Abstract
Aims
To evaluate the efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes mellitus and inadequate glycemic control and investigate the impact of maintaining exercise habits during treatment.
Materials and methods
A total of 20 patients were enrolled. Patients aged 20–70 years with type 2 diabetes mellitus were administered 50 mg of ipragliflozin once daily for 12 weeks. The primary endpoint was the change in glycated hemoglobin levels from baseline to week 12. Key secondary endpoints included changes in total body weight, body composition, serum lipid levels, and diabetes therapy-related quality of life from baseline to week 12. Adverse events were recorded throughout the study.
Results
The patients’ glycated hemoglobin levels were 0.69% lower at week 12 versus baseline (adjusted mean difference from baseline; P < 0.01, n = 18). Mean total body weight, body composition, and serum lipid levels also improved significantly from baseline. Of note, stratification analysis by physical activity level revealed slight but significant reductions in skeletal muscle mass and muscle mass, but not body fat mass, in the minimal exercise group compared to the data for the moderate exercise group. One of the subdomain structures in diabetes therapy-related quality of life questionnaire, “Anxiety and dissatisfaction with treatment,” was significantly improved. Although no major hypoglycemic episodes occurred, two adverse events were reported.
Conclusions
Daily treatment with ipragliflozin was associated with marked improvements in glycemic control and body composition without major side effects, and this improvement was affected by exercise habits. This study was registered with UMIN CTR (no. UMIN000014388).
Keywords: Exercise, Ipragliflozin, Type 2 diabetes mellitus, Initial SGLT-2 inhibitor treatment, Patient education
Introduction
A number of oral and injectable agents are available clinically for alleviating hyperglycemia; however, only 53% of patients with diabetes mellitus (DM) achieve the glycemic target of a glycated hemoglobin (HbA1c) level <7.0% [1]. Type 2 DM (T2DM) is a progressive condition involving a gradual and continuous loss of β-cell function [2, 3], resulting in deterioration of glycemic control and an eventual need for insulin-replacement therapy or multiple anti-hyperglycemic therapies. In addition to insufficient glycemic control, side effects such as hypoglycemia, body weight gain, gastric symptoms, edema, heart failure, and fluid retention are associated with the use of these agents [4–7]. Thus, a number of factors must be considered concerning glycemic control in patients with T2DM when individualizing first-line therapy. Therefore, there is a high unmet medical need for novel treatment options with acceptable safety profiles that can help to overcome these difficulties.
Sodium-glucose cotransporter 2 (SGLT2) is a low-affinity, high-capacity transporter that mediates glucose reabsorption from the proximal convoluted tubule in the kidneys [8]. Ipragliflozin, a competitive and highly selective inhibitor of SGLT2, reduces renal glucose reabsorption and increases renal glucose excretion in a dose-dependent manner [9].
In previous preclinical and pilot clinical trials, ipragliflozin improved glycemic control in animal models of diabetes [10, 11] and in patients with T2DM [12–14]. Notably, in addition to improving glycemic control, ipragliflozin had beneficial effects on body weight, and it was well tolerated in these clinical studies [12–14]. Furthermore, SGLT2 inhibitors such as ipragliflozin function independently of insulin. This allows SGLT2 inhibitors to be co-administered with other insulin-dependent oral hypoglycemic agents, and enhanced glycemic control can be expected [14]. However, a considerable number of serious adverse events (SAEs) including urogenital infections, hypoglycemia, and dehydration were reported in patients receiving SGLT2 inhibitors in Japan [15]. Thus, based on these properties, ipragliflozin may be useful as an add-on therapy to metformin or another first-line agent as a component of dual- or triple-therapy regimens in daily clinical use. However, it remains unclear whether ipragliflozin can be used with acceptable safety in Japanese patients with T2DM who are receiving conventional therapy.
The aim of our study was to evaluate glycemic efficacy, weight loss, quality of life (QOL), and safety over 12 weeks of ipragliflozin therapy in patients with inadequate glycemic control despite dietary modification and exercise or conventional anti-hyperglycemic therapy for more than 6 weeks.
Materials and methods
Study design
The present single arm, open, prospective intervention study assessed the efficacy and safety of ipragliflozin in Japanese patients with T2DM. The protocol for the study was approved by the Medical Corporation Dojin Memorial Foundation Meiwa Hospital Ethics Committee, and it conforms to the provisions of the Declaration of Helsinki-Ethical Guidelines for Clinical Research (2013). This study was conducted in accordance with the ethical guidelines for medical and health research involving human subjects (31 March 2015) as well as local laws and regulations. All patients provided written informed consent prior to all procedures related to this study.
Patients
The inclusion criteria of the present study were a diagnosis of T2DM, age of 20–70 years, inadequate glycemic control despite diet modification and exercise or conventional anti-hyperglycemic therapy for more than 6 weeks, HbA1c ≥7.0%, body mass index (BMI) ≥22.0 kg/m2, and written consent to participate in this study. The patients were administered 50 mg ipragliflozin once daily before or after breakfast for 12 weeks. The patients of this study were recruited from outpatients treated at Kato Clinic of Internal Medicine, Tokyo, Japan. As the impact of SGLT2 inhibitors on body weight reduction is well established [13, 14], we actively recruited obese patients. The exclusion criteria of the present study were as follows: type 1 diabetes mellitus; perioperative status; presence of ketoacidosis; diabetic coma or precoma; severe infection; severe trauma; moderate-to-severe renal dysfunction (serum creatinine level ≥1.5 mg/dl in males and ≥1.3 mg/dl in females); current use of SGLT2 inhibitors or insulin; women who are currently pregnant, nursing, or trying for pregnancy; hypersensitivity to the ingredients of the medication used or a determination of inappropriateness to participate in the study by the principal investigator.
