by studies wherein cessation of insulin administration induced glucagon secretion in response to hypoglycemia in pancreatic perfusion experiments in insulinopenic diabetic rats [49, 50].
While these reports indicate a pivotal role for insulin in the regulation of glucagon secretion, direct molecular evidence that accredits insulin signaling in α cells in vivo has been lacking. We provided direct genetic in vivo evidence for the significance of insulin signaling in α cells in the regulation of glucagon secretion by generating and investigating the same in α-cell specific insulin receptor knockout (αIRKO) mice [41]. The αIRKO mice exhibited glucose intolerance, hyperglycemia, and hyperglucagonemia in the fed state, along with enhanced glucagon secretion in response to l-arginine. These results indicated that disruption of the insulin receptor in α cells enhanced glucagon secretion by diminishing the glucagonostatic effect of insulin, thereby providing direct in vivo evidence for the suppression of glucagon secretion by insulin from β cells via an intraislet paracrine mechanism. Interestingly, the mutant mice also displayed a blunted glucagon response to hypoglycemia, indicating that the disruption of insulin signaling in α cells impaired the insulin “switch-off” mechanism, which gives a defective glucagon response [49, 50]. The results from the experiments with the αIRKO mice clearly demonstrate a critical role for insulin in the regulation of α-cell function in both normo- and hypoglycemic states in vivo [51].
Model for the intraislet regulation of glucagon secretion from α cells by insulin
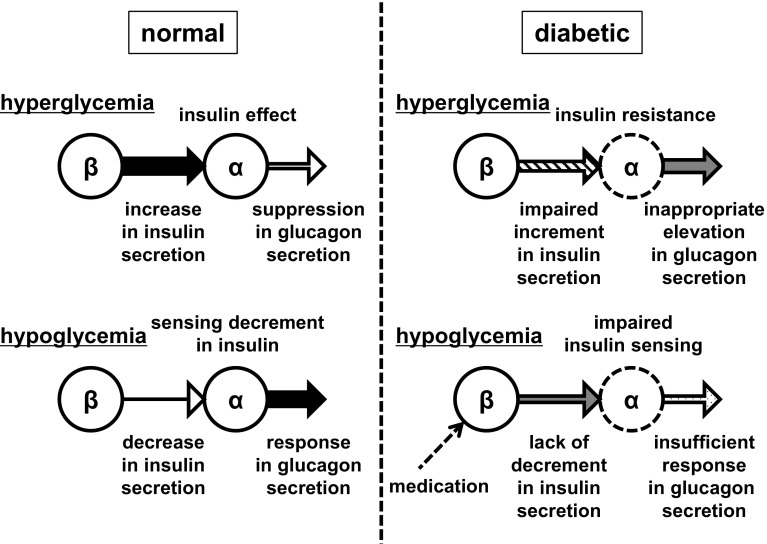
From these findings, a model for the intraislet regulation of glucagon secretion by insulin has been proposed. In states of hyperglycemia, β cells are induced to secrete greater amounts of insulin, which activate insulin signaling in the α cells via a paracrine mechanism and, in turn, repress glucagon secretion. Conversely, in the hypoglycemic state, the reduction in the ambient level of insulin is sensed by the α cells, leading to the inactivation of insulin signaling and the subsequent stimulation of glucagon secretion (Fig. 2). This occurs in addition to the direct stimulation that is possible with low glucose levels. This proposed mechanism has been reported to be feasible in humans by a clinical study [52]. Thus, it is conceivable that chronic and postprandial hyperglucagonemia, observed in diabetes patients, is partly due to a lack of direct suppression of glucagon secretion by insulin, induced either by an absolute shortage of insulin and/or α-cell insulin resistance [53, 54]. It is also possible that the defective secretory response of glucagon to hypoglycemia occurs secondary to a defect in insulin sensing in α cells in diabetes patients (Fig. 2). Thus, insulin can be considered a central player not only in the suppression of glucagon secretion but also in the stimulation of glucagon secretion.
Fig. 2.
