Introduction
The development of a sensitive assay using an extracellular flux analyzer has enabled the determination of lactate, the end product of glycolysis, by measuring the pH (extracellular acidification rate; ECAR) of living cells, such as circulating leukocytes. Mitochondrial oxidative phosphorylation can also be simultaneously measured by measuring the oxygen consumption rate (OCR). The basal OCR/ECAR ratio is a marker of the relative use of glycolysis and oxidative phosphorylation (OXPHOS). Among various immune-mediated cells, OXPHOS is highest in lymphocytes (2.87 ± 0.22 pmol O2/mpH), followed in decreasing OXPHOS by monocytes (1.24 ± 0.13 pmol O2/mpH) and neutrophils (0.47 ± 0.08 pmol O2/mpH) [1]. The amount of energy produced and the balance between glycolysis and mitochondrial OXPHOS are critical factors in the immune responses mediated by the activation of the various immune-mediated cells. Hyperglycemia, chronic inflammation and hyperinsulinemia observed in type 2 diabetes and obesity may affect the metabolic program in immune-mediated cells and generate harmful effects on the immune defense against infection and carcinogenesis.
Neutrophils
Neutrophils contain relatively few mitochondria, and most of the energy of these cells is derived from glycolysis. The effects of hyperglycemia and/or other metabolic abnormalities can increase susceptibility and the predisposition to infection. Both resting neutrophils and those stimulated by phorbol 12-myristate 13-acetate (PMA) and zymosan from diabetic individuals produce more superoxide and have enhanced respiratory burst activities compared to those from healthy individuals. The increased oxidative respiratory burst activity in patients with diabetes may predispose to infection and lead to chronic inflammation [2]. In 2004, a new neutrophil-mediated defense mechanism, neutrophil extracellular traps (NETs), was reported. These traps are networks of extracellular fibers composed of DNA, histones and anti-microbial peptides that are able to kill extracellular pathogens and minimize damage to host cells. The formation of NETs is strictly dependent on glucose and to a lesser extent on glutamine, and is also enhanced by the glucose transporter-1 (GLUT-1), glucose uptake, and glycolysis rate under PMA stimulation [3]. NET formation can metabolically be divided into two phases, the first being the chromatin decondensation process, which is independent of exogenous glucose, and the second being NET release, which is strictly dependent on exogenous glucose and glycolysis [3]. Interleukin-6 (IL-6) is a potent inducer of energy-dependent NET formation, and hyperglycemia mimics the proinflammatory condition which may lead to a blunted response to external pathogens and susceptibility to infections [4].
Monocytes and macrophages
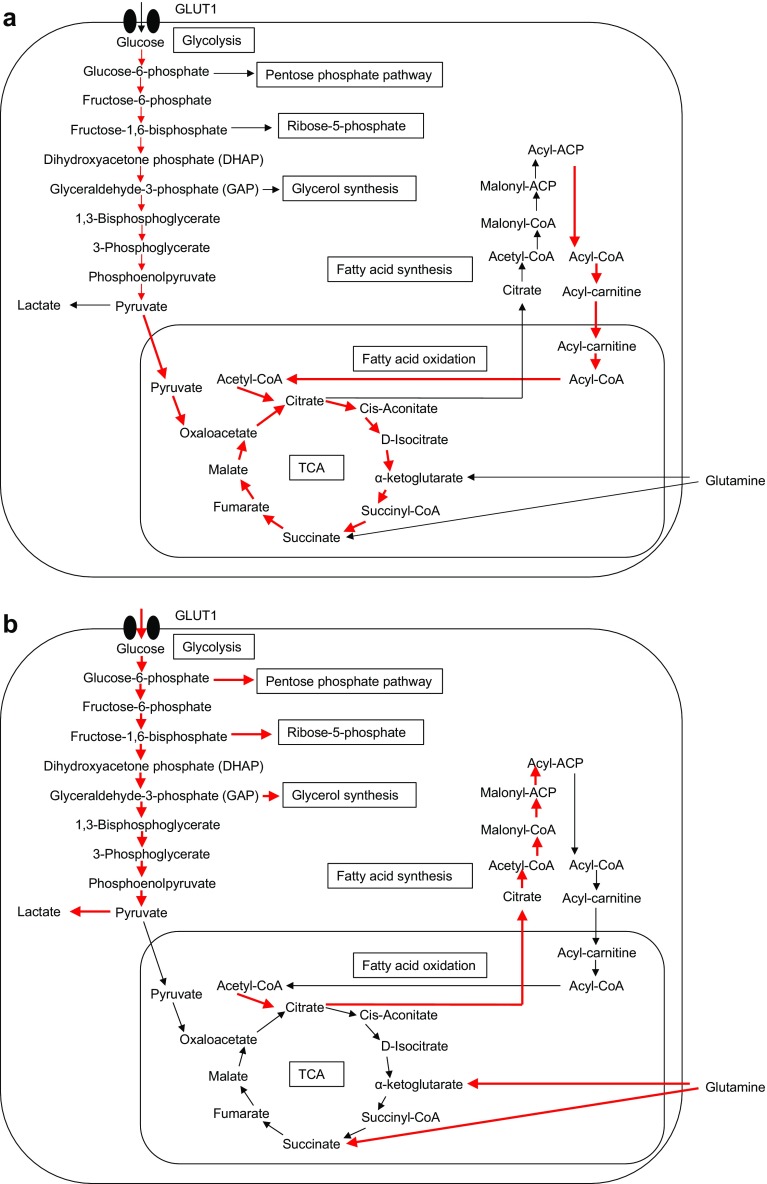