Clinical evaluations
During the treatment period, blood and urine samples were collected at weeks 0, 4, 8, and 12. The primary efficacy variable was the change in HbA1c levels from baseline to week 12. The secondary efficacy variables included the changes in body composition [body fat mass (BFM), muscle mass (MM), skeletal muscle mass (SkMM), body water (BW)], total body weight (TBW), waist circumference (WC), serum lipids, glycoalbumin (GA), blood pressure, other clinical laboratory test results such as diet records, and other patient information including diabetes therapy-related quality of life (DTR-QOL) from baseline to week 12.
Body weight, BFM, MM, SkMM, and BW were measured using the InBody 430 body composition analyzer (Biospace Co., Ltd., Seoul, Korea).
Concerning the stratification analysis of HbA1c, the cutoff value was set at −0.7%, which was decided by the mean reduction of HbA1c after 12 weeks of ipragliflozin administration [12, 13] and our clinical experiences. Patients whose HbA1c levels declined by at least 0.7% were considered to be in the HbA1c improvement group, and those whose HbA1c levels did not decline by at least 0.7% were considered to be in the no improvement group. To investigate the influence of exercise habits on glycemic control and body composition, patients were stratified by their physical activity levels into two groups: a moderate exercise group consisting of patients who did regular exercise ≥2 times/week, in addition to making efforts to increase exercise in daily life (e.g., using stairs instead of an escalator, not using a car wherever possible) and a minimal exercise group, which included patients with no exercise habit or patients who did not exercise at all. To quantitate the physical activity level, we used the International Physical Activity Questionnaire-Short Form (IPAQ-SF) [16]. The IPAQ is an index developed by a WHO working group for comparing physical activity volume that is internationally applicable. It has questions regarding the number of days and amount of time spent in an average week on moderate and vigorous intensity physical activity. The Long Form (LF) has detailed questions regarding each life situation, and the Short Form (SF) only has questions regarding different physical activity intensity levels. In the present study, we used the IPAQ-SF, because it was considered adequate for the purpose of the study. The IPAQ-SF data were used to estimate the total weekly physical activity level [metabolic equivalents (METS)/hour/week], and then we divided by 7 days to calculate the daily mean. One MET was defined as an oxygen consumption rate of 3.5 ml/kg/min in adults.
The DTR-QOL questionnaire was used to measure the influence of the intervention on patient QOL [17] using 29 items rated on a 7-point Likert scale (1: strongly agree; 7: strongly disagree). The questionnaire consisted of four domain structures: factor 1: burden on social activities and daily activities; factor 2: anxiety and dissatisfaction with treatment; factor 3: hypoglycemia; factor 4: satisfaction with treatment. The score of each item was reversed, and thus 7 represented the highest QOL score. High DTR-QOL scores indicate good QOL for their diabetes treatment.
Safety assessments included adverse events (AEs), which were recorded throughout the study.
Statistical analysis
In the present study, the target sample size was determined as 30 patients. Kashiwagi reported a change in HbA1c of Δ0.79 at 12 weeks after treatment with ipragliflozin 50 mg/day [18]. Based on this value, eight patients were considered necessary to obtain the results with a statistical power of 90%. However, considering the changes in BFM and MM, consequently, a total of 30 patients was determined as a target sample size.
Efficacy variables were analyzed in 18 patients after excluding 2 because of AEs. The safety analysis set included all patients who started treatment except those who did not receive the study medication or those for whom safety data were lacking after treatment initiation. Efficacy variables were presented descriptively for changes over time as mean ± standard deviation. For graphic presentations including HbA1c and body composition over the 12-week treatment period, the changes from baseline were analyzed at each interval using Tukey’s multiple comparison test for comparisons between strata and Dunnett’s multiple comparison test for comparisons of data at various time points with baseline data within a stratum. Statistical and data analyses were performed using SAS version 9.4 (SAS Institute Japan, Ltd., Tokyo, Japan). Statistical comparisons were made using two-sided tests at α = 0.05.
For analyses of DTR-QOL, Wilcoxon’s signed rank test was used to analyze the response to individual questions, and a paired t test was used to analyze the results of subdomains and the total score.
AEs were coded using the Medical Dictionary for Regulatory Activities (version 18.0) by system organ class and preferred term, and the number and rates of AEs were summarized.
Results
Patients
A total of 20 patients were enrolled. The safety analysis set consisted of all 20 patients. Two patients withdrew from the study because of AEs, namely folliculitis and genital pruritus, and were excluded from the full analysis set, resulting in 18 patients. The patients’ demographics and baseline characteristics are summarized in Table 1. Sixteen patients (80.0%) were male, their mean age was 55.2 ± 9.6 years, and the mean duration of diabetes was 11.5 ± 7.1 years. The baseline mean HbA1c level was 8.50 ± 1.00%, and the mean fasting plasma glucose (FPG) level was 174.2 ± 49.0 mg/dl. The baseline mean lipid profiles including triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels were 158.4 ± 108.0, 211.0 ± 49.3, 123.8 ± 40.7, and 57.3 ± 14.7 mg/dl, respectively. The patients’ mean body weight was 81.5 ± 21.1 kg, and the mean BMI was 29.0 ± 5.0 kg/m2, with 85.0% of patients classified as obese (BMI ≥25) according to the definition of obesity and visceral fat obesity in Japan [19]. Fifteen patients (75.0%) were taking other oral antidiabetic agents. The following antidiabetic oral agents were concomitantly administered to patients during the treatment period: sulfonylureas (SUs) (60.0%), biguanides (50.0%), thiazolidinediones (15.0%), dipeptidase-4 inhibitors (15.0%), and glinides (5.0%). Seventeen patients (85.0%) were also taking therapeutic agents for lifestyle-related disease, including antihypertensive agents (40.0%), antidyslipidemic agents (45.0%), and antihyperuricemic agents (5.0%).