Model for the intraislet regulation of glucagon secretion from α cells by insulin. Normal state: in hyperglycemia, increased insulin secretion from β cells acts on α cells to suppress glucagon secretion; in hypoglycemia, decreased insulin secretion from β cells is sensed by α cells, which increases glucagon secretion in response. Diabetic state: in hyperglycemia, the lack of suppression of glucagon secretion due to impaired insulin secretion from β cells and insulin resistance in α cells leads to an inappropriate elevation of glucagon; in hypoglycemia, the lack of decrease in insulin secretion from β cells, in addition to the insulin insensitivity of α cells, results in insufficient glucagon secretion in response
Molecular mechanisms underlying pancreatic α-cell dysfunction
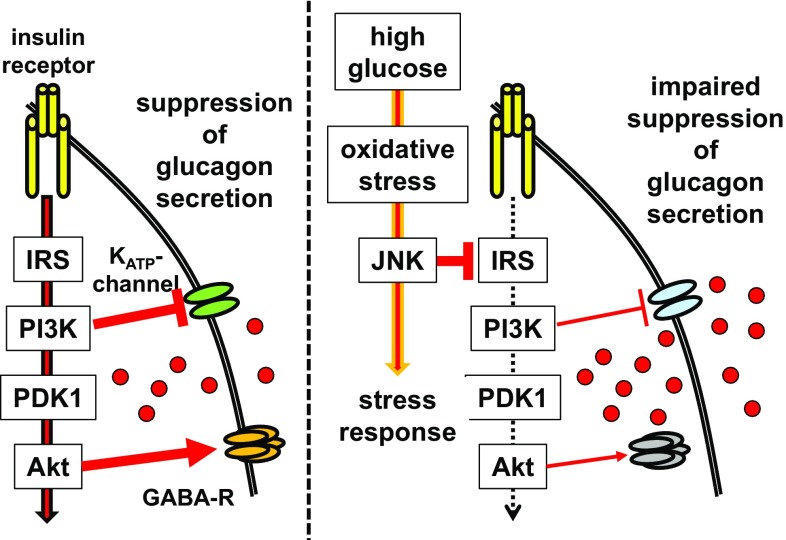
Insulin has been reported to directly suppress glucagon secretion by either (1) reducing the sensitivity of channels [26], which regulate the glucagon secretion machinery via phosphatidylinositol 3-kinase (PI3K) [55], or (2) modulating Akt, a critical downstream effector of PI3K, leading to the recruitment of the GABA-A receptor to the cellular membrane, thereby allowing its ligand, GABA, to inhibit glucagon secretion [42, 43]. Indeed, the extreme reduction in intraislet insulin caused by the destruction of β cells in streptozotocin (STZ)-treated mice resulted in massive hyperglucagonemia under severe hyperglycemia, again hinting at the significance of insulin in the suppression of glucagon secretion in the hyperglycemic state [56]. Surprisingly, in STZ-treated hypoinsulinemic hyperglycemic mice, normalization of hyperglycemia by phloridzin treatment decreased the plasma glucagon level to those found in normoglycemic untreated mice. These findings suggest that, similar to the in vitro observations [26–28], hyperglycemia itself stimulates glucagon secretion from α cells in vivo in the absence of insulin, and that the intraislet effect of insulin plays a central role in the physiological suppression of glucagon by high glucose [54, 57].
These findings also suggest an involvement of impaired insulin signaling in α cells in the dysregulation of glucagon secretion in diabetes. Despite extensive investigations of various physiological regulatory mechanisms of glucagon secretion, the mechanism(s) underlying abnormal glucagon secretion in diabetes is (are) still not completely elucidated [2]. Therefore, we next explored the potential molecular mechanisms responsible for the dysregulated glucagon secretion using hamster-derived glucagon-secreting InR1G cells [58]. High-glucose exposure to isolated mouse islets and the InR1G cells induced increased glucagon secretion during high-glucose stimulation, which is a hallmark of diabetes mellitus. This treatment reduced the phosphorylation of Akt, indicating the deterioration of insulin signaling, and simultaneously increased the oxidative stress and JNK activity. These findings, which indicated that the high-glucose treatment abnormally elevated glucagon secretion in these cells by impairing insulin signaling, demonstrated the involvement of the high glucose–oxidative stress–JNK–insulin signaling pathway axis (Fig. 3). These data elucidate, at least partly, the previously unclear mechanism of abnormal glucagon secretion in diabetes.
Fig. 3.
