Abstract
In 169 Japanese patients with type 2 diabetes mellitus with blood glucose levels that were inadequately controlled with diet and exercise therapy alone, or with diet and exercise therapy plus a sulfonylurea (SU) drug, we evaluated the safety and efficacy of global standard dose metformin given up to a maximum daily dose of 2250 mg for 54 weeks. The changes in HbA1c from baseline to the final evaluation visit were −1.32 ± 0.76% for metformin monotherapy and −1.29 ± 0.81% for metformin plus SU, both significantly lower than baseline. The incidences of adverse events and adverse drug reactions were 91.1% (154/169 patients) and 67.5% (114/169 patients), respectively. The most common adverse events were gastrointestinal symptoms, and most of the gastrointestinal symptoms were considered by investigators to be related to metformin treatment. An increased blood lactic acid level was observed in three subjects (1.8%); however, no clinical symptoms were reported, and there was no increase in mean lactic acid concentration throughout the evaluation period. Symptoms of hypoglycemia were reported in 16 patients, all receiving metformin plus SU, but none received metformin monotherapy. There was a decrease in mean body weight. Global standard dose metformin may be useful for maintaining good blood glucose control over the long term in the treatment of type 2 diabetes mellitus in Japanese patients.
Keyword: Metformin, Long-term treatment, BMI, Blood lipid level
Introduction
Since the metabolic abnormalities caused by diabetes mellitus can elicit the onset of microangiopathic (microvascular) and macroangiopathic (macrovascular) complications, the early detection and prompt treatment of this disease are vital.
Oral hypoglycemic drugs for the treatment of type 2 diabetes mellitus involve various approaches with the aim of correcting the deficient action of insulin. The biguanides are unique among them, in that their main mechanism of action involves inhibition of gluconeogenesis in the liver. The biguanide metformin hydrochloride (hereunder, metformin) has been used in Japan since 1961. Before April 2009, the approved metformin indication in the Japanese package insert was to use it only for type 2 diabetic patients who do not adequately respond to sulfonylurea therapy. After May 2009, monotherapy has been added to the metformin indication in the package insert. In the 1970s, deaths attributable to lactic acidosis due to the first available biguanide, phenformin hydrochloride, were reported overseas and led to phenformin's withdrawal. On the basis of these reports, restrictions of the use of metformin were instituted in 1977–1978. For a long time now, metformin has been prescribed up to a maximum daily dose of 750 mg in Japan, whereas it has been used at doses of 2550–3000 mg in other countries, including the US, and in Europe. Clinical studies in Western countries have yielded copious evidence showing that metformin has a potent anti-hyperglycemic action equivalent to that of SU without causing hypoglycemia or weight gain [1, 2], and it reduces the risk of onset and progression of diabetes-associated complications [3], etc. Given these results, the algorithm for the treatment of type 2 diabetes mellitus jointly published by the diabetes associations of the US and Europe [4] proposes that if type 2 diabetes mellitus is diagnosed and there are no contraindications such as a severe renal disorder or intolerability, metformin should be prescribed together with lifestyle interventions as first-line treatment, highlighting the key position of metformin in the treatment of diabetes.
Therefore, to establish an appropriate dosing regimen in Japanese individuals, we evaluated the efficacy and safety of metformin (brand name: Metgluco tablets, commercialized by Sumitomo Dainippon Pharma since 2010), given to type 2 diabetes mellitus patients with blood glucose levels that were inadequately controlled with diet and exercise therapy alone, or with diet and exercise therapy plus an SU.
Subjects and methods
This multicenter, open-label, uncontrolled study was conducted at 21 sites across Japan, in compliance with the Ordinance on Standards for the Conduct of Clinical Studies on Drugs (GCP) and other relevant notifications.
The HbA1c was measured as the Japan Diabetes Society (JDS) value and was converted into the National Glycohemoglobin Standardization Program (NGSP) value based on the conversion table [5]. The subjects were patients aged 20 years or older, with type 2 diabetes mellitus (with immediate pre-enrolment HbA1c ≥6.9% and <12.5% [6]) who, before starting the study drug, were being treated with an unchanging regimen of diet and exercise alone, or an unchanging regimen of diet and exercise plus an SU drug at a fixed dose. Patients with impaired renal function (immediate pre-enrollment creatinine level ≥1.3 mg/dl for males and ≥1.2 mg/dl for females), hepatic function (immediate pre-enrollment AST (GOT) or ALT (GPT) ≥ 2.5 times the study site’s upper limit of normal), cardiovascular function, or pulmonary function were excluded.
When the blood lactic acid level exceeded two times the study site’s upper limit of normal, re-examination was conducted as soon as possible. When the blood lactic acid level exceeded two times the study site’s upper limit of normal again, the patient discontinued this study.
Metformin was taken orally, either post-prandially or immediately pre-prandially, at a starting dose of 500 mg/day, titrated gradually to 1500 mg/day taken in two or three divided doses. Patients for whom the investigator concluded that a higher dose was necessary, based on safety and efficacy considerations, received 2250 mg/day. During the treatment period, 1500 mg/day was regarded as the maintenance dose, and the dose could be varied in the range from 750 to 2250 mg/day, depending on the patient’s need and tolerability.
