Abstract
Aims
Our purpose was to clarify the predictive clinical characteristics of add-on therapy using dapagliflozin in patients with type 2 diabetes mellitus.
Methods
This single-center, open-label, pilot study was conducted in 50 patients with type 2 diabetes mellitus. They were treated with 50 mg dapagliflozin, once daily. A total of 46 patients were successfully followed over 12 weeks of treatment with dapagliflozin. They were assessed for several clinical parameters before the start of the study and at 12 weeks. Multiple linear regression analysis was used to search for independent predictors of reduction of hemoglobin A1c (HbA1c) levels after 12 weeks of dapagliflozin add-on treatment (ΔHbA1c).
Results
Dapagliflozin administration for 12 weeks resulted in significant reductions in baseline body mass index, systolic blood pressure, fasting plasma glucose, HbA1c, alanine aminotransferase, and uric acid. We were also able to show that HbA1c levels and low-density lipoprotein cholesterol (LDL-C) significantly correlated with ΔHbA1c. Furthermore, multiple linear regression analysis showed that baseline HbA1c and LDL-C significantly correlated with ΔHbA1c.
Conclusion
Our prospective 12-week study showed that baseline HbA1c and LDL-C significantly contribute to the HbA1c-lowering effect of dapagliflozin.
Trial registration
UMIN Clinical Trials Registry (UMIN000014922).
Keywords: Type 2 diabetes mellitus, Dapagliflozin, Sodium-glucose cotransporter 2 inhibitor, Predictive clinical characteristics
Introduction
There is a continuing unmet need for novel glucose-lowering therapies that provide durable glycemic control while avoiding hypoglycemia, weight gain, and fluid retention, which are recognized problems with a number of existing glucose-lowering drugs [1–3]. Dapagliflozin is a new orally active sodium-glucose cotransporter 2 (SGLT2) inhibitor under development by Bristol-Myers Squibb and AstraZeneca. Dapagliflozin’s mechanism of action is different from and complementary to the mechanism of currently available antidiabetic medications, resulting in direct and insulin-dependent elimination of glucose by the kidneys. The resulting glycosuria is associated with plasma glucose reductions and caloric loss, leading to weight reduction, and mild osmotic diuresis, leading to blood pressure reduction [4, 5]. Furthermore, because SGLT2 is almost exclusively expressed in the kidney, the highly selective nature of dapagliflozin minimizes the risk of off-target effects [6]. As such, dapagliflozin offers an important additional strategy for improving glycemic control in patients with type 2 diabetes mellitus.
In order to use dapagliflozin as an add-on therapy for patients with type 2 diabetes mellitus, it is important to know the predictive clinical characteristics for its therapeutic efficacy. Therefore, we performed multiple linear regression analyses, with the index of reduction in the hemoglobin A1c (HbA1c) level as the dependent variable, to search for independent predictors of the reduction in the HbA1c level after 12 weeks of dapagliflozin treatment in Japanese patients with type 2 diabetes mellitus.
Materials and methods
Subjects
This single-center, open-label, pilot study was conducted in 50 patients with type 2 diabetes mellitus whose HbA1c was higher than 6.0 % at Iwasaki Naika Clinic. Patients were enrolled between August 2014 and January 2015. The specific patient eligibility criteria for inclusion were as follows: male and female patients with type 2 diabetes mellitus; age ≥20 years; treated with hypoglycemic agents that had not been changed for at least 6 months. Patients were excluded if they had type 1 diabetes; had nephropathy (serum creatinine: >1.1 mg/dl); were pregnant or breast-feeding; intended to become pregnant or were female and of childbearing age and not using adequate contraceptive methods; had known/suspected allergy to trial medication(s), excipients, or related products; and had any contraindications to dapagliflozin-therapy.
The 50 patients with type 2 diabetes mellitus were treated with 50 mg dapagliflozin once daily. They were assessed for several clinical parameters, including body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting plasma glucose (FPG), HbA1c levels, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), creatinine, and uric acid before the start of the study and at 12 weeks. The primary outcome of the study was changes in HbA1c at 12 weeks after the start of the study (ΔHbA1c).
The study protocol conformed to the principles of the Declaration of Helsinki, and the study was approved by an institutional review committee at Iwasaki Naika Clinic. Written informed consent was obtained from all the patients. This study is registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000014922).