Table 1.
Baseline characteristics of patients (n = 20)
| Characteristic | Mean ± SD | Median (min–max) |
|---|---|---|
| Age (years) | 55.2 ± 9.6 | 57.5 (30–69) |
| Sex, n (%) | ||
| Male | 16 (80.0) | – |
| Female | 4 (20.0) | – |
| HbA1c (%) | 8.50 ± 1.00 | 8.40 (7.20–10.80) |
| FPG (mg/dl) | 174.2 ± 49.0 | 168.0 (89.0–274.0) |
| TG (mg/dl) | 158.4 ± 108.0 | 123.5 (50.0–484.0) |
| TC (mg/dl) | 211.0 ± 49.3 | 196.5 (133.0–303.0) |
| LDL-C (mg/dl)a | 123.8 ± 40.7 | 117.0 (67.0–198.0) |
| HDL-C (mg/dl) | 57.3 ± 14.7 | 54.0 (38.0–110.0) |
| TBW (kg) | 81.5 ± 21.1 | 75.9 (57.2–135.8) |
| BMI (kg/m2) | 29.0 ± 5.0 | 27.4 (24.0–43.5) |
| <25 kg/m2, n (%) | 3 (15.0) | – |
| ≥25 kg/m2, n (%) | 17 (85.0) | – |
| WC (cm) | 97.5 ± 13.5 | 94.8 (81.0–136.0) |
| SBP (mmHg) | 131.5 ± 16.4 | 131.0 (104.0–160.0) |
| DBP (mmHg) | 72.5 ± 9.9 | 70.0 (60.0–90.0) |
| Duration of diabetes (years) | 11.5 ± 7.1 | 10.3 (1.0–35.0) |
| Anti-diabetic treatment, n (%) | 15 (75.0) | – |
| Drug-naive | 5 (25.0) | – |
| Sulfonylureas | 12 (60.0) | – |
| Biguanides | 10 (50.0) | – |
| Dipeptidase-4 inhibitors | 3 (15.0) | – |
| Thiazolidinediones | 3 (15.0) | – |
| Glinides | 1 (5.0) | – |
| Concomitant medications, n (%) | 17 (85.0) | – |
| Antihypertensive agents | 8 (40.0) | – |
| Antidyslipidemic agents | 9 (45.0) | – |
| Antihyperuricemic agents | 1 (5.0) | – |
Values are shown as n (%) or mean ± SD
HbA1c glycated hemoglobin, FPG fasting plasma glucose, TG triglyceride, TC total cholesterol, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, TBW total body weight, BMI body mass index, WC waist circumference, SBP systolic blood pressure, DBP diastolic blood pressure, SD standard deviation
aThe number of patients was 19
Efficacy
Glycemic efficacy endpoints
Figure 1a–c shows the changed in HbA1c, FPG, and GA levels over time. The adjusted mean change in HbA1c levels from baseline was −0.47% (P < 0.05) at week 8 and −0.69% (P < 0.01) at week 12, and it did not reach a plateau during the treatment period (Fig. 1a). Significant improvements from baseline in FPG and GA levels were observed as early as week 4 (Fig. 1b and 1c). The magnitude of these significant reductions in FPG and GA levels was maintained throughout the treatment period (Fig. 1b and 1c).
Fig. 1.
Efficacy of ipragliflozin. Mean changes from baseline in HbA1c (%) (a), FPG (mg/dl) (b), GA (%) (c), and total body weight (kg) (d). HbA1c glycated hemoglobin, FPG fasting plasma glucose, GA glycoalbumin. Data are expressed as mean ± standard deviation (n = 18). P values denote differences between baseline data and week 4, 8, or 12 data. *** P < 0.001, ** P < 0.01, * P < 0.05
Other efficacy endpoints
Table 2 presents the changes in body composition variables including TBW, BMI, WC, BFM, SkMM, MM, and BW from baseline to the end of treatment at week 12. A significant reduction in TBW was confirmed at week 4 (−1.40 kg, P < 0.01) and maintained through the end of treatment (week 8, −1.73 kg, P < 0.01; week 12, −1.86 kg, P < 0.01) (Fig. 1d; Table 2). BMI, SkMM, MM, and BW were also reduced significantly, with reductions maintained throughout the study period (Table 2).
Table 2.
Mean change from baseline in body compositions over time
| Week 4 | Week 8 | Week 12 | |
|---|---|---|---|
| TBW (kg) | –1.40 ± 2.00** | –1.73 ± 1.96** | –1.86 ± 2.32** |
| BMI (kg/m2) | –0.48 ± 0.70** | –0.56 ± 0.69** | –0.79 ± 0.83*** |
| WC (cm) | –1.21 ± 1.98* | –1.11 ± 2.53 | –1.99 ± 2.48** |
| BFM (kg) | –0.65 ± 1.35 | –1.26 ± 1.68** | –1.31 ± 1.69** |
| SkMM (kg) | –0.38 ± 0.70* | –0.34 ± 0.62* | –0.57 ± 0.65** |
| MM (kg) | –0.67 ± 1.22* | –0.60 ± 1.00* | –0.92 ± 1.02** |
| BW (kg) | –0.49 ± 0.98* | –0.48 ± 0.80* | –0.70 ± 0.80** |
Dunnett’s multiple comparison test was used for comparisons between data at various time points and baseline data. Data are shown as the mean ± standard deviation (n = 18)
TBW total body weight, BMI body mass index, WC waist circumference, BFM body fat mass, SkMM skeletal muscle mass, MM muscle mass, BW body water
*** P < 0.001, ** P < 0.01, * P < 0.05
A significant reduction in systolic blood pressure (SBP) was transiently confirmed at week 4 (Table 3); however, no significant changes versus baseline were observed at weeks 8 and 12. In addition, diastolic blood pressure at weeks 8 and 12 was lower than the baseline value, albeit not significantly. Hematocrit content was increased significantly from baseline at weeks 8 and 12.