The primary efficacy endpoint was the changes from baseline (before starting the study drug) to the final visit in HbA1c, and the secondary endpoints were the changes of glycoalbumin (GA), fasting plasma glucose (FPG), fasting insulin (IRI), total cholesterol (T-Cho), HDL cholesterol (HDL-Cho), LDL cholesterol (LDL-Cho) and triglycerides (TG), which were calculated in the same manner. The efficacy analysis set was the full analysis set (FAS). Assessments were also made as a secondary endpoint of the percentage of patients achieving HbA1c levels classified as excellent or good (<6.9%; hereunder, treatment goal achievement rate), in accordance with the Guidelines for the Treatment of Diabetes Mellitus.
Summary statistics and two-sided 95% confidence intervals (CI) were calculated for each efficacy endpoint. Hypotheses were not statistically tested in this study, but if the two-sided 95% CI of a change from baseline did not include zero, the difference was regarded as significant at the level of 0.05, two sided, and the change was labeled as “significant” in this report. No multiplicity was adjusted in multipoint measurements.
For safety assessments, the numbers of patients with AEs and ADRs and their incidences, and the numbers of patients with individual AEs and ADRs, were tabulated by event and severity, and their incidences were calculated. Diarrhea, nausea, vomiting, abdominal pain and anorexia are defined as gastrointestinal symptoms. Changes in blood lactic acid levels and body weight were also ascertained.
Results
Disposition of patients and patient characteristics
Among the 174 patients enrolled in this study, 5 did not receive metformin and were excluded, and 169 patients started treatment with metformin, comprising 83 patients on diet and exercise therapy alone (metformin monotherapy group) and 86 on diet and exercise therapy, plus an SU drug (metformin plus SU group). Of the 169 patients who started treatment with metformin, 3 were excluded because they failed to meet the selection criteria and 1 was excluded because of missing post-baseline HbA1c values, leaving 165 patients (80 in the metformin monotherapy group and 85 in the metformin plus SU group) in the FAS. The frequency distribution for the most common doses was as follows: 2 patients in the 500 mg/day group (both in the metformin plus SU group), 9 patients in the 750 mg/day group (3 in the metformin monotherapy group and 6 in the metformin plus SU group; similarly hereunder), 126 in the 1500 mg/day group (62 and 64 patients) and 28 in the 2250 mg/day group (15 and 13 patients). Comparison of the clinical characteristics of the patients in the FAS showed that the disease duration was longer and the percentage of patients aged 65 years or older was higher (elderly patients) in the metformin plus SU group than in the metformin monotherapy group (Table 1). Furthermore, HbA1c, GA, FPG and IRI were all higher in patients in the metformin plus SU group. There were no other major differences in patient characteristics.
Table 1.
Characteristics of the safety population at baseline
| Monotherapy (n = 83) | SU concomitant therapy (n = 86) | Total (n = 169) | |
|---|---|---|---|
| Male/female | 51/32 | 48/38 | 99/70 |
| Age (years) | 58.0 ± 9.2 | 61.3 ± 9.7 | 59.7 ± 9.6 |
| <40 | 1(1.2) | 2(2.3) | 3(1.8) |
| ≥40 to <50 | 16(19.3) | 7(8.1) | 23(13.6) |
| ≥50 to <60 | 26(31.3) | 25(29.1) | 51(30.2) |
| ≥60 to <70 | 34(41.0) | 36(41.9) | 70(41.4) |
| ≥70 | 6(7.2) | 16(18.6) | 22(13.0) |
| <65 | 61(73.5) | 48(55.8) | 109(64.5) |
| ≥65 | 22(26.5) | 38(44.2) | 60(35.5) |
| Diabetes duration (years) | 5.4 ± 6.8 | 8.9 ± 7.2 | 7.2 ± 7.2 |
| BMI (kg/m2) | 25.56 ± 4.11 | 25.07 ± 3.83 | 25.31 ± 3.96 |
| <25 | 42(50.6) | 46(53.5) | 88(52.1) |
| ≥25 | 41(49.4) | 40(46.5) | 81(47.9) |
| HbA1c (NGSP) (%) | 7.72 ± 0.85 | 7.96 ± 0.91 | 7.84 ± 0.88 |
| <7.4 | 34(41.0) | 25(29.1) | 59(34.9) |
| ≥7.4 to <8.4 | 31(37.3) | 36(41.9) | 67(39.6) |
| ≥8.4 <9.4 | 15(18.1) | 19(22.1) | 34(20.1) |
| ≥9.4 to <10.5 | 3(3.6) | 5(5.8) | 8(4.7) |
| ≥10.5 to <11.5 | 0(0.0) | 0(0.0) | 0(0.0) |
| ≥11.5 | 0(0.0) | 1(1.2) | 1(0.6) |
| GA (%) | 21.73 ± 3.65 | 23.29 ± 3.96 | 22.52 ± 3.88 |
| FPG (mg/dl) | 148.8 ± 33.6 | 161.1 ± 48.7 | 155.1 ± 42.3 |
| IRI (μU/ml) | 7.23 ± 4.40 | 7.87 ± 5.93 | 7.56 ± 5.23 |
Data are mean ± standard deviation or n (%)
BMI body mass index, HbA1c hemoglobin A1c, NGSP National Glycohemoglobin Standardization Program, GA glycoalbumin, FPG fasting plasma glucose, IRI immunoreactive insulin, SU sulfonylurea
The safety analysis set consisted of all 169 patients who received treatment with metformin (83 and 86 patients), with more than 80% of patients receiving treatment for ≥50 weeks. A maximum dose of 1500 mg/day was taken by 120 patients (60 and 60 patients), and 2250 mg/day was taken by 43 patients (22 and 21 patients). Thirty-eight patients (16 and 22 patients) changed from post-prandial to immediately pre-prandial dosing during the treatment period, and 131 patients (67 and 64 patients) maintained post-prandial dosing throughout the treatment period. The distributions of patients in the safety analysis set and the FAS were almost identical.