Model of the mechanism underlying excessive glucagon secretion induced by high glucose. Left: Insulin signaling suppresses glucagon secretion by reducing the sensitivity of channels via PI3K, or by recruiting the GABA-A receptor to the cellular membrane via Akt. Right: Insulin signaling is impaired through JNK, which is upregulated by high glucose, and following oxidative stress, which in turn induces stress responses of the cell. Glucagon secretion becomes excessive due to the impaired suppression of its secretion by insulin signaling
Exploring therapeutic approaches targeting dysfunctional islet cells
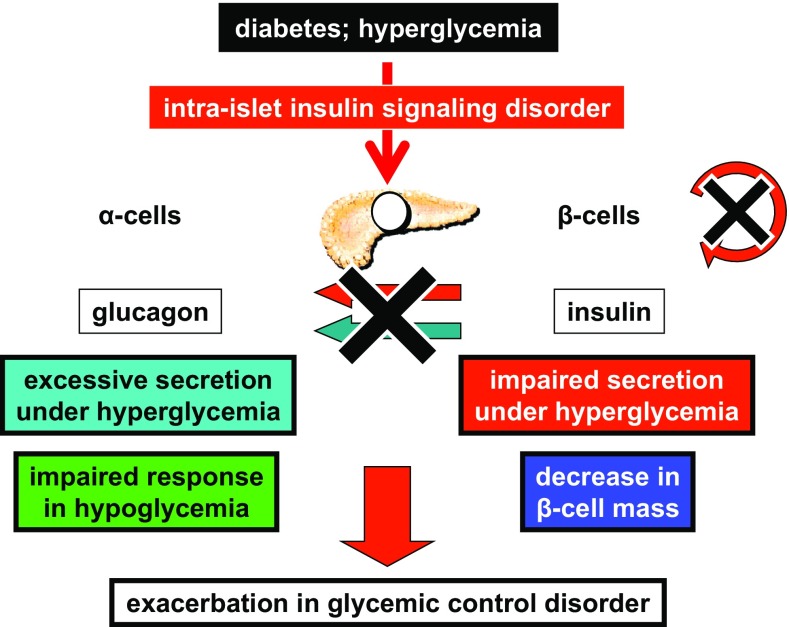
Through the studies that explored the molecular mechanisms underlying α- and β-cell dysfunction, the deterioration in insulin signaling in islets caused by high glucose and oxidative stress was identified as the common responsible element (Fig. 4). In other words, these studies indicate that insulin resistance is not limited to the “classical” insulin target organs but also occurs in pancreatic islets, which are the critical controllers of glucose homeostasis. Therefore, it is important to investigate prospective therapeutic approaches for dysfunctional islet cells from the perspective of future therapies for diabetes.
Fig. 4.
Significance of intraislet insulin signaling in the pathophysiology of both α- and β-cell dysfunction in diabetes. The disordered insulin signaling in islets caused by high glucose and oxidative stress was identified as the common element responsible for both α- and β-cell dysfunction in diabetes
Deterioration in β-cell insulin signaling by chronic hyperglycemia-evoked oxidative stress has been found to induce cellular dysfunction through the impaired functioning of a critical transcription factor, PDX1, consequent to FOXO1 interaction [15, 17]. Indeed, the β-cell-specific insulin receptor knockout (βIRKO) mouse exhibits impaired β-cell function such as glucose-stimulated insulin secretion and a progressive reduction in β-cell mass, both of which are features of the progressive prediabetic state [59], thus indicating the pathophysiological significance of β-cell insulin signaling. On the other hand, novel therapeutic approaches involving the use of GLP-1 analogs and incretin enhancers have emerged as important players in the treatment of type 2 diabetes [60]. Some reports suggest that the effects of GLP-1 on β-cell proliferation and secretory function occur as a consequence of crosstalk with proteins in the insulin signaling pathway and by modulating transcription factors, including PDX1 [61, 62]. Therefore, to investigate the therapeutic potential of incretin-related therapies for dysfunctional β cells, the βIRKO mice were treated with the DPP-4 inhibitor vildagliptin [63]. Treatment of the knockout mice with vildagliptin significantly improved glucose tolerance secondary to enhanced insulin secretion and proliferation of β cells. In molecular studies with a β-cell line lacking functional insulin receptors, treatment with a GLP-1 analog, exendin-4, upregulated Akt phosphorylation. Upregulated protein expression of cyclins A, D1, and E was identified in addition to the upregulation in cyclin D2. These data indicate that GLP-1 signaling can compensate for impaired insulin signaling and enhance β-cell function and proliferation by promoting the cell cycle. Overall, these data verify the potential of GLP-1-related therapies to enhance β-cell proliferation in addition to well-known anti-apoptotic properties, and to promote beneficial outcomes in models with dysfunctional β cells. Indeed, GLP-1 has also been shown to upregulate IGF-1R expression in β cells and to enhance β-cell proliferation by the IGF-2/IGF-1R autocrine loop [64].