Table 3.
Mean change from baseline in blood pressures and laboratory test values over time
| Week 4 | Week 8 | Week 12 | |
|---|---|---|---|
| SBP (mmHg) | –8.2 ± 15.8* | –7.2 ± 16.8 | –5.9 ± 16.4 |
| DBP (mmHg) | 0.9 ± 9.9 | –1.6 ± 13.5 | –1.0 ± 11.4 |
| Hematocrit (%) | 0.62 ± 1.73 | 1.44 ± 1.99** | 1.41 ± 2.03** |
| Serum creatinine (mg/dl) | 0.029 ± 0.042** | 0.022 ± 0.068 | 0.003 ± 0.060 |
| Serum uric acid (mg/dl) | –0.31 ± 0.94 | –0.25 ± 0.76 | –0.45 ± 0.59** |
| eGFR (ml/min/1.73 m2) | –3.96 ± 4.58** | –3.69 ± 7.16* | –1.15 ± 5.67 |
| Triglyceride (mg/dl) | –10.2 ± 57.7 | –19.2 ± 93.4 | –28.9 ± 53.3* |
| TC (mg/dl) | –6.3 ± 23.2 | –4.9 ± 23.5 | –17.2 ± 31.4* |
| LDL-C (mg/dl) | –4.3 ± 18.0 | –1.9 ± 20.5 | –13.8 ± 24.0* |
| HDL-C (mg/dl) | 0.5 ± 4.5 | 1.9 ± 5.6 | 2.4 ± 7.8 |
Dunnett’s multiple comparison test was used for comparisons between data at various time points and baseline data. Data are shown as the mean ± standard deviation (n = 18)
SBP systolic blood pressure, DBP diastolic blood pressure, eGFR estimated glomerular filtration rate, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TC total cholesterol
*** P < 0.001, ** P < 0.01, * P < 0.05
Lipid parameters including LDL-C, TG, and TC levels were significantly reduced versus baseline at week 12 (all P < 0.05; Table 3), whereas no significant change in HDL-C level was observed. Concerning the effect of ipragliflozin on renal function, a significant reduction from baseline of serum uric acid levels was confirmed at week 12. Estimated glomerular filtration rate (eGFR) was transiently decreased significantly at weeks 4 and 8; however, it was not significant at 12 weeks.
Stratification analysis of HbA1c
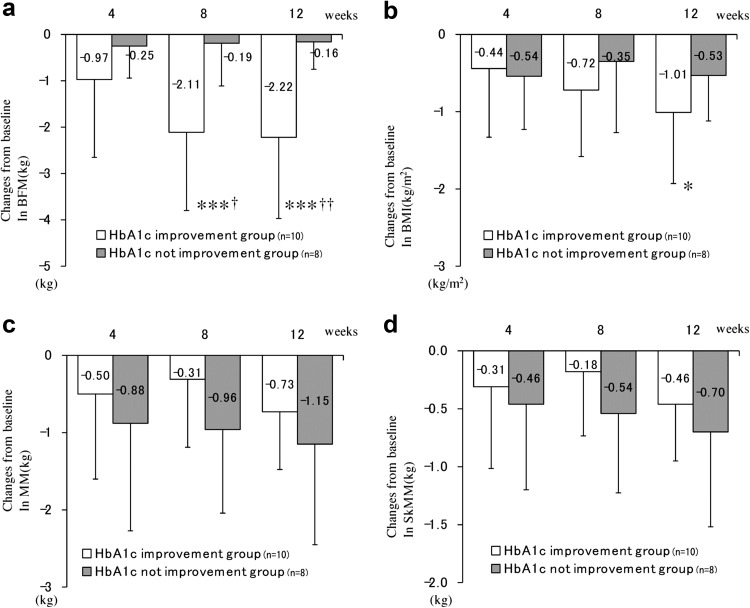
To elucidate the relationship between HbA1c and body composition, body composition data were also analyzed using the degree of HbA1c improvement as a stratification factor (Fig. 2). Patients who experienced an improvement in HbA1c levels displayed significant reductions in BFM at weeks 8 (−2.11 kg, P < 0.001) and 12 (−2.22 kg, P < 0.001), whereas no differences in BFM were observed in patients whose HbA1c levels remained unchanged from baseline (Fig. 2a). In addition, a significant reduction in BMI was observed among patients whose HbA1c levels improved at week 12 (−1.01 kg/m2, P < 0.05) (Fig. 2b). Although the patients whose HbA1c levels did not improve tended to lose more MM and SkMM than those whose HbA1c levels improved, there were no significant differences in MM and SkMM between the two strata (Fig. 2c and 2d). Of note, significant differences in BFM, but not BMI, MM, and SkMM, were confirmed between the two strata at weeks 8 and week 12 (Fig. 2).
Fig. 2.