Efficacy
HbA1c
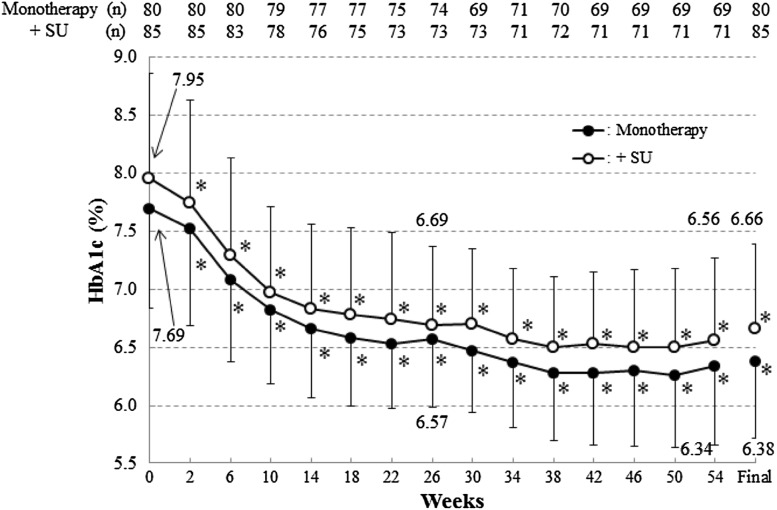
The time profile of HbA1c levels is shown in Fig. 1.
Fig. 1.
Mean HbA1c values during treatment. SD values are indicated by bars. *p < 0.05, change from baseline (no multiplicity adjustment)
In the FAS, good blood glucose control was maintained over the 54-week period, with HbA1c 7.42 ± 0.88% (mean ± standard deviation, similarly hereunder) at baseline and 6.12 ± 0.71% at the final visit. The HbA1c level decreased significantly, with a change of −1.30 ± 0.78% (95% CI −1.42 to −1.18%, similarly hereunder) at the final visit.
Metformin monotherapy
Good blood glucose control was maintained over the 54-week period, with HbA1c 7.69 ± 0.85% (mean ± standard deviation, similarly hereunder) at baseline and 6.38 ± 0.67% at the final visit. The HbA1c level decreased significantly, with a change of −1.32 ± 0.76% (95% CI −1.48 to −1.15%, similarly hereunder) at the final visit. The treatment goal achievement rates at 14, 26 and 54 weeks and at the final visit were 72.7, 79.7, 82.6 and 80.0%, respectively, showing that continued treatment led to an increase in the rate of goal achievement and that most patients achieved the treatment goal. The change in HbA1c at the final visit for the 15 patients receiving a most common dose (among the daily doses prescribed for the patient, the dose taken for the longest time) of 2250 mg/day was −1.78 ± 1.11%. Furthermore, in patients who were prescribed 2250 mg/day for 12 weeks or longer, the HbA1c significantly decreased because of the increase in treatment dose from 1500 to 2250 mg/day (Table 2).
Table 2.
HbA1c values in patients prescribed 2250 mg/day of metformin for 12 weeks or longer continuously
| HbA1c (%) | Monotherapy (n = 17) | SU concomitant (n = 15) | Total (n = 32) |
|---|---|---|---|
| At baseline | 8.55 ± 1.08 | 8.35 ± 1.39 | 8.46 ± 1.22 |
| At just before increase to 2250 mg/daya | 7.26 ± 0.67 | 7.42 ± 1.01 | 7.34 ± 0.84 |
| At last visit dosed 2250 mg/day | 6.51 ± 0.67 | 6.74 ± 0.98 | 6.62 ± 0.82 |
| Change from at just before increase to 2250 mg/day | −0.76 ± 0.62 | −0.68 ± 0.46 | −0.72 ± 0.54 |
| 95% CI | (−1.08, −0.44) | (−0.93, −0.43) | (−0.92, −0.53) |
Data are mean ± standard deviation or (lower limit, upper limit)
aAll the patients were dosed at 1500 mg/day of metformin at this time
Metformin plus SU
Good blood glucose control was maintained over the 54-week period, with HbA1c 7.95 ± 0.91% at baseline and 6.66 ± 0.73% at the final visit. The HbA1c level decreased significantly, with a change of −1.29 ± 0.81% (−1.47 to −1.12%) at the final visit. The treatment goal achievement rates at 14, 26 and 54 weeks and at the final visit were 57.9, 67.1, 78.9 and 69.4%, respectively. Like the results for metformin monotherapy alone, continued treatment led to an increase in the rate of goal achievement, and most patients achieved the treatment goal. The change in HbA1c at the final visit for the 13 patients receiving a most common dose of 2250 mg/day was −1.55 ± 0.70%. Furthermore, in patients who were prescribed 2250 mg/day for 12 weeks or longer, the HbA1c significantly decreased because of the increase in treatment dose from 1500 to 2250 mg/day (Table 2).