Regarding the regulation of β-cell mass, it is widely recognized that β-cell mass is already decreased in type 2 diabetes patients at the time of diagnosis [65], indicating its relevance to the development of diabetes. Therefore, it is important to explore the possible methods of retaining or expanding β-cell mass in vivo. Despite the widely recognized fact that β cells exhibit compensatory hyperplasia in response to systemic insulin resistance, details on the regulation mechanism for β-cell mass, especially for compensatory hyperplasia, have been obscure. Some mechanisms such as glucose metabolism, the insulin and IGF-1 signaling pathways, signals from other organs such as neural signals, adipocytokines, and incretins are reported to regulate β-cell proliferation. Among these, we focused on the interorgan crosstalk between β cells and the liver, one of the most important physiological targets of insulin, and explored the interaction using the liver-specific insulin receptor knockout (LIRKO) mouse model, a unique model that exhibits severe insulin resistance accompanied by dramatic islet hyperplasia [66]. In parabiosis and the transplantation assays, enhanced proliferation of normal β cells was presented under the LIRKO environment, indicating that the hepatocyte-derived humoral factor induced β-cell proliferation in a non-neural and non-cell-autonomous manner. Through these data, serpinB1, a protease inhibitor, was identified as the liver-derived secretory protein that regulates β-cell proliferation [67] and is involved in the compensatory β-cell mass expansion in response to insulin resistance. On the other hand, as the β-cell autonomous mechanisms for compensatory β-cell hyperplasia against insulin resistance, the involvement of cell cycle proteins has been shown to be important [68, 69], hinting at the complementary regulation of β-cell proliferation by external and internal factors.
GLP-1 and related therapies are widely applied to the treatment of diabetes because incretin hormones enhance glucose-stimulated insulin secretion and regulate β-cell mass through the modulation of cellular proliferation and death [60]. In addition to these effects on β cells, GLP-1 is reported to suppress glucagon secretion. One study has indicated that the suppression of glucagon secretion and the augmentation of insulin secretion contribute equally to the glycemic control properties of GLP-1 [70]. Thus, in type 2 diabetes, defects in GLP-1 secretion and activity likely affect the pathophysiology of the disease via the abnormal regulation of both insulin and glucagon secretion [71]. Therefore, to investigate the therapeutic potential of GLP-1 for dysfunctional α cells that display abnormal regulation of glucagon secretion, αIRKO mice were treated with GLP-1 [56]. GLP-1 treatment significantly suppressed plasma glucagon in the αIRKO mice, implying that GLP-1 can suppress glucagon secretion independent of the action of insulin on α cells and therefore pointing to the therapeutic potential of GLP-1 for glucagon suppression in patients with diabetes who exhibit dysfunctional α cells with an impaired response to insulin, which is similar to other organs.
Implications of DPP-4 and incretin therapies for type 1 diabetes patients
The results of the basic islet studies indicate the importance of insulin signaling to both α- and β-cell function, and the therapeutic potential of GLP-1 for dysfunctional islets in type 2 diabetes. Next, we provided the scientific basis for the future application of incretin-based therapies to type 1 diabetes patients who exhibit nonfunctional β cells with dysfunctional persistent α cells, by assessing incretin-degrading dipeptidyl peptidase-4 (DPP-4) enzymatic activity [72]. We examined serum DPP-4 activity in Japanese young-adult type 1 diabetes and in healthy controls, and reported that subjects with type 1 diabetes displayed significantly higher serum DPP-4 activity than healthy controls, indicating the pathophysiological significance of the enzyme in type 1 diabetes. Currently, DPP-4 inhibition is widely used in the treatment of type 2 diabetes, and the application of this strategy is awaited as a new therapeutic approach for type 1 diabetes. The clinical evidence for the influence of DPP-4 on type 1 diabetes should facilitate the development of future therapies for the disease.