Changes from baseline in body composition stratified by HbA1c response. Changes from baseline in BFM (kg), BMI (kg/m2), MM (kg), and SkMM (kg) are shown in a, b, c, and d, respectively. Red and blue lines indicate the group in which HbA1c levels improved and the group in which HbA1c levels did not improve, respectively (n = 10 and n = 8, respectively). BFM body fat mass, BMI body mass index, MM muscle mass, SkMM skeletal muscle mass. Error bars represent standard deviations. The changes from baseline were analyzed at each interval using Tukey’s multiple comparison test for comparisons between strata. P values denote differences between baseline data and week 4, 8, or 12 data. †† P < 0.01, † P < 0.05. Dunnett’s multiple comparison test was used to compare data time points with those measured at baseline within a stratum. P values denote differences between baseline and week 4, 8, or 12. ***P < 0.001, **P < 0.01, *P < 0.05
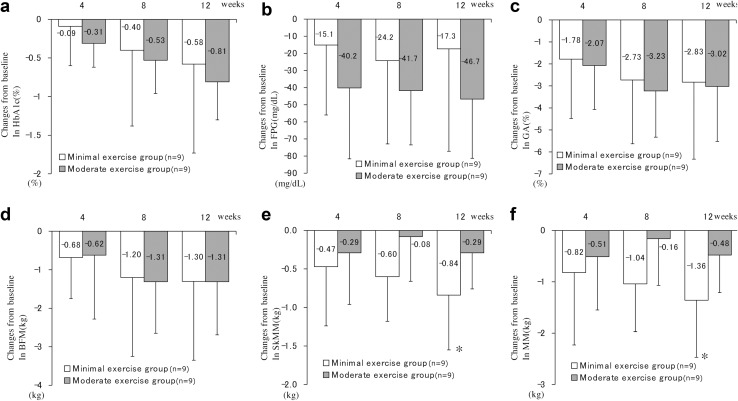
Stratification analysis by exercise
The benefits of exercise as a therapy for diabetes are well recognized. To investigate the influence of exercise habits on HbA1c levels and body composition in ipragliflozin-treated patients, patients were stratified by their physical activity levels, and their glycemic control and body composition were analyzed (Fig. 3). We compared the energy consumption of the moderate exercise group and minimal exercise group. The mean MET energies of the moderate exercise group and minimal exercise group were 348 ± 152 and 115 ± 63 kcal/day, respectively (n = 9). A significant difference between the two groups was confirmed (P < 0.01). The magnitudes of reductions in HbA1c, FPG, and GA levels were larger for the moderate exercise group, albeit not significantly, than for the minimal exercise group (Fig. 3a–c). Similar patterns of changes from baseline in BFM were observed between the moderate and minimal exercise groups (Fig. 3d). By contrast, the magnitudes of reductions in SkMM and MM in the minimal exercise group were larger, albeit not significantly, than those in the moderate exercise groups (Fig. 3e, f). In addition, the minimal exercise group displayed significant decreases in SkMM (−0.84 kg, P < 0.05) and MM (−1.36 kg, P < 0.05) at week 12 versus baseline (Fig. 3e and 3f), whereas no significant changes in these parameters were recorded in the moderate exercise group.
Fig. 3.
Changes in HbA1c, FPG, GA, BFM, SkMM, and MM stratified by physical activity levels. Changes from baseline in HbA1c (%), FPG (mg/dl), GA (%), BFM (kg), SkMM (kg), and MM (kg) are shown in a, b, c, d, e, and f, respectively. Red and blue lines denote the moderate and minimal exercise groups, respectively (n = 9, respectively). Error bars represent standards deviations. P values denote differences between baseline data and week 4, 8, or 12 data. *P < 0.05
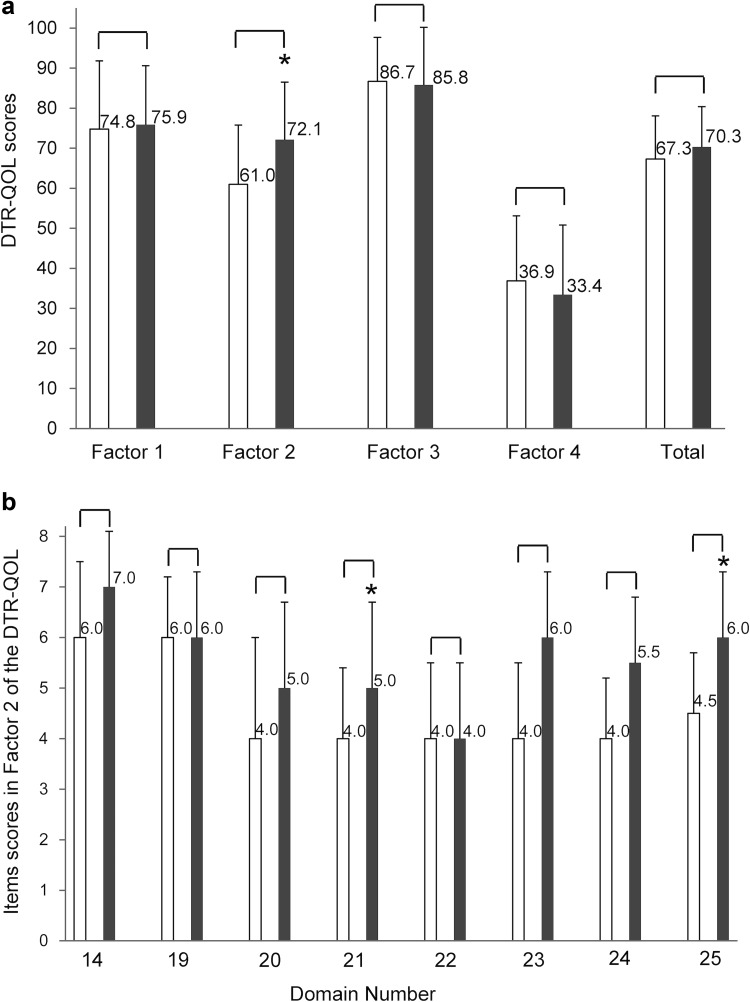
DTR-QOL
The DTR-QOL questionnaire is a disease-specific, self-administered questionnaire that assesses the influence of diabetes treatment on patient QOL regardless of the treatment method [17]. Although factor 1 and total scores slightly increased and factor 3 and 4 slightly decreased at week 12, these changes from baseline did not show any significant differences (Fig. 4a). Of note, there were significant improvements in DTR-QOL subdomain scores, including the description of anxiety and dissatisfaction with diabetes treatment as factor 2, compared to baseline (P < 0.05; n = 18). Among the items within factor 2, significant improvements from baseline at week 12 were seen in the scores of item nos. 21 and 25 (P < 0.05) (Fig. 4b).