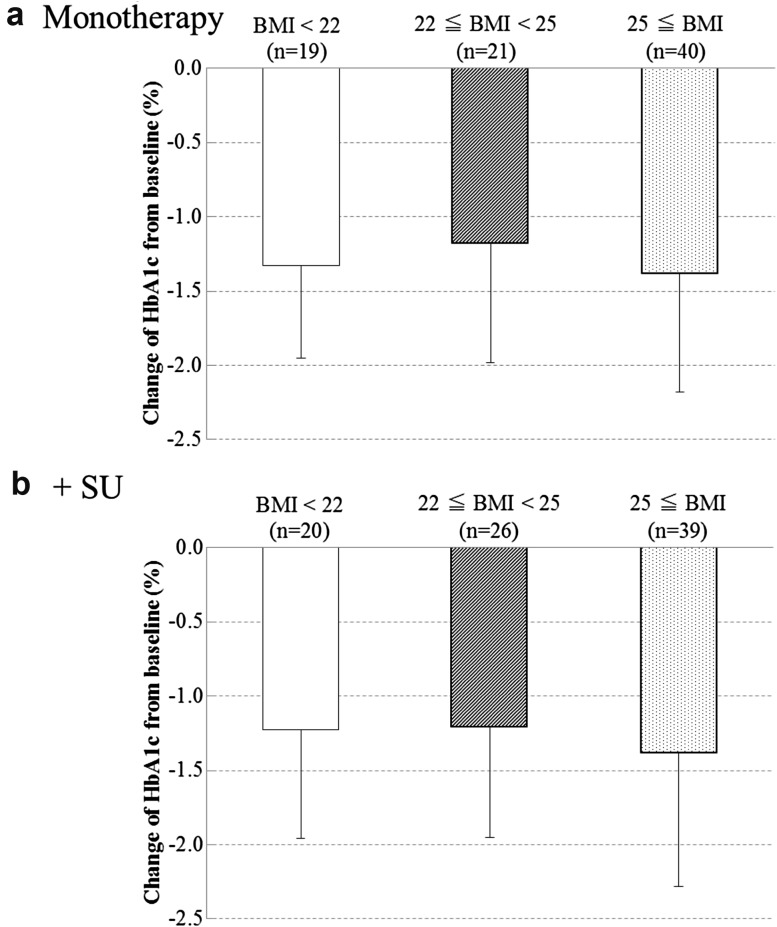
Change in HbA1c at the final visit, stratified by BMI
Change in HbA1c at the final visit, stratified by BMI, was analyzed as the post hoc analysis. Stratified by BMI (<22, ≥22 and <25, and ≥25; results presented in this order hereunder), the changes from baseline in HbA1c at the final visit were −1.33 ± 0.62% (n = 19), −1.18 ± 0.80% (n = 21) and −1.38 ± 0.80% (n = 40), respectively, in the metformin monotherapy group and −1.23 ± 0.73% (n = 20), −1.21 ± 0.74% (n = 26) and −1.38 ± 0.90% (n = 39), respectively, in the metformin plus SU group (Fig. 2). In both the metformin monotherapy and the metformin plus SU groups, differences in BMI had no effect on the change in HbA1c at the final visit.
Fig. 2.
Change of mean HbA1c from baseline in each BMI level. SD values are indicated by bars
FPG, GA and IRI
Metformin monotherapy
Mean values of both FPG and GA were significantly lower than baseline at the final visit, with changes of −27.4 ± 28.9 mg/dl (−33.8 to −20.9) for FPG and −3.96 ± 2.57% (−4.53 to −3.39) for GA. Analyzed by visit, mean values were significantly lower from 2 weeks, and the decreases in both FPG and GA were maintained at 14, 26 and 54 weeks. IRI was 7.12 ± 4.42 μU/ml at baseline and 6.44 ± 3.92 μU/ml at the final visit.
Metformin plus SU
Mean values of both FPG and GA were significantly lower than baseline at the final visit, with changes of −29.5 ± 36.2 mg/dl (−37.3 to −21.7) for FPG and −4.33 ± 2.83% (−4.94 to −3.72) for GA. Analyzed by visit, mean values were significantly lower from 2 weeks, and the decreases in both FPG and GA were maintained at 14, 26, 38 and 54 weeks. IRI was 7.88 ± 5.97 μU/ml at baseline and 7.58 ± 6.14 μU/ml at the final visit.
Serum lipids (T-Cho, TG, HDL-Cho and LDL-Cho)
Metformin monotherapy
T-Cho and TG values were significantly lower than baseline at 54 weeks and at the final visit; however, the LDL-Cho values at the final visit were not significantly different from baseline (Table 3). HDL-Cho values at the final visit were not significantly different from baseline (Table 3).
Table 3.