Monocytes and macrophages are also active constituents of the innate immune system. Macrophages consist of a functionally heterogeneous cell population that includes pro-inflammatory M1 macrophages induced by the classical activation program by interferon γ (IFN-γ), IL-1β and lipopolysaccharide (LPS) and anti-inflammatory M2 macrophages induced through an alternative activation program by IL-4 and IL-13. M1 macrophages increase glycolysis and lactate release and decrease the OCR (Fig. 1a). In contrast, M2 macrophages mainly use OXPHOS in metabolic pathways [5] (Fig. 1b). Obesity induces lipolysis and the release of pro-inflammatory free fatty acids and chemokine (C–C motif) ligand 2, which in turn recruit circulating monocytes into adipose tissues where they are converted into pro-inflammatory M1 macrophages. Although the precise mechanism for the metabolic switch between M1 and M2 macrophages is still unexplored, Granulocyte-macrophage colony-stimulating factor increases LPS-induced acute glycolysis in murine bone marrow-derived macrophages associated with de novo synthesis of GLUT-1, -3, and -4 [6].
Fig. 1.
Metabolic pathways of aerobic glycolysis and mitochondrial oxidative phosphorylation (OXPHOS). a Normal resting cells, such as M2 macrophages, conventional dendritic cells (DCs) and naïve and memory T cells, generate ATP through a combination of glycolysis and mitochondrial OXPHOS by the tricarboxylic acid (TCA) cycle. b Actively proliferating cells, such as cancer cells, activated DCs, activated M1 macrophages, and effector T cells, switch metabolism from OXPHOS to aerobic glycolysis. GLUT1 Glucose transporter-1, ACP acyl-carrier protein, CoA coenzyme A
Conventional dendritic cells (DCs) that are non-activated are rather catabolic in nature and use fatty acid oxidation (FAO) and OXPHOS to generate ATP through the combination of glycolysis and tricarboxylic acid (TCA) cycles. The activation of DCs by toll-like receptor ligands results in a switch from OXPHOS to glycolysis, with a resulting increased glycolysis rate (Fig. 1b), by increasing the transcription activities of inducible nitric oxide synthase expression, which in turn produces nitric oxide and inhibits the mitochondrial respiratory chain [7]. Inflammatory DCs also produce IFN-α/β, and they inhibit the transcriptional activities of OXPHOS genes while increasing those of glycolytic genes via hypoxia-inducible factor 1-alpha [7].
Lymphocytes
In cancer cells, tumorigenesis promotes the metabolic pathway termed aerobic glycolysis, i.e. Warburg effects. The quiescent lymphocytes, such as naïve and memory lymphocytes, store ATP reserves via OXPHOS and FAO to prepare for activation. Like cancer cells, once activated lymphocytes escape the quiescent state, proliferate, produce various cytokines and shift to aerobic glycolysis (Fig. 1b). Although they are inefficient in terms of ATP production, they do provide the important metabolites for the synthesis of the macromolecules required for proliferation, such as glucose-6-phosphate and fructose-1,6-biphosphate for the pentose phosphate pathway and ribose synthesis and glyceraldehyde-3-phosphate (G3P) for glycerol synthesis. The influx of pyruvate into the TCA cycle is reduced, while these cells display a large influx of succinate derived from glutaminolysis via α-ketoglutarate, which is ultimately converted to citrate and used in fatty acid synthesis.