Fig. 4.
Changes in factor and total scores in patients with type 2 diabetes mellitus (T2DM) according to ipragliflozin treatment. DTR-QOL scores (a) and item scores in factor 2 (b). The questions for each item are as follows: 14: I am bothered by weight gain with my current diabetes treatment; 19: I have uncomfortable symptoms due to hyperglycemia (high blood glucose); 20: I am worried about high blood glucose; 21: I am dissatisfied that my blood glucose is unstable (high and low); 22: I am worried that complications might get worse with my current diabetes treatment; 23: I get anxious thinking about living while on my current diabetes treatment; 24: I find it unbearable to think that even if I continue my current diabetes treatment, my diabetes may not be cured; 25: I am concerned that if I continue my current diabetes treatment, the efficacy may diminish. The white and gray columns indicate item scores at baseline and week 12, respectively. Data are expressed as mean ± standard deviation for all assigned participants (n = 18). P values denote significant differences between baseline data and week 12 data. *P < 0.05
Safety
No major hypoglycemic episodes occurred, and no patients discontinued treatment with the study medication because of hypoglycemia. Two AEs (folliculitis and genital pruritus) (10.0%) were reported. One AE (genital pruritus, 5.0%) was related to the study treatment. Both patients discontinued treatments because of these AEs. No SAEs were reported. No cancers and deaths were reported in this small-scale and short-duration study.
Discussion
In the present study, we investigated the efficacy of ipragliflozin via a multilateral approach in terms of body composition, exercise habits, and patient QOL. Despite the small number of patients, 12-week administration of ipragliflozin in Japanese patients with T2DM and inadequate glycemic control resulted in clinically meaningful improvements in glycemic control; mean TBW, BMI, and WC; and serum lipid levels during the study. Although no exercise intervention was conducted for patients, the minimal exercise group exhibited slight but significant reductions in SkMM and MM, but not BFM, compared to the findings in the moderate exercise group. Furthermore, the score for factor 2 in the DTR-QOL questionnaire was significantly improved at week 12.
The mean changes in HbA1c and FPG levels from baseline to week 12 obtained in this study were comparable to those observed in earlier studies of ipragliflozin [12, 13] as well as a metformin add-on dose finding study with ipragliflozin [14]. Consistent with the previous observation that GA levels decreased more rapidly than HbA1c levels as glycemic control improved [20], ipragliflozin rapidly reduced GA levels by week 4, and its levels plateaued by week 8. Importantly, the proportion of patients achieving HbA1c <7.0% was 4/18 (22.2%), which was comparable to the finding of a previous report [12]. Thus, these results suggested that the administration of ipragliflozin alone or in combination with existing oral hypoglycemic agents efficiently improved glycemic control in Japanese patients with T2DM receiving conventional therapy.
A reduction in TBW following ipragliflozin treatment was observed progressively in this study even though patients received several oral anti-hyperglycemic agents that were associated with weight gain. Notably, our stratified analysis of HbA1c revealed that patients whose HbA1c levels improved exhibited significant reductions in BFM and BMI, but not MM and SkMM, compared with patients whose HbA1c levels were not improved. Thus, ipragliflozin could be a useful anti-hyperglycemic medication for obese Japanese patients because it efficiently reduces BFM.
Our analysis of body composition stratified by physical activity level uncovered significant reductions in SkMM and MM, but not BFM, in the minimal exercise group compared to the findings in the moderate exercise group. This result suggests that the instructions to intensify the exercise (e.g., substantially exercise more than twice a week) for T2DM patients undergoing ipragliflozin have a beneficial effect on glycemic control or body composition. It is important to thoroughly inform patients that intensifying exercise therapy at the beginning of use of SGLT2 inhibitors will reduce fat and any decrease in body weight, loss of MM will be kept to a minimum. As far as we know, this is the first time that such a specific proposal has been made.
However, it remained unclear (1) whether ipragliflozin enhances SkMM and MM loss in T2DM patients and (2) whether the impact of other SGLT2 inhibitors on improving or maintaining SkMM through exercise is comparable to that of ipragliflozin. Bolinder et al. reported a positive change in MM in terms of the percentage of body fat when dapagliflozin was taken for 2 years and also found that in body weight changes, a reduction in total lean tissue mass occurs as body fat decreases [21]. Considering this in terms of the effects of SGLT2 inhibitors on urinalysis, this finding indicates the possibility that absolute MM decreases along with a decrease in total fat mass.
Recently, Sano et al. (reference attached; Sano Motoaki) reported that use of SGLT2 inhibitors resulted in increased maximal hand grip strength [22]. This was assumed to be associated with the reduced chronic inflammation and improved adipokine balance caused by reduced visceral adipose tissue mass and reduced oxidative stress due to lipotoxicity. Although their study assessed the MM of the upper limbs indirectly and used three types of SGLT2 inhibitors, I think that it does not contradict our result because this result was the index only for hand grip strength.