Blood lipid parameters
| (mg/dl) | Monotherapy | SU concomitant | Total |
|---|---|---|---|
| T-Cho | |||
| At baseline | 200.9 ± 33.0 (n = 80) | 201.4 ± 34.2 (n = 85) | 201.1 ± 33.5 (n = 165) |
| Change from baseline at 54 weeks | −9.5 ± 24.9 (n = 69) | −7.9 ± 27.0 (n = 71) | −8.7 ± 25.9 (n = 140) |
| 95% CI | (−15.5, −3.5) | (−14.3, −1.5) | (−13.0, −4.4) |
| Change from baseline at last visit | −9.1 ± 26.3 (n = 80) | −10.3 ± 27.6 (n = 85) | −9.7 ± 26.9 (n = 165) |
| 95% CI | (−15.0, −3.3) | (−16.3, −4.4) | (−13.9, −5.6) |
| TG | |||
| At baseline | 140.5 ± 105.6 (n = 80) | 150.4 ± 160.9 (n = 85) | 145.6 ± 136.6 (n = 165) |
| Change from baseline at 54 weeks | −20.7 ± 78.3 (n = 69) | −15.6 ± 63.7 (n = 71) | −18.1 ± 71.1 (n = 140) |
| 95% CI | (−39.5, −1.9) | (−30.7, −0.5) | (−30.0, −6.2) |
| Change from baseline at last visit | −20.7 ± 73.6 (n = 80) | −25.0 ± 125.4 (n = 85) | −22.9 ± 103.3 (n = 165) |
| 95% CI | (−37.0, −4.3) | (−52.1, 2.0) | (−38.8, −7.0) |
| HDL-Cho | |||
| At baseline | 56.8 ± 13.6 (n = 80) | 54.3 ± 12.9 (n = 85) | 55.5 ± 13.3 (n = 165) |
| Change from baseline at 54 weeks | −0.9 ± 7.7 (n = 69) | −1.0 ± 7.4 (n = 71) | −1.0 ± 7.3 (n = 140) |
| 95% CI | (−2.8, 0.9) | (−2.6, 0.6) | (−2.2, 0.3) |
| Change from baseline at last visit | −1.4 ± 7.9 (n = 80) | −1.7 ± 7.4 (n = 85) | −1.5 ± 7.6 (n = 165) |
| 95% CI | (−3.2, 0.4) | (−3.3, −0.1) | (−2.7, −0.4) |
| LDL-Cho | |||
| At baseline | 117.8 ± 29.4 (n = 80) | 118.8 ± 30.4 (n = 85) | 118.3 ± 29.8 (n = 165) |
| Change from baseline at 54 weeks | −5.9 ± 22.8 (n = 69) | −4.0 ± 22.6 (n = 71) | −5.0 ± 22.6 (n = 140) |
| 95% CI | (−11.4, −0.4) | (−9.4, 1.3) | (−8.7, −1.2) |
| Change from baseline at last visit | −4.9 ± 23.9 (n = 80) | −4.5 ± 23.3 (n = 85) | −4.7 ± 23.5 (n = 165) |
| 95% CI | (−10.2, 0.5) | (−9.6, 0.5) | (−8.3, −1.1) |
Data are mean ± standard deviation (n) or (lower limit, upper limit)
T-Cho total cholesterol, TG triglyceride, HDL-Cho high-density lipoprotein cholesterol, LDL-Cho low-density lipoprotein cholesterol
Metformin plus SU
T-Cho values at 54 weeks and at the final visit were significantly lower than at baseline; however, LDL-Cho values at 54 weeks and at the final visit were not significantly different (Table 3). The HDL-Cho value at the final visit was lower than at baseline, but there was no consistent tendency throughout the treatment period, with non-significant differences at 54 weeks. The TG value at 54 weeks was lower than baseline, but the change at the final visit was not significant.
Safety
Overall assessment
The incidences of AEs and ADRs were 91.1% (154/169) and 67.5% (114/169), respectively (Table 4). More than 10% of metformin monotherapy patients or metformin plus SU patients had experienced gastrointestinal symptoms [63.9% (53/83) or 59.3% (51/86)], nasopharyngitis [38.6% (32/83) or 32.6% (28/86)], abdominal discomfort [8.4% (7/83) or 11.6% (10/85)] or hypoglycemia symptoms [0 or 22.1% (19/86)]. Except for one subject, the investigator considered all of the nasopharyngitis cases not to be related to metformin treatment. On the other hand, most of the gastrointestinal symptoms were considered by an investigator to be related to metformin treatment. All ADRs were either mild or moderate in severity, and none were severe. Twelve serious AEs occurred in 10 patients (5.9%), 4 of 60 elderly patients (6.7%) and 6 of 109 non-elderly patients (5.5%), and investigators considered all of these serious AEs not to be related to metformin treatment. Analysis of ADR data by time of occurrence showed no increase in the frequency or worsening of severity or the occurrence of new ADRs with long-term treatment.
Table 4.