Conclusions and future perspectives
Through basic molecular studies that explored the underlying mechanisms of pancreatic endocrine islet dysfunction, we identified a deterioration in insulin signaling induced by chronic high glucose followed by the upregulation of oxidative stress in both α- and β cells. In β cells, oxidative stress induced the dysfunction of PDX1 through its nucleocytoplasmic translocation via interactions with the insulin and JNK signaling pathways and FOXO1. The significance of α-cell insulin signaling in the physiological and pathological regulation of α-cell biology was demonstrated by αIRKO mice with dysregulated glucagon secretion. Besides the impairment of insulin signaling, a high-glucose load induced dysregulation of glucagon secretion. In addition, the use of GLP-1 proved to be beneficial when tested on dysfunctional α and β cells, indicating its therapeutic potential in the treatment of diabetes patients with insulin resistance in islets. These studies explain the molecular mechanisms underlying α- and β-cell dysfunction in diabetes, and provide important guidance towards a future therapeutic approach to the disease.
Previously, glucagon was believed to simply elevate or decline in response to blood glucose levels. Recent data have revealed sophisticated regulatory mechanisms for glucagon secretion, as well as new, wide-ranging roles for α cells. In particular, the concept of the intraislet regulation of glucagon secretion via an insulin-related paracrine mechanism is now widely appreciated as an important pathway that determines α-cell function. Given the significance of insulin resistance in diabetes, it is important to investigate insulin resistance at the level of α cells; possible insulin dysregulation in the diabetic state is likely to be closely connected to the pathophysiology of glucagon dysregulation. α Cells not only receive input signals from various neighboring cells, but they also regulate the functions of other cells by secreting various bioactive substances. The close interactions between islet endocrine cells that occur through paracrine and autocrine crosstalk allow the cells to respond immediately to changes in systemic energy status, as well as to effectively maintain appropriate levels of cellular secretion of various hormones.
Currently, research on glucagon and α cells faces significant challenges pertaining to the accessibility and availability of glucagon assays and α cells in vivo. Indeed, the accuracy of the radioimmunoassay previously used for glucagon evaluation is now being questioned because of cross-reactivity with related substances that share the same amino acid sequence as glucagon [73]. The development of an assay system with improved specificity and sensitivity is needed to progress research in this field. Thus, it is also possible that new knowledge will contradict commonly accepted knowledge concerning glucagon.
Acknowledgements
The contents of this review were presented by the author in the Lilly Award Lecture at the 60th Annual Meeting of the Japan Diabetes Society, Nagoya, Japan. The author sincerely thanks Profs. Iichiro Shimomura, Taka-aki Matsuoka (Osaka University), Rohit N. Kulkarni, C. Ronald Kahn, Edward S. Horton, Gordon C. Weir (Joslin Diabetes Center), Munehide Matsuhisa (Tokushima University), and Hideaki Kaneto (Kawasaki Medical School) for their support and mentoring, and also colleagues in the Department of Metabolic Medicine, Graduate School of Medicine, Osaka University, and the Kulkarni Laboratory, Section of Islet Cell and Regenerative Biology, Joslin Diabetes Center for their support. The author is partially supported by JSPS KAKENHI (Grant Number 26461336), by the Ministry of Education, Culture, Sports, Science & Technology in Japan, by fellowship from Manpei Suzuki Diabetes Foundation and JDRF, and by research grants from the Kowa Life Science Foundation, the Banyu Life Science Foundation International, the Suzuken Memorial Foundation, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Japan Diabetes Foundation.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Ethical approval
In the presented animal studies in which the author was involved [41, 56, 63, 64, 66, 68, 69], all protocols were approved by the Institutional Animal Care and Use Committee of the Joslin Diabetes Center, MA, USA, and were in accordance with NIH guidelines. The presented clinical study [72] was approved by the local ethics committee of the Osaka University Hospital, Osaka, Japan (#14001), and was conducted in accordance with the principles of the Helsinki Declaration.
