Abstract
The JDCP study is a large-scale, prospective observational study designed to elucidate risk factors for diabetic complications as they become manifest in Japanese type 1 and 2 diabetic patients in the course of their observation and follow-up. Of the 6338 patients enrolled in the study, all patients with type 1 diabetes (n = 394) were examined for baseline clinical characteristics, which were summarized as follows: men, n = 174 (44 %); mean age (men/women), 55.3/56.8 years; duration of diabetes, 11.9/11.1 years; and those with a family history of diabetes, 27.7 %/35.6 %; BMI, 22.4/21.8 kg/m2 (P = 0.048); HbA1c, 7.9/7.7 %; those with HbA1c < 7 %, 23.1/26.9 %; SBP, 126.0/124.9 mmHg; and LDL-C, 106.1/107.7 mg/dL. Additionally, the insulin dose per patient was shown to be 33.0 U or 0.58 U/kg body weight with self-monitoring of blood glucose being used in 95.8 % of the patients.
Keywords: JDCP study, Diabetic complications, Nationwide observational study, Glycemic control, Type 1 diabetes
Introduction
Type 1 diabetes has been repeatedly reported to be increasing in incidence among children, particularly among those in Western countries [2–4] Furthermore, the latest reports on prognosis in affected children suggest that they are placed at two to fourfold risk of death from the disease compared to that in the general population [5–9]. Again, 8-year follow-up in Sweden has also shown that the hazard ratio (HR) for cardiovascular death is higher among children with type 1 diabetes at 4.6 compared to that among the general population [5]. In contrast, to date, very few prospective observational studies have evaluated the status of type 1 diabetes and its complications, as well as its risk factors, among adults.
In October 2004, the Japan Diabetes Society (JDS) launched the Diabetes Registry Configuration Committee (DRCC) as a permanent committee and initiated the Japan Diabetes Complication and its Prevention prospective (JDCP) study as a prospective 5-year observational research project [10], jointly conducted by the JDS, the Japanese Society of Nephrology (JSN), Japanese Society of Ophthalmic Diabetology (JSOD), and Japanese Society of Periodontology (JSP), given that the evaluation of the risk for the onset and progression of diabetic complications entailed enlisting the assistance of experts in specialties of interest to diabetes complications.
The JDCP study was launched with the approval of the JDS Ethics Committee for Research in May 2007 as a Ministry of Health, Labour and Welfare (MHLW)-subsidized study over a 2-year period since 2009, and has continued since 2011 as a core scientific research project to be conducted by the DRCC.
The objective of the study was to survey the status of management and treatment of patients with type 1 and 2 diabetes, as well as to clarify the risk factors for the onset or progression of diabetic complications, so that the resulting insights could be drawn on in the future JDS Treatment Guideline for Diabetes. This report focuses on the baseline clinical characteristics of the type 1 diabetic patients included in the JDCP study.
Patients and methods
Patients and their accrual
The study enrolled patients with type 1 and 2 diabetes ≥40 years old but <75 years of age currently being treated on an outpatient basis at healthcare facilities specializing in diabetes all over the country, including university hospitals, local base hospitals, and clinics. A total of 7700 patients who met the study eligibility criteria and gave informed consent were enrolled between June 2007 and November 2009 from the participating facilities, to which a batch of case report forms (CRFs) was sent by mail at patient enrollment and once a year thereafter, with the request that the CRFs be completed and returned.
Patients were excluded from the study: ① if they were unable to visit their outpatient clinic on a regular basis; ② if they had proliferative retinopathy; ③ if they were currently on dialysis; ④ if they had been diagnosed with malignancy in the past five years; and ⑤ if they were judged by the study investigator to be ineligible for study entry. This led to a total of 6338 patients who met the study eligibility criteria being included in the study.
This report included for analysis a total of 394 (6.2 %) patients with type 1 diabetes currently being treated at 185 facilities all over the country. The diagnosis of type 1 diabetes was made in these patients based on the judgment of their attending physicians. All patients whose diagnosis was thought to be less definitive due to factors such as their age of onset or BMI were followed up by contacting their attending physicians to establish their diagnosis as type 1 diabetes.
Methods
The CRF used in the study covered the following items for follow-up and evaluation: ① patient information at baseline (mandatory at study initiation); and ② patient information at follow-up (at study enrollment and once every year), i.e., findings from physical/hematological examinations and electrocardiogram, findings associated with nephropathy, retinopathy, neuropathy, and periodontal disease, as well as diabetes treatments implemented. Besides, diet (Brief-type, self-administered Diet History Questionnaire, BDHQ), exercise (International Physical Activity Questionnaire, IPAQ), and periodontal disease (oral examinations; orthopantomography) were investigated in a subset of patients. History examinations as part of patient information covered both past and current diseases.
The primary endpoint for the study was the occurrence of nephropathy, retinopathy, neuropathy, and macroangiopathy (i.e., ischemic heart disease, cerebrovascular disease, cardiac failure, peripheral artery disease, and lower extremity amputation). The secondary endpoints for the study were described in detail in the JDCP study 1 [9]. The occurrence of each event was assessed by the relevant working groups, each of which was organized to assess a particular diabetic complication.