Adverse events and adverse drug reactions
| Adverse events | Adverse drug reactions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy (n = 83) | SU concomitant (n = 86) | Total (n = 169) | Monotherapy (n = 83) | SU concomitant (n = 86) | Total (n = 169) | |||||||
| Adverse events or adverse drug reactions | 76 | (91.6) | 78 | (90.7) | 154 | (91.1) | 54 | (65.1) | 60 | (69.8) | 114 | (67.5) |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Serious events | 7 | (8.4) | 3 | (3.5) | 10 | (5.9) | 0 | 0 | 0 | |||
| Gastrointestinal symptomsa | 53 | (63.9) | 51 | (59.3) | 104 | (61.5) | 49 | (59.0) | 49 | (57.0) | 98 | (58.0) |
| Hypoglycemia symptomsb | 0 | 19 | (22.1) | 19 | (11.2) | 0 | 16 | (18.6) | 16 | (9.5) | ||
| Blood lactic acid increased | 3 | (3.6) | 0 | 3 | (1.8) | 1 | (1.2) | 0 | 1 | (0.6) | ||
| Lactic acidosis | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Common adverse drug reactions (occurring ≥5% of patients in any therapy) | ||||||||||||
| Diarrhea | 48 | (57.8) | 48 | (55.8) | 96 | (56.8) | 46 | (55.4) | 46 | (53.5) | 92 | (54.4) |
| Nausea | 15 | (18.1) | 12 | (14.0) | 27 | (16.0) | 14 | (16.9) | 12 | (14.0) | 26 | (15.4) |
| Abdominal pain | 10 | (12.0) | 9 | (10.5) | 19 | (11.2) | 8 | (9.6) | 9 | (10.5) | 17 | (10.1) |
| Abdominal discomfort | 7 | (8.4) | 10 | (11.6) | 17 | (10.1) | 6 | (7.2) | 9 | (10.5) | 15 | (8.9) |
| Vomiting | 7 | (8.4) | 9 | (10.5) | 16 | (9.5) | 6 | (7.2) | 6 | (7.0) | 12 | (7.1) |
| Anorexia | 9 | (10.8) | 18 | (20.9) | 27 | (16.0) | 8 | (9.6) | 17 | (19.8) | 25 | (14.8) |
| Hypoglycemia | 0 | 13 | (15.1) | 13 | (7.7) | 0 | 10 | (11.6) | 10 | (5.9) | ||
MedDRA/J Ver.10.1
Data are mean ± standard deviation or n (%)
aDiarrhea, nausea, vomiting, abdominal pain and anorexia are defined as gastrointestinal symptoms
bHypoglycemia symptoms include hypoglycemia and hypoglycemia-related symptoms, e.g., excessively hungry with sweating, palpitation, tachycardia, yawning or twilight state
Gastrointestinal symptoms
Gastrointestinal symptoms led to discontinuation of treatment in ten patients (5.9%), whereas most patients who experienced such symptoms continued taking the study drug.
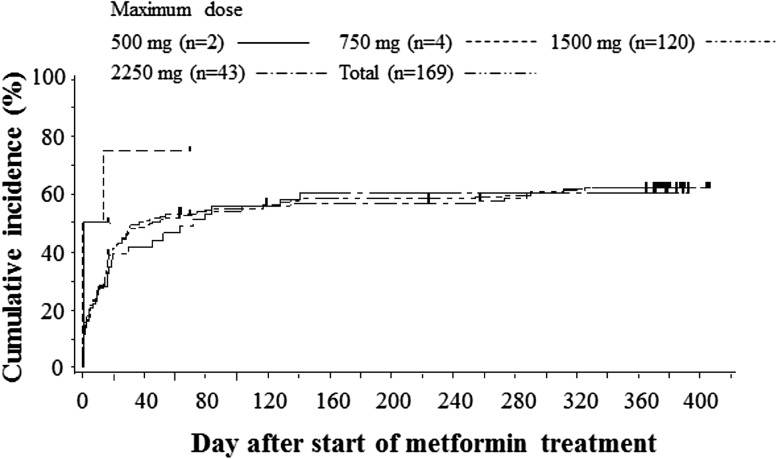
Figure 3 showed the Kaplan-Meier plot for the accumulation incidence of gastrointestinal symptom AEs in patient groups stratified by the maximum received dose. The horizontal axis indicated the first observation day of any gastrointestinal symptom AEs. The cumulative incidences of gastrointestinal symptoms in patients whose maximum doses were 1500 and 2250 mg/day were similar and were 61.7% (74/120) and 60.5% (26/43), respectively. Most of the first gastrointestinal symptoms occurred within 6 weeks.
Fig. 3.
Kaplan-Meier plot for gastrointestinal symptom adverse events. The patients are stratified by the maximum treatment dose
Gastrointestinal symptoms either occurred or continued in the following periods: 29.6% (50/169) at <2 weeks; 41.7% (70/168) at 2–6 weeks; 21.8% (36/165) at 6–10 weeks; 23.6% (38/161) at 10–14 weeks; 30.4% (48/158) at 14–28 weeks; 20.0% (30/150) at 28–42 weeks; 14.8% (21/142) at ≥42 weeks. The frequency of occurrence was highest in the 2–6 week period, and while the frequencies increased in the 10–14 and 14–28 week periods when dose levels were increased to 2250 mg/day, they decreased from 28 weeks onwards. Apart from one patient in the 1500 mg/day population with gastrointestinal symptoms who reported moderate diarrhea, nausea, vomiting and anorexia, all other symptoms were mild in severity.
The incidences of gastrointestinal symptom AEs in elderly and non-elderly patients were 68.3% (41/60) and 57.8% (63/109), respectively, and were similar.
Blood lactic acid levels
Lactic acidosis, an important ADR of former biguanides such as phenformin, did not occur at all with metformin in this study. Mean blood lactic acid levels did not show any clinically meaningful increase throughout the assessment period, with levels of 10.60 ± 4.27 mg/dl (n = 166) at baseline, 10.60 ± 4.45 mg/dl (n = 150) at 26 weeks, 10.37 ± 4.34 mg/dl (n = 140) at 54 weeks and 10.28 ± 4.29 g/dl (n = 168) at the final visit. Stratification of blood lactic acid values by age into elderly (≥65 years) and non-elderly patients revealed no difference in the pattern of occurrence. The maximum blood lactic acid concentration observed in this study was 39.0 mg/dl in a 55-year-old male patient who was under monotherapy of 2250 mg/day metformin. The AE “blood lactic acid increased” occurred transiently in three patients (1.8%) (Table 5). None of the patients had any clinical symptoms such as respiratory distress or increased drowsiness. In all three case, the blood lactic acid level in the next measurement recovered to normal range.
Table 5.