Informed consent
All patients and healthy volunteers provided written informed consent.
References
- 1.Unger RH. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism. 1978;27:1691–1709. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- 2.Kawamori D, Welters HJ, Kulkarni RN. Molecular pathways underlying the pathogenesis of pancreatic alpha-cell dysfunction. Adv Exp Med Biol. 2010;654:421–445. doi: 10.1007/978-90-481-3271-3_18. [DOI] [PubMed] [Google Scholar]
- 3.Kaneto H, Kawamori D, Matsuoka TA, Kajimoto Y, Yamasaki Y. Oxidative stress and pancreatic beta-cell dysfunction. Am J Ther. 2005;12:529–533. doi: 10.1097/01.mjt.0000178773.31525.c2. [DOI] [PubMed] [Google Scholar]
- 4.Kaneto H, Matsuoka TA, Miyatsuka T, Kawamori D, Katakami N, Yamasaki Y, et al. PDX-1 functions as a master factor in the pancreas. Front Biosci. 2008;13:6406–6420. doi: 10.2741/3162. [DOI] [PubMed] [Google Scholar]
- 5.Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 6.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 7.Matsuoka T, Kajimoto Y, Watada H, Kaneto H, Kishimoto M, Umayahara Y, et al. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Invest. 1997;99:144–150. doi: 10.1172/JCI119126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, et al. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- 10.Ihara Y, Yamada Y, Toyokuni S, Miyawaki K, Ban N, Adachi T, et al. Antioxidant alpha-tocopherol ameliorates glycemic control of GK rats, a model of type 2 diabetes. FEBS Lett. 2000;473:24–26. doi: 10.1016/S0014-5793(00)01489-7. [DOI] [PubMed] [Google Scholar]
- 11.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, et al. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 12.Gorogawa S, Kajimoto Y, Umayahara Y, Kaneto H, Watada H, Kuroda A, et al. Probucol preserves pancreatic beta-cell function through reduction of oxidative stress in type 2 diabetes. Diabetes Res Clin Pract. 2002;57:1–10. doi: 10.1016/S0168-8227(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 14.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 15.Kawamori D, Kajimoto Y, Kaneto H, Umayahara Y, Fujitani Y, Miyatsuka T, et al. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes. 2003;52:2896–2904. doi: 10.2337/diabetes.52.12.2896. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, 3rd, Wright CV, et al. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, et al. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem. 2006;281:1091–1098. doi: 10.1074/jbc.M508510200. [DOI] [PubMed] [Google Scholar]
- 18.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 20.Nakatani Y, Kaneto H, Kawamori D, Hatazaki M, Miyatsuka T, Matsuoka TA, et al. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem. 2004;279:45803–45809. doi: 10.1074/jbc.M406963200. [DOI] [PubMed] [Google Scholar]
- 21.Exton JH, Jefferson LS Jr, Butcher RW, Park CR. Gluconeogenesis in the perfused liver. The effects of fasting, alloxan diabetes, glucagon, epinephrine, adenosine 3′,5′-monophosphate and insulin. Am J Med. 1966;40:709–15. [DOI] [PubMed]
- 22.Unger RH, Orci L. The role of glucagon in the endogenous hyperglycemia of diabetes mellitus. Annu Rev Med. 1977;28:119–130. doi: 10.1146/annurev.me.28.020177.001003. [DOI] [PubMed] [Google Scholar]
- 23.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 24.Vieira E, Salehi A, Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia. 2007;50:370–379. doi: 10.1007/s00125-006-0511-1. [DOI] [PubMed] [Google Scholar]
- 25.Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes. 2005;54:1789–1797. doi: 10.2337/diabetes.54.6.1789. [DOI] [PubMed] [Google Scholar]
- 26.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- 27.Salehi A, Vieira E, Gylfe E. Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes. 2006;55:2318–2323. doi: 10.2337/db06-0080. [DOI] [PubMed] [Google Scholar]
- 28.Olsen HL, Theander S, Bokvist K, Buschard K, Wollheim CB, Gromada J. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology. 2005;146:4861–4870. doi: 10.1210/en.2005-0800. [DOI] [PubMed] [Google Scholar]