Statistical analysis
Data are expressed as mean ± standard deviation, unless indicated otherwise. Differences between two variables were examined for statistical significance using two-tailed Student’s paired or unpaired t tests where appropriate. Baseline HbA1c level minus HbA1c level at 12 weeks after the start of add-on dapagliflozin is expressed as ΔHbA1c. Baseline BMI level minus BMI level at 12 weeks after the start of add-on dapagliflozin is expressed as ΔBMI. Because the relationships between continuous variables (i.e., age, duration of diabetes, BMI, SBP, DBP, FPG, HbA1c, AST, ALT, GGT, LDL-C, HDL-C, TG, creatinine, uric acid, and ΔHbA1c) were nonlinear, correlations between sets of two independent continuous variables were determined using Spearman’s rank correlation coefficient. Multiple stepwise regression analysis was used to determine independent predictors of ΔHbA1c and ΔBMI. All variables considered a clinically meaningful parameter for a given patient’s background were employed as independent variables in multivariate analysis (i.e., sex, age, duration of diabetes, BMI, HbA1c, and LDL-C). The F value for the inclusion of the variables was set at 4.0. The level of statistical significance was set at P < 0.05. All statistical analyses were performed with StatView version 5.0 for Windows (SAS Institute, Cary, NC, USA).
Results
Patient demographics and baseline characteristics
A total of 50 patients were enrolled in the present study, and 46 were successfully followed over the 12-week treatment with dapagliflozin. Four patients were excluded from analysis: one with cystitis, one with a feeling of exhaustion, and two who dropped out (Fig. 1). The baseline demographics and clinical characteristics of the study subjects are shown in Table 1. The mean age was 62.5 ± 10.8 years, mean HbA1c was 7.71 ± 0.925 %, mean BMI was 26.4 ± 4.85 kg/m2, and mean disease duration was 14.8 ± 8.08 years. Table 1 also shows the number of participants who received antidiabetic agent(s) orally prior to the study: sulfonylurea, 38; metformin, 30; thiazolidinedione, 7; dipeptidyl peptidase-4 (DPP-4) inhibitor, 47; insulin injection, 11; glucagon-like peptide-1 (GLP-1) analogue injection, 2.
Fig. 1.
Patient attrition
Table 1.
Baseline demographics and characteristics of the patients
| Variables | |
|---|---|
| N | 46 |
| Sex (male/female) | 15/31 |
| Age (years) | 62.5 ± 10.8 |
| Duration of diabetes (years) | 14.3 ± 8.08 |
| BMI (kg/m2) | 26.0 ± 4.85 |
| SBP (mmHg) | 131 ± 12.0 |
| DBP (mmHg) | 71.3 ± 10.9 |
| FPG (mg/dl) | 139 ± 18.1 |
| HbA1c (%) | 7.72 ± 0.925 |
| AST (IU/l) | 23.8 ± 13.2 |
| ALT (IU/l) | 31.7 ± 21.1 |
| GGT (IU/l) | 49.6 ± 34.8 |
| LDL-C (mg/dl) | 89.9 ± 20.9 |
| HDL-C (mg/dL) | 59.7 ± 18.5 |
| TG (mg/dl) | 124 ± 55.1 |
| Creatinine (mg/dl) | 0.667 ± 0.156 |
| Uric acid (mg/dl) | 4.94 ± 1.39 |
| Antidiabetic medications (n) | |
| Sulfonylurea | 36 |
| Metformin | 27 |
| Thiazolidinedione | 6 |
| DPP-4 inhibitor | 43 |
| Insulin injection | 10 |
| GLP-1 analog injection | 2 |
| Hypolipidemic agents (n) | |
| Statins | 35 |
| Fibrates | 5 |
| Antihypertensive agents (n) | |
| ARBs/ACEIs | 29 |
| CCBs | 23 |
| β-blockers | 0 |
| Diuretics | 4 |
Data are mean ± SD
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, FPG fasting plasma glucose, HbA1c hemoglobin A1c, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT γ-glutamyltransferase, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, TG triglycerides, DPP-4 dipeptidyl peptidase-4, GLP-1 glucagon-like peptide-1, ARBs angiotensin II receptor blockers, ACEIs angiotensin-converting enzyme inhibitors, CCBs calcium channel blockers
Clinical characteristics of 46 patients who received dapagliflozin at baseline and after 12 weeks of treatment
Dapagliflozin administration for 12 weeks resulted in significant reductions in BMI (26.0 ± 4.85 to 25.9 ± 4.99 kg/m2, P < 0.0001), SBP (131 ± 12.0 to 124 ± 12.6 mmHg, P < 0.0001), FPG (139 ± 18.1 to 123 ± 16.9 mg/dL, P < 0.0001), HbA1c (7.72 ± 0.925 to 7.22 ± 0.730 %, P < 0.0001), ALT (31.7 ± 21.1 to 27.9 ± 16.1 IU/l, P = 0.0447), and uric acid (4.94 ± 1.39 to 4.55 ± 1.36 mg/dl, P = 0.0101) (Table 2).