Details of blood lactic acid increase adverse events
| Basal therapy | Male/female | Age | AE start day | Metformin dose at AE start (mg/day) | Durationa (day) | Lactic acid level at baseline (mg/dl) | Creatinine lebel at baseline (mg/dl) | Lactic acid level at AE (mg/dl) | Creatinine level at AE (mg/dl) | Lactic acidosis symptomsb | Relation to metformin treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy | Male | 55 | 78 | 1500 | 5 | 12.4 | 1.13 | 36.6 | 1.14 | No | No |
| Monotherapy | Male | 62 | 189 | 1500 | 29 | 6.2 | 0.69 | 23.0 | 0.64 | No | No |
| Monotherapy | Female | 39 | 343 | 2250 | 36 | 23.4 | 0.44 | 33.2 | 0.53 | No | Unknown |
AE adverse event
aFrom the AE start day to the day confirmed the outcome
bPotentially associated with lactic acidosis such as respiratory distress or increased drowsiness
Nephropathy and cardiovascular events
Serum creatine levels before and after 54-week treatment were 0.696 ± 0.161 mg/dl (n = 169) and 0.712 ± 0.173 mg/dl (n = 140), respectively. Serum BUN levels before and after 54-week treatment were 14.74 ± 3.47 mg/dl (n = 169) and 14.45 ± 3.67 mg/dl (n = 140), respectively. No change of these parameters was observed during this study. On the other hand, five cardiovascular adverse events were found in five subjects in total. Three of the five were palpitation, and the others were atrial fibrillation and coronary artery disorder. The incidence of cardiovascular adverse events in this study is reasonable for a 1-year treatment study in patients with diabetes mellitus.
Hypoglycemia symptoms
In this study, 19 of 169 patients (11.2%) experienced hypoglycemia symptoms, a cause of concern with hypoglycemic drugs (Table 4). All the hypoglycemia symptoms were mild in severity. ADRs related to hypoglycemia symptoms occurred in 16 patients (9.5%). All instances of hypoglycemia symptoms occurred in patients receiving metformin plus SU, with none in patients receiving metformin monotherapy. Analysis by the maximum dose level in metformin plus SU showed that the frequencies of occurrence were similar in the 1500 mg/day population and the 2250 mg/day population, 12 of 60 patients (20.0%) and 4 of 21 patients (19.0%), respectively.
The incidences of hypoglycemia symptom AEs in elderly and non-elderly patients with metformin plus SU were 18.4% (7/38) and 25.0% (12/48), respectively, and were similar.
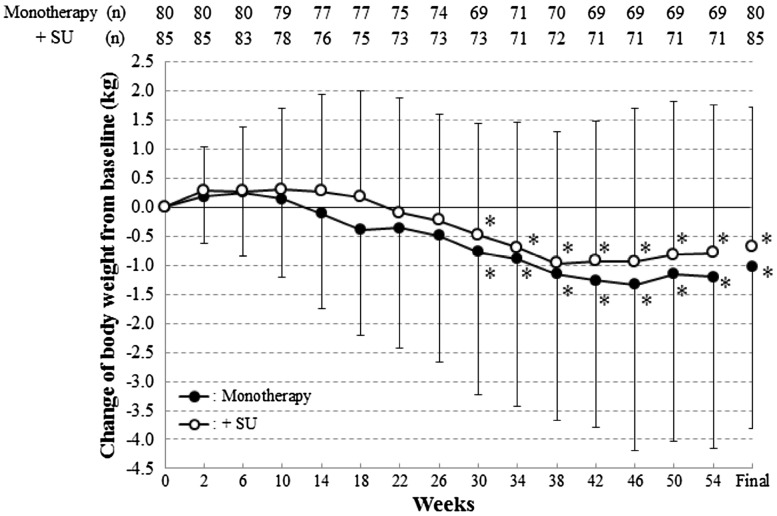
Effects on body weight
The time course of body weight data are shown in Fig. 4. In the metformin monotherapy group, the changes in body weight were −0.49 ± 2.17 kg (−0.98 to 0.00) at 26 weeks, −1.20 ± 2.95 kg (−1.90 to −0.49) at 54 weeks and −1.03 ± 2.79 kg (−1.64 to −0.42) at the final visit and in the metformin plus SU group, −0.23 ± 1.83 kg (−0.66 to 0.19) at 26 weeks, −0.79 ± 2.55 kg (−1.39 to −0.18) at 54 weeks and −0.68 ± 2.40 kg (−1.20 to −0.17) at the final visit. Body weights were significantly lower after 26 weeks in the metformin monotherapy group and after 30 weeks in the metformin plus SU group. In the metformin monotherapy group, body weight changes in obesity (BMI ≥ 25) and non-obesity (25 > BMI) patients at 54 weeks were −2.31 ± 3.38 kg (n = 37) and 0.09 ± 1.60 kg (n = 32), respectively. In the metformin plus SU group, body weight changes in obesity and non-obesity patients at 54 weeks were −0.86 ± 2.70 kg (n = 35) and −0.72 ± 2.44 kg (n = 36), respectively.
Fig. 4.
Change of mean body weight values during treatment. SD values are indicated by bars. *p < 0.05, change from baseline (no multiplicity adjustment)
Discussion
In this study, we evaluated the safety and efficacy of global standard dose metformin in long-term treatment at a starting dose of 500 mg/day and a maintenance dose of 1500 mg/day, with dose variations permitted in the range of 750–2250 mg/day.