- 29.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes. 1995;44:180–184. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- 30.McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes. 2004;53:1953–1958. doi: 10.2337/diabetes.53.8.1953. [DOI] [PubMed] [Google Scholar]
- 31.Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC, et al. Hypothalamic ATP-sensitive K+ channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes. 2004;53:2542–51. [DOI] [PubMed]
- 32.Gerich JE, Karam JH, Forsham PH. Stimulation of glucagon secretion by epinephrine in man. J Clin Endocrinol Metab. 1973;37:479–481. doi: 10.1210/jcem-37-3-479. [DOI] [PubMed] [Google Scholar]
- 33.Iversen J. Effect of acetyl choline on the secretion of glucagon and insulin from the isolated, perfused canine pancreas. Diabetes. 1973;22:381–387. doi: 10.2337/diab.22.5.381. [DOI] [PubMed] [Google Scholar]
- 34.Pederson RA, Brown JC. Interaction of gastric inhibitory polypeptide, glucose, and arginine on insulin and glucagon secretion from the perfused rat pancreas. Endocrinology. 1978;103:610–615. doi: 10.1210/endo-103-2-610. [DOI] [PubMed] [Google Scholar]
- 35.Meier JJ, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt WE, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46:798–801. doi: 10.1007/s00125-003-1103-y. [DOI] [PubMed] [Google Scholar]
- 36.de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51:2263–2270. doi: 10.1007/s00125-008-1149-y. [DOI] [PubMed] [Google Scholar]
- 37.Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weir GC, Knowlton SD, Atkins RF, McKennan KX, Martin DB. Glucagon secretion from the perfused pancreas of streptozotocin-treated rats. Diabetes. 1976;25:275–282. doi: 10.2337/diab.25.4.275. [DOI] [PubMed] [Google Scholar]
- 39.Asplin CM, Paquette TL, Palmer JP. In vivo inhibition of glucagon secretion by paracrine beta cell activity in man. J Clin Invest. 1981;68:314–318. doi: 10.1172/JCI110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest. 1984;74:2296–2299. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9:350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rorsman P, Berggren PO, Bokvist K, Ericson H, Mohler H, Ostenson CG, et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- 43.Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- 45.Ishihara H, Wollheim CB. Is zinc an intra-islet regulator of glucagon secretion? Diabetol Int. 2016;7:106–110. doi: 10.1007/s13340-016-0259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhathena SJ, Oie HK, Gazdar AF, Voyles NR, Wilkins SD, Recant L. Insulin, glucagon, and somatostatin receptors on cultured cells and clones from rat islet cell tumor. Diabetes. 1982;31:521–531. doi: 10.2337/diab.31.6.521. [DOI] [PubMed] [Google Scholar]
- 47.Patel YC, Amherdt M, Orci L. Quantitative electron microscopic autoradiography of insulin, glucagon, and somatostatin binding sites on islets. Science. 1982;217:1155–1156. doi: 10.1126/science.6126003. [DOI] [PubMed] [Google Scholar]
- 48.Stagner JI, Samols E. Retrograde perfusion as a model for testing the relative effects of glucose versus insulin on the A cell. J Clin Invest. 1986;77:1034–1037. doi: 10.1172/JCI112356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, et al. Regulation of alpha-cell function by the beta-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes. 2004;53:1482–1487. doi: 10.2337/diabetes.53.6.1482. [DOI] [PubMed] [Google Scholar]
- 50.Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP. Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the “switch-off” hypothesis. Diabetes. 2004;53:1488–1495. doi: 10.2337/diabetes.53.6.1488. [DOI] [PubMed] [Google Scholar]
- 51.Kawamori D, Kulkarni RN. Insulin modulation of glucagon secretion: the role of insulin and other factors in the regulation of glucagon secretion. Islets. 2009;1:276–279. doi: 10.4161/isl.1.3.9967. [DOI] [PubMed] [Google Scholar]
- 52.Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes. 2010;59:2936–2940. doi: 10.2337/db10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raju B, Cryer PE. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes. 2005;54:757–764. doi: 10.2337/diabetes.54.3.757. [DOI] [PubMed] [Google Scholar]