Table 2.
Clinical characteristics of 46 patients who received dapagliflozin at baseline and after 12 weeks of treatment
| Characteristic | Before treatment | After 12 weeks | P value |
|---|---|---|---|
| BMI (kg/m2) | 26.0 ± 4.85 | 25.9 ± 4.99 | <0.0001 |
| SBP (mmHg) | 131 ± 12.0 | 124 ± 12.6 | <0.0001 |
| DBP (mmHg) | 71.3 ± 10.9 | 70.5 ± 11.1 | 0.5383 |
| FPG (mg/dl) | 139 ± 18.1 | 123 ± 16.9 | <0.0001 |
| HbA1c (%) | 7.72 ± 0.925 | 7.22 ± 0.730 | <0.0001 |
| AST (IU/l) | 23.8 ± 13.2 | 22.4 ± 9.26 | 0.3315 |
| ALT (IU/l) | 31.7 ± 21.1 | 27.9 ± 16.1 | 0.0447 |
| GGT (IU/l) | 49.6 ± 34.8 | 44.8 ± 40.6 | 0.0967 |
| LDL-C (mg/dl) | 89.9 ± 20.9 | 91.0 ± 21.4 | 0.6848 |
| HDL-C (mg/dl) | 59.7 ± 18.5 | 60.8 ± 18.9 | 0.4554 |
| TG (mg/dl) | 124 ± 55.1 | 125 ± 58.3 | 0.9411 |
| Creatinine (mg/dl) | 0.667 ± 0.156 | 0.691 ± 0.164 | 0.1541 |
| Uric acid (mg/dl) | 4.94 ± 1.39 | 4.55 ± 1.36 | 0.0101 |
Data are means ± SD
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, FPG fasting plasma glucose, HbA1c hemoglobin A1c, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT γ-glutamyltransferase, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, TG triglycerides
Correlation between baseline characteristics and change in HbA1c levels and BMI levels
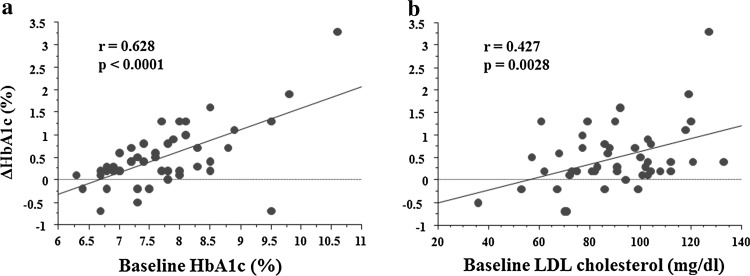
We examined the correlation between the baseline characteristics and the change in HbA1c levels (ΔHbA1c) and found that baseline HbA1c levels and LDL-C significantly correlated with ΔHbA1c (Table 3). The distribution plots among ΔHbA1c and baseline HbA1c as well as baseline LDL-C are shown in Fig. 2. We also examined the correlation between the baseline characteristics and the change in BMI levels (ΔBMI) and found that baseline SBP, DBP, and HDL-C significantly correlated with ΔBMI (Table 3).
Table 3.