The results showed that metformin exerted a potent anti-hyperglycemic effect, reducing the HbA1c level from baseline by more than 1%, and even in long-term treatment for 1 year, HbA1c continued to stay below 6.5%, with no attenuation of the effect. Furthermore, the treatment goal of excellent or good was achieved by high percentages of patients, 80.0% of those receiving metformin monotherapy and 69.4% of those receiving metformin plus SU. According to the UKPDS results obtained overseas [3], metformin was shown to be effective in patients with high BMI values. However, since differences in BMI had no effect on the change in HbA1c at the final visit in our study, it appears that in Japanese individuals, metformin is also effective in patients with low BMIs.
The dose was increased to 2250 mg/day in patients for whom it was concluded that an increase from the maintenance dose of 1500 mg/day was necessary for efficacy and safety considerations. For both metformin monotherapy and metformin plus SU, the HbA1c level decreased significantly compared with the immediate pre-dose increasing level, and despite the occurrence of gastrointestinal symptoms when the dose was increased to 2250 mg/day, there were no major concerns. Additionally, body weights decreased significantly for patients receiving either metformin monotherapy or metformin plus SU. In large-scale clinical studies such as the UKPDS [7–10], it has been shown that good blood glucose control leads to lower risk of onset and progression of macroangiopathic (macrovascular) and microangiopathic (microvascular) complications. To date, the maximum dose permitted for metformin in Japan has been 750 mg/day, about 1/3–1/4 of the approved dose in the Western countries. However, it is anticipated that using doses above 750 mg/day will provide evidence on aspects such as the inhibition of macroangiopathic (macrovascular) events in Japan, too.
Regarding safety, the main ADRs were gastrointestinal symptoms such as diarrhea and nausea, but discontinuations of the study drug due to gastrointestinal ADRs were necessary only in a few patients. If gastrointestinal symptoms do occur, it should be possible to continue treatment through measures such as drug holds or dose reductions, and there appear to be no concerns about tolerability. However, since the frequencies of occurrence of symptoms were higher at the start of treatment and when the dose was increased from 1500 to 2250 mg/day, it will be necessary to pay close attention to the possibility of gastrointestinal symptoms at the start of treatment with metformin or when increasing the dose. Hypoglycemia symptoms, which are a cause of concern with hypoglycemic drugs, were reported for patients taking metformin plus SU but not metformin alone, and no severe or protracted hypoglycemia symptoms occurred.
According to the recent literature, the highest risk for onset of lactic acidosis is not any medication, but acute renal failure or severe dehydration [11–13].
The efficacy and safety-related results were similar for elderly/non-elderly and obese/non-obese patients and were independent of sex, duration of illness and presence of comorbidities. The results of our study suggested that the global standard dose metformin will be useful for many patients with type 2 diabetes, regardless of age or obesity status, in whom metformin is not contraindicated.
Hence, it appears that metformin can potentially make a major contribution to the treatment of type 2 diabetes mellitus, because administering metformin at doses higher than up to 750 mg/day, which has been the practice in Japan for a considerable length of time, will enable the maintenance of more stringent blood glucose control over an extended period. However, this study had an open-label design and had a 54-week treatment period that did not allow assessment of the effect of treatment on micro- and macroangiopathic complications. Accordingly, a longer-term double-blind trial will help to evaluate the inhibition of the onset and progression of these complications.
Acknowledgements
We thank the study participants, the investigators and contributors from each of the study sites.
Conflict of interest
These studies were funded by Sumitomo Dainippon Pharma Co., Ltd. MO, RK, NT and YI are members of a coordinating committee of this study. SK and NH are members of a safety data monitoring committee. All of these committee members have received honoraria from Sumitomo Dainippon Pharma. No other potential conflicts of interest relevant to this article were reported. YY and FU are employees of Sumitomo Dainippon Pharma and carried out statistical analysis and managed this study, respectively.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
References
- 1.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G, ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Goodman AM, The Multicenter Metformin Study Group Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:541–549. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H, Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes Society International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol Int. 2012;3:8–10. doi: 10.1007/s13340-012-0069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, Nanjo K, Sasaki A, Seino Y, Ito C, Shima K, Nonaka K, Kadowaki T, The Committee of Japan Diabetes Society for the Diagnostic Criteria of Diabetes Mellitus Report of the Committee of Japan Diabetes Society on the Classification and Diagnostic Criteria of Diabetes Mellitus. J. Jpn Diabetes Soc. 2010;53:450–467. [Google Scholar]
- 7.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes 4 years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-K. [DOI] [PubMed] [Google Scholar]
- 11.Duong JK, Furlong TJ, Roberts DM, Graham GG, Greenfield JR, Williams KM, Day RO, et al. The role of metformin in metformin-associated lactic acidosis (MALA): cases series and formulation as a model of pathogenesis. Drug Saf. 2013;36:733–746. doi: 10.1007/s40264-013-0038-6. [DOI] [PubMed] [Google Scholar]
- 12.Huang W, Castelino RL, Petersen GM. Adverse event notifications implicating metformin with lactic acidosis in Australia. J Diab Compl. 2015;29:1261–1265. doi: 10.1016/j.jdiacomp.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Schernthaner G, Schernthaner-Reiter MH. Risk of metformin use in patients with T2DM and advanced CKD. Nat Rev Endocrinol. 2015 doi: 10.1038/nrendo.2015.132. [DOI] [PubMed] [Google Scholar]