These unresolved issues in this study are worth addressing in future studies in larger population studies.
Consistent with a previous report claiming that SGLT2 inhibitor treatment reduced SBP by ~2–6 mmHg [23], the administration of ipragliflozin reduced SBP by ~6–8 mmHg through the study period. This reduction of SBP could be explained by osmotic diuresis induced by glucose in the urine and sodium loss [8]. We also confirmed (1) a significant increment of hematocrit level caused by an osmotic diuretic effect at week 12, consistent with a previous report [12], and (2) reduction of the serum uric acid level. Furthermore, ipragliflozin treatment significantly reduced eGFR at weeks 4 and 8, consistent with the previous reports [24, 25]. By inhibiting SGLT2 activity with SGLT2 inhibitors, NaCl reabsorption in the proximal tubule is decreased, resulting in the increment of Na+ or Cl− delivery to the macula densa, augmenting the tubuloglomerular feedback signal and reducing the single-nephron glomerular filtration rate [26]. Thus, SGLT2 inhibitors potentially exhibit renal protection by lowering intraglomerular pressure through the improvement of afferent arteriolar vasodilation.
Several other lipid parameters including LDL-C, TG, and TC levels were also improved by ipragliflozin. In particular, although some previous studies reported that LDL-C levels were increased by the administration of SGLT-2 inhibitors [27, 28], our study uncovered a significant reduction of LDL-C levels at week 12. Taken together with findings that ipragliflozin improved hyperlipidemia and hepatic steatosis and decreased epididymal adipose tissue and body weights in T2DM mice [29], these findings suggest that ipragliflozin has therapeutic potential for improving lipid abnormalities and obesity in patients with T2DM.
Although this study included relatively young obese patients with a long diabetes duration who had difficulty in maintaining glycemic control, factor 2 in the DTR-QOL subdomain scores was significantly improved at week 12. Factor 2 in the DTR-QOL subdomain scores at the baseline should have been low because the patients were notified of the possible occurrence of dehydration, rash, and a feeling of hunger when they were initially administered ipragliflozin. However, the patients had practically none of these AEs after ipragliflozin administration. Therefore, the significant improvement of factor 2 in the DTR-QOL subdomain scores might be attributed to the removal of anxiety with treatment after 12 weeks of ipragliflozin administration. In particular, a significant improvement was observed after 12 weeks from baseline for “No. 21: I am dissatisfied that my blood glucose is unstable (high and low)” and “No. 25: I am concerned that if I continue my current diabetes treatment the efficacy (effectiveness) may diminish.” One of the possible reasons for this may be because, in Japan, when a medicine with a new mechanism of action is introduced, patients usually receive a detailed explanation of potential AEs. However, they often experience no AEs, making them feel that the medicine is safe. Another major reason may be associated with the treatment effects such as “decrease in weight” and “well-controlled blood glucose” with no development of hypoglycemia and improvement in blood glucose levels at every hospital visit.
Ishii claimed that glycemic control, hypoglycemia, weight gain, overall health status, and communication with their physician influence the QOL of patients receiving diabetes treatment [17]. To elucidate what kinds of clinical parameters contribute to the improvement of the DTR-QOL score, we performed correlation analysis between DTR-QOL and HbA1c and body composition parameters determined in this study, but we found no such correlations between factor 2 in the DTR-QOL and HbA1c or body composition parameters (data not shown). Intriguingly, Yamazaki et al. recently reported a correlation between the total DTR-QOL scores and HbA1c levels but not body weight in patients receiving dapagliflozin [30]. Future studies will need to address this discrepancy.
Two medication-related AEs (genital pruritus and folliculitis) occurred in this study. After the recommendations from the committee on the proper use of SGLT2 inhibitors were announced [31], we discontinued their administration as soon as possible when erythema or alternative skin symptoms occurred because of the administration of SGLT2 inhibitors. However, genital itching disappeared after discontinuation and the patient with genital pruritus did not have to go to a gynecologist. The other skin lesion was a furuncle, but after discontinuation of the drug, a dermatologist determined that this was not an adverse drug reaction. Therefore, these AEs are considered to be minor side effects. Of note, there were no episodes of severe hypoglycemia even among patients receiving ipragliflozin in combination with an SU, suggesting that ipragliflozin, through its insulin-independent action, can safely be given in combination with other established diabetes medications.
Study limitations
This was an open-label observational study. This short duration is a limitation to assessing the long-term usefulness of ipragliflozin administration. However, the study investigated an association between exercise and the effects of ipragliflozin in the clinical setting, and we feel that this is of great significance because it could be generally applied in daily clinical practice, drawing patients’ attention to the association in medication guidance, for other SGLT2 inhibitors as well.
Conclusion
Regardless of the background anti-hyperglycemic therapy, ipragliflozin efficiently improved glycemic control, body weight, and lipid parameters in patients with T2DM in clinical practice in Japan, with a low incidence of AEs. These findings provide further support of the clinical utility of ipragliflozin as an add-on therapy in Japanese T2DM patients with inadequate glycemic control despite diet modification and exercise therapy or conventional anti-hyperglycemic therapy. Also, in future medication guidance for SGLT2 inhibitors, it should be effective to thoroughly inform patients that intensifying exercise therapy at the beginning of use of SGLT2 inhibitors will keep loss of MM to a minimum.
Acknowledgements
This study was supported by a research grant from Astellas Pharma Inc. (Tokyo, Japan).