Correlation of the ΔHbA1c and ΔBMI with other associated baseline variables
| ΔHbA1c | ΔBMI | |||
|---|---|---|---|---|
| r value | P value | r value | P value | |
| Age | −0.033 | 0.8267 | 0.246 | 0.0999 |
| Duration of diabetes | −0.066 | 0.6648 | 0.277 | 0.0624 |
| BMI | −0.152 | 0.3164 | −0.232 | 0.1206 |
| SBP | 0.234 | 0.1173 | −0.323 | 0.0279 |
| DBP | 0.057 | 0.7050 | −0.307 | 0.0374 |
| FPG | 0.118 | 0.4376 | 0.073 | 0.6339 |
| HbA1c | 0.628 | <0.0001 | −0.046 | 0.7615 |
| AST | 0.139 | 0.3602 | −0.011 | 0.9429 |
| ALT | 0.191 | 0.2059 | −0.116 | 0.4448 |
| GGT | −0.038 | 0.8028 | −0.135 | 0.3742 |
| LDL–C | 0.427 | 0.0028 | 0.129 | 0.3932 |
| HDL–C | −0.211 | 0.1591 | 0.336 | 0.0219 |
| TG | −0.023 | 0.8779 | −0.133 | 0.3801 |
| Creatinine | −0.125 | 0.4111 | 0.021 | 0.8927 |
| Uric acid | 0.042 | 0.7845 | −0.105 | 0.4875 |
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, FPG fasting plasma glucose, HbA1c hemoglobin A1c, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT γ-glutamyltransferase, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, TG triglycerides
Fig. 2.
Distribution plots of a ΔHbA1c (%) versus baseline HbA1c and b baseline LDL cholesterol
Multivariate linear regression analysis of baseline characteristics to assess the contribution to change in HbA1c levels
To assess the contribution to change in HbA1c levels, we performed multivariate linear regression analysis of the baseline clinical characteristics. In the analysis, HbA1c levels and LDL-C significantly correlated with the change in HbA1c levels (ΔHbA1c), suggesting that these characteristics may be predictive factors for the efficacy of dapagliflozin add-on therapy (Table 4). To assess the contribution to change in BMI levels, we also performed multivariate linear regression analysis of baseline clinical characteristics; however, there were no significant correlations with change in BMI levels (ΔBMI) (data not shown).
Table 4.
Multiple regression analysis of the relationship between ΔHbA1c and other associated variables
| Risk factors | Regression coefficient | Standard error | Standardized regression coefficient | P |
|---|---|---|---|---|
| Age (years) | 0.001 | 1.102 | 0.009 | 0.9476 |
| Gender | 0.205 | 0.167 | 0.138 | 0.2274 |
| Duration of diabetes | −0.021 | 0.011 | −0.246 | 0.0594 |
| BMI | −0.012 | 0.018 | −0.081 | 0.5161 |
| HbA1c | 0.460 | 0.087 | 0.606 | <0.0001 |
| LDL-C | 0.011 | 0.004 | 0.317 | 0.0079 |
The dependent variable is the ΔHbA1c. The independent variables are age, gender, duration of diabetes, BMI, HbA1c, and LDL-C. R 2 for the entire model = 0.561
BMI body mass index, LDL-C low-density lipoprotein cholesterol
Discussion
In this single-center, open-label, pilot study, we investigated the efficacy of dapagliflozin treatment in 50 Japanese outpatients with type 2 diabetes mellitus; 46 patients were successfully followed over a 12-week treatment period. We demonstrated that dapagliflozin administration for 12 weeks resulted in significant reductions in BMI, SBP, FPG, HbA1c, ALT, and uric acid (Table 2). Kaku et al. also reported that in Japanese patients with type 2 diabetes mellitus, dapagliflozin administration for 12 weeks resulted in significant reductions in FPG and HbA1c [7]. Kashiwagi et al. reported that ipragliflozin as an add-on to a sulfonylurea or pioglitazone significantly improved glycemic control in Japanese type 2 diabetes patients [8, 9]. We were also able to show that HbA1c levels and LDL-C significantly correlated with ΔHbA1c (Table 3). Furthermore, multiple linear regression analysis showed that baseline HbA1c and LDL-C significantly correlated with ΔHbA1c. Therefore, we demonstrated that baseline HbA1c and LDL-C contributed to the HbA1c-lowering effect of dapagliflozin in Japanese patients with type 2 diabetes mellitus (Table 4), confirming the previously reported association between the baseline HbA1c and the HbA1c-lowering effect of dapagliflozin [10].
Among the 46 patients who completed the study, 35 (76 %) received statin prior to the study. Therefore, we performed multiple linear regression analysis that included LDL-C and statin therapy prior to the study. LDL-C was significantly associated with ΔHbA1c, including statin therapy (data not shown).