Under low glycolytic flux in resting CD4+ cells, G3P dehydrogenase (GAPDH) associates with the 3′ untranslated region of IFNγ mRNA and prevents its translation. Upon the activation of CD4+ cells, signaling through the T-cell receptor stimulates aerobic glycolysis, and the G3P provided by glycolysis binds to GAPDH as a substrate and inhibits the binding of GAPDH to IFNγ mRNA [8]. The glycolytic activities also regulate the differentiation and effector function of CD8+ T cells (Fig. 1b). Following antigen stimulation, naïve CD8+ T cells rapidly increase their uptake of glucose and glutamine and provide ATP and fatty acids, a metabolic reprogramming which supports cell proliferation that is characterized by short survival and reduced anti-tumor activities. As effector T-cell response subsides, and memory T cells increase mitochondrial integrity and metabolism and activate OXPHOS and FAO to sustain prolonged cell survival and anti-tumor activities. The memory CD8+ cells engage in fatty acid synthesis via lysosome-based lipid storage and the concurrent FAO pathway [9]. Investigation of mitochondrial morphology demonstrated that memory T cells have fused mitochondria while effector T cells have fissed mitochondria. Fusion protein Opa1 is required for memory T cells [10].
Conclusion
The metabolic switch between aerobic glycolysis and OXPHOS is critical for the proper functioning of various immune-mediated cells, such as macrophages and T lymphocytes. The metabolic shift to OXPHOS and FAO is important to sustain prolonged cell survival of memory T cells, while aerobic glycolysis is crucial for the proliferation and rapid production of bio-substances for the synthesis of macromolecules in effector T cells. Insulin resistance and microinflammation may influence the metabolic pathways in various immune-mediated cells of patients with diabetes; these effects may be linked to the development of susceptibility for various infection and malignancies. In addition, certain pharmaceutical interventions may have beneficial effects on such metabolic pathways. For example, metformin increases the number of CD8+ tumor-infiltrating lymphocytes and recovers the multiple functionality of these cells with recovered production of IL-2, TNFα, and IFNγ [11]. Further studies are required to clarify the relation between aspects of intracellular metabolic programing in various immune cells in patients with diabetes and obesity.
Conflict of interest
The author declares that he has no conflicts of interest.
Human rights statement and informed consent
This article does not report any studies with human or animal subjects performed by the author.
References
- 1.Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hand WL, Hand DL, Vasquez Y. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res Clin Pract. 2007;76(1):44–50. doi: 10.1016/j.diabres.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Espinosa O, Rojas-Espinosa O, Moreno-Altamirano MM, Lopez-Villegas EO, Sanchez-Garcia FJ. Metabolic requirements for neutrophil extracellular traps formation. Immunology. 2015;145(2):213–224. doi: 10.1111/imm.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi MB, Lad A, Bharath Prasad AS, Balakrishnan A, Ramachandra L, Satyamoorthy K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013;587(14):2241–2246. doi: 10.1016/j.febslet.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 5.Zhu L, Zhao Q, Yang T, Ding W, Zhao Y. Cellular metabolism and macrophage functional polarization. Int Rev Immunol. 2015;34(1):82–100. doi: 10.3109/08830185.2014.969421. [DOI] [PubMed] [Google Scholar]
- 6.Na YR, Gu GJ, Jung D, Kim YW, Na J, Woo JS, Cho JY, Youn H, Seok SH. GM-CSF induces inflammatory macrophages by regulating glycolysis and lipid metabolism. J Immunol. 2016;197(10):4101–4109. doi: 10.4049/jimmunol.1600745. [DOI] [PubMed] [Google Scholar]
- 7.Everts B, Pearce EJ. Metabolic control of dendritic cell activation and function: recent advances and clinical implications. Front Immunol. 2014;5:203. doi: 10.3389/fimmu.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41(1):75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJ, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166(1):63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci USA. 2015;112(6):1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]