- 54.Meier JJ, Kjems LL, Veldhuis JD, Lefebvre P, Butler PC. Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes. 2006;55:1051–1056. doi: 10.2337/diabetes.55.04.06.db05-1449. [DOI] [PubMed] [Google Scholar]
- 55.Leung YM, Ahmed I, Sheu L, Gao X, Hara M, Tsushima RG, et al. Insulin regulates islet alpha-cell function by reducing KATP channel sensitivity to adenosine 5′-triphosphate inhibition. Endocrinology. 2006;147:2155–2162. doi: 10.1210/en.2005-1249. [DOI] [PubMed] [Google Scholar]
- 56.Kawamori D, Akiyama M, Hu J, Hambro B, Kulkarni RN. Growth factor signalling in the regulation of alpha-cell fate. Diabetes Obes Metab. 2011;13(Suppl 1):21–30. doi: 10.1111/j.1463-1326.2011.01442.x. [DOI] [PubMed] [Google Scholar]
- 57.Greenbaum CJ, Havel PJ, Taborsky GJ, Jr, Klaff LJ. Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J Clin Invest. 1991;88:767–773. doi: 10.1172/JCI115375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katsura T, Kawamori D, Aida E, Matsuoka TA, Shimomura I. Glucotoxicity induces abnormal glucagon secretion through impaired insulin signaling in InR1G cells. PLoS One. 2017;12:e0176271. doi: 10.1371/journal.pone.0176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/S0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 60.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–748. doi: 10.2337/diabetes.49.5.741. [DOI] [PubMed] [Google Scholar]
- 62.Habener JF, Stoffers DA. A newly discovered role of transcription factors involved in pancreas development and the pathogenesis of diabetes mellitus. Proc Assoc Am Physicians. 1998;110:12–21. [PubMed] [Google Scholar]
- 63.Kawamori D, Shirakawa J, Liew CW, Hu J, Morioka T, Duttaroy A, et al. GLP-1 signalling compensates for impaired insulin signalling in regulating beta cell proliferation in betaIRKO mice. Diabetologia. 2017. doi:10.1007/s00125-017-4303-6. [DOI] [PMC free article] [PubMed]
- 64.Cornu M, Modi H, Kawamori D, Kulkarni RN, Joffraud M, Thorens B. Glucagon-like peptide-1 increases beta-cell glucose competence and proliferation by translational induction of insulin-like growth factor-1 receptor expression. J Biol Chem. 2010;285:10538–10545. doi: 10.1074/jbc.M109.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 66.El Ouaamari A, Kawamori D, Dirice E, Liew CW, Shadrach JL, Hu J, et al. Liver-derived systemic factors drive beta cell hyperplasia in insulin-resistant states. Cell Rep. 2013;3:401–410. doi: 10.1016/j.celrep.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El Ouaamari A, Dirice E, Gedeon N, Hu J, Zhou JY, Shirakawa J, et al. SerpinB1 promotes pancreatic beta cell proliferation. Cell Metab. 2016;23:194–205. doi: 10.1016/j.cmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Georgia S, Hinault C, Kawamori D, Hu J, Meyer J, Kanji M, et al. Cyclin D2 is essential for the compensatory beta-cell hyperplastic response to insulin resistance in rodents. Diabetes. 2010;59:987–996. doi: 10.2337/db09-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hinault C, Kawamori D, Liew CW, Maier B, Hu J, Keller SR, et al. Delta40 Isoform of p53 controls beta-cell proliferation and glucose homeostasis in mice. Diabetes. 2011;60:1210–1222. doi: 10.2337/db09-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hare KJ, Vilsboll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2010;59:1765–1770. doi: 10.2337/db09-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holst JJ, Vilsboll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297:127–136. doi: 10.1016/j.mce.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 72.Osawa S, Kawamori D, Katakami N, Takahara M, Sakamoto F, Katsura T, et al. Significant elevation of serum dipeptidyl peptidase-4 activity in young-adult type 1 diabetes. Diabetes Res Clin Pract. 2016;113:135–142. doi: 10.1016/j.diabres.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Bak MJ, Albrechtsen NW, Pedersen J, Hartmann B, Christensen M, Vilsboll T, et al. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol. 2014;170:529–538. doi: 10.1530/EJE-13-0941. [DOI] [PubMed] [Google Scholar]