Dapagliflozin had varied effects on plasma lipids. Changes in LDL-C (−0.5 to +9.5 %), HDL-C (+2.1 to +9.3 %), and TG (−0.9 to −10.6 %) were noted in clinical trials [11]. In a pooled analysis of 13 clinical trials, patients treated with 10 mg of dapagliflozin had increased LDL-C (2.9 versus −1.0 %) at 24 weeks compared with patients treated with placebo [12]. In our study, LDL-C was also increased after 12 weeks of treatment with dapagliflozin, but not significantly.
It was reported that conditions with increased glucagon levels decreased plasma LDL-C by inducing the number of hepatic LDL receptors [13] and that dapagliflozin increased the postprandial plasma glucagon concentration [14, 15]. As LDL-C is associated with a component of glucagon, baseline LDL-C might be related to the HbA1c-lowering effect of dapagliflozin.
The major limitation of this study is the limited number of subjects studied. All the subjects received add-on therapy of various kinds. Drug-naïve patients should be included in our study. In addition, the study duration may be a limitation, because the extent of glycemic response may not have been fully elucidated over this time frame. Furthermore, other markers that could have affected the HbA1c reduction due to dapagliflozin were not measured in this study, such as waist circumference as a marker of central obesity, markers for the assessment of β-cell function in homeostatic models, and basal active GLP-1. Therefore, more subjects and more detailed clinical parameters are needed in the future to avoid overestimation of the data obtained.
In conclusion, this prospective 12-week study showed that baseline HbA1c and LDL-C significantly contribute to the HbA1c-lowering effect of dapagliflozin. More long-term analysis of a larger patient population is warranted to confirm these findings.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revision. Informed consent or substitute for it was obtained from all patients for being included in the study.
Acknowledgments
The authors declare that they have no conflict of interest. This work was not supported by any grant.
References
- 1.Brunton SA. Hypoglycemic potential of current and emerging pharmacotherapies in type 2 diabetes mellitus. Postgrad Med. 2012;124:74–83. doi: 10.3810/pgm.2012.07.2570. [DOI] [PubMed] [Google Scholar]
- 2.Morgan CL, Jenkins-Jones S, Evans M, et al. Weight change in people with type 2 diabetes: secular trends and the impact of alternative antihyperglycaemic drugs. Diabetes Obes Metab. 2012;14:424–432. doi: 10.1111/j.1463-1326.2011.01552.x. [DOI] [PubMed] [Google Scholar]
- 3.Mudaliar S, Chang AR, Aroda VR, et al. Effects of intensive insulin therapy alone and with added pioglitazone on renal salt/water balance and fluid compartment shifts in type 2 diabetes. Diabetes Obes Metab. 2010;12:133–138. doi: 10.1111/j.1463-1326.2009.01126.x. [DOI] [PubMed] [Google Scholar]
- 4.List JF, Woo V, Morales E, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–657. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilding JP, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405–415. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 6.Chao EC, Henry RR. SGLT2 inhibition—a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 7.Kaku K, Inoue S, Matsuoka O, et al. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2013;15:432–440. doi: 10.1111/dom.12047. [DOI] [PubMed] [Google Scholar]
- 8.Kashiwagi A, Akiyama N, Shiga T, et al. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetol Int (in press).
- 9.Kashiwagi A, Shiga T, Akiyama N, et al. Efficacy and safety of ipragliflozin as an add-on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study (the SPOTLIGHT study). Diabetol Int (in press).
- 10.Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–2224. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013;125:181–189. doi: 10.3810/pgm.2013.05.2667. [DOI] [PubMed] [Google Scholar]
- 12.Bristol-Myers Squibb Company Farxiga [package insert]. Princeton: Bristol-Myers Squibb Company; 2014.
- 13.Rudling M, Angelin B. Stimulation of rat hepatic low density lipoprotein receptors by glucagon. Evidence of a novel regulatory mechanism in vivo. J Clin Invest. 1993;91:2796–2805. doi: 10.1172/JCI116522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen PL, Iqbal N, Ekholm E, Cook W, et al. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr Pract. 2014;20:1187–1197. doi: 10.4158/EP14489.OR. [DOI] [PubMed] [Google Scholar]
- 15.Cefalu WT. Paradoxical insights into whole body metabolic adaptations following SGLT2 inhibition. J Clin Invest. 2014;124:485–487. doi: 10.1172/JCI74297. [DOI] [PMC free article] [PubMed] [Google Scholar]