Conflict of interest
All authors except one (M.K.) report no conflicts of interest. One author (M.K.) has received honoraria and travel expenses for speaking at an event organized by Astellas Pharma Inc. and Mitsubishi Tanabe Pharma (Osaka, Japan). The sponsor had no role in the study design; collection, analysis, and interpretation of data; writing of the manuscript; or the decision to submit the manuscript for publication.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients for being included in the study.
References
- 1.Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–2279. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman RR. Assessing the potential for alpha-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract. 1998;40:S21–S25. doi: 10.1016/S0168-8227(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 3.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429–442. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 5.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Buse JB, Davidson MB, American Diabetes Association. European Association for Study of Diabetes et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilding J. Thiazolidinediones, insulin resistance and obesity: finding a balance. Int J Clin Pract. 2006;60:1272–1280. doi: 10.1111/j.1742-1241.2006.01128.x. [DOI] [PubMed] [Google Scholar]
- 8.Chao EC, Henry RR. SGLT2 inhibition—a novel strategy for diabetes treatment. Nat Rev Medication Discov. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 9.Veltkamp SA, Kadokura T, Krauwinkel WJ, et al. Effect of -pragliflozin (ASP1941), a novel selective sodium-dependent glucose co-transporter 2 inhibitor, on urinary glucose excretion in healthy subjects. Clin Medication Investig. 2011;31:839–851. doi: 10.1007/BF03256922. [DOI] [PubMed] [Google Scholar]
- 10.Tahara A, Kurosaki E, Yokono M, et al. Antidiabetic effects of SGLT2-selective inhibitor ipragliflozin in streptozotocin-nicotinamide-induced mildly diabetic mice. J Pharmacol Sci. 2012;120:36–44. doi: 10.1254/jphs.12089FP. [DOI] [PubMed] [Google Scholar]
- 11.Tahara A, Kurosaki E, Yokono M, et al. Pharmacological profile of ipragliflozin (ASP1941), a novel selective SGLT2 inhibitor, in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:423–436. doi: 10.1007/s00210-011-0713-z. [DOI] [PubMed] [Google Scholar]
- 12.Kashiwagi A, Kazuta K, Yoshida S, et al. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:382–391. doi: 10.1111/jdi.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonseca VA, Ferrannini E, Wilding JP, et al. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complicat. 2013;27:268–273. doi: 10.1016/j.jdiacomp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Wilding JP, Ferrannini E, Fonseca VA, et al. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes Obes Metab. 2013;15:403–409. doi: 10.1111/dom.12038. [DOI] [PubMed] [Google Scholar]
- 15.Yabe D, Nishikino R, Kaneko M, et al. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin Medication Saf. 2015;14:795–800. doi: 10.1517/14740338.2015.1034105. [DOI] [PubMed] [Google Scholar]
- 16.Murase N, Katsumura T, Ueda C, et al. Validity and reliability of Japanese version of International Physical Activity Questionnaire. Journal of Health and Welfare Statistics. 2002;49:1–9. [Google Scholar]
- 17.Ishii H. Development and psychometric validation of the Diabetes Therapy-Related QOL (DTR-QOL) questionnaire. J Med Econ. 2012;15:556–563. doi: 10.3111/13696998.2012.665111. [DOI] [PubMed] [Google Scholar]
- 18.Kashiwagi A, Kazuta K, Yoshida S, et al. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:382–391. doi: 10.1111/jdi.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Japan Society for the Study of Obesity Examination Committee of Criteria for ‘Obesity Disease’ in Japan. Circ J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi S, Uchino H, Shimizu T, et al. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J. 2007;54:139–144. doi: 10.1507/endocrj.K06-103. [DOI] [PubMed] [Google Scholar]
- 21.Bolinder J, Ljunggren Ö, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16:159–169. doi: 10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- 22.Sano M, Meguro S, Kawai T, Suzuki Y, et al. Increased grip strength with sodium–glucose cotransporter 2. J Diabetes. 2016;8:736–737. doi: 10.1111/1753-0407.12402. [DOI] [PubMed] [Google Scholar]
- 23.Ohkura T. Ipragliflozin: a novel sodium–glucose cotransporter 2 inhibitor developed in Japan. World J Diabetes. 2015;6:136–144. doi: 10.4239/wjd.v6.i1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–1027. doi: 10.1111/dom.12348. [DOI] [PubMed] [Google Scholar]
- 25.Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert RE. Sodium–glucose linked transporter-2 inhibitors: potential for renoprotection beyond blood glucose lowering? Kidney Int. 2014;86:693–700. doi: 10.1038/ki.2013.451. [DOI] [PubMed] [Google Scholar]
- 27.Bode B, Stenlöf K, Harris S, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17:294–303. doi: 10.1111/dom.12428. [DOI] [PubMed] [Google Scholar]
- 28.Matthaei S, Bowering K, Rohwedder K, et al. Durability and tolerability of dapagliflozin over 52 weeks as add-on to metformin and sulphonylurea in type 2 diabetes. Diabetes Obes Metab. 2015 doi: 10.1111/dom.12543. [DOI] [PubMed] [Google Scholar]
- 29.Tahara A, Kurosaki E, Yokono M, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–255. doi: 10.1016/j.ejphar.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki M, Higo N, Kaneko T, et al. SGLT2 inhibitors as the trigger for diabetes care: reconfirmed importance of behavior modification after drug administration. J Jpn Diab Soc. 2015;58:745–752. [Google Scholar]
- 31.Japan Diabetes Society Committee on the Proper Use of SGLT2 inhibitors [Recommendations on the proper use of SGLT2 inhibitors] (2014). http://www.jds.or.jp/modules/important/index.php?page=article&storyid=48. Accessed 08 Feb 2016.