Statistical analysis
Continuous variables were expressed as mean ± SD and tested for significance by using Student’s t test. Non-normally distributed data were expressed as median (interquartile range) and tested for significance by using the Wilcoxon rank-sum test. All differences in frequency were tested for significance by using the χ 2 test or Fisher’s exact test. A P value of <0.05 (two-sided) was considered to indicate significance. All data sets were analyzed independently at least twice by multiple researchers inside or outside the working groups to verify that each data set being analyzed constituted the same data set. All statistical analyses were performed by using SPSS ver. 20.
Ethical considerations
The Declaration of Helsinki and all relevant guidelines, including those by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare of Japan (as applicable during the study period), i.e., “Ethical Guidelines in Epidemiological Research”, were followed in this study. The informed consent form as part of the study protocol clearly stated that ophthalmologic and dental examinations were to occur in routine care settings to avoid any additional time or financial burden on the participants being examined. The current study was approved by the JDS Ethics Review Committee for Scientific Surveys and Studies and the Ethics Committee of each participating institution (or an ad hoc Ethics Committee convened at the request of the principal investigator if the required review process could not be put in place at any of the participating institutions), and registered with the University Hospital Medical Information Network Center (UMIN) with the identifier UMIN000016519.
Results
Patient characteristics
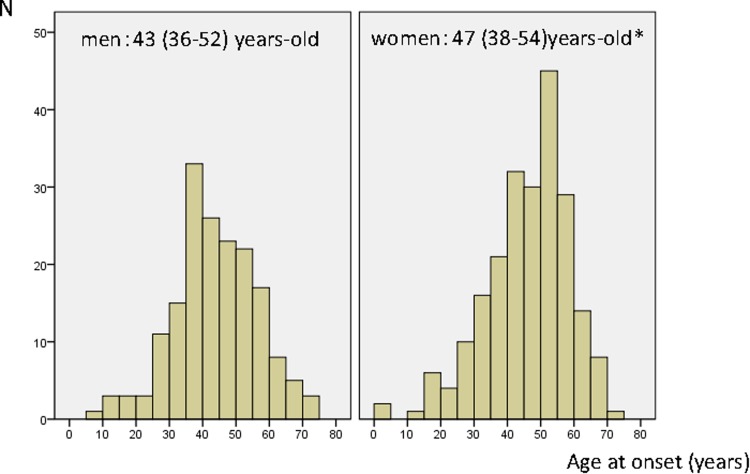
Of the 6338 patients who comprised the JDCP cohort, a total of 394 (6.2 %) patients had type 1 diabetes [mean age (men/women), 55.3 ± 9.5/56.8 ± 9.0 years old; and duration of diabetes, 11.9 ± 9.0/11.1 ± 9.4 years]. Their median age (interquartile range) at onset of disease was 43 (36–52) years old in men versus 47 (38–54) years old in women, with the age at onset of disease significantly older among women (P = 0.016) (see also Fig. 1 for a histogram showing the distribution of ages at onset of disease). The small number of patients with childhood-onset disease in this population may be accounted for in part by the JDCP criteria for age at study entry, i.e., 40 years old or older, which may have led to fewer patients with childhood-onset disease being included in the study.
Fig. 1.
Histograms for age at onset by sex. Age at onset was presented as median (interquartile range) and tested for significance by using the Wilcoxon rnak-sum test* (p = 0.016)
Men/women with a history of dyslipidemia and hypertension accounted for 19.5 %/29.5 % (P = 0.023) and 23.6 %/22.7 %, respectively, with dyslipidemia significantly more common among women. Again, men/women with a history of cerebrovascular disease and myocardial infarction accounted for 1.1 %/1.8 % and 0.6 %/0.5 %, respectively, while men/women with a family history of diabetes accounted for 27.7 %/35.6 %. Additionally, men/women with past and current smoking and drinking accounted for 42.8 %/34.5 % and 44.8 %/11.4 % (P < 0.001), respectively, suggesting that while there was no gender difference in smoking, significantly more men drank than did women (Table 1).
Table 1.
Patients’ characteristics
| N (men %) | All n = 394 | Men n = 174 | Women n = 220 | P values | |
|---|---|---|---|---|---|
| Age (years) | 394 (44.2 %) | 56.2 ± 9.3 | 55.3 ± 9.5 | 56.8 ± 9.0 | 0.104 |
| Duration of diabetes (years) | 392 (44.1 %) | 11.5 ± 9.2 | 11.9 ± 9.0 | 11.1 ± 9.4 | 0.361 |
| Age at onset | 392 (44.1 %) | 45 (37–54) | 43 (36–52) | 47 (38–54) | 0.016 |
| Past history (%) | 394 (44.2 %) | ||||
| Dyslipidemia | 25.1 | 19.5 | 29.5 | 0.023 | |
| Hypertension | 23.1 | 23.6 | 22.7 | 0.845 | |
| Stroke | 1.5 | 1.1 | 1.8 | 0.698 | |
| Myocardial infarction | 0.5 | 0.6 | 0.5 | 1.000 | |
| Others | 21.1 | 20.1 | 21.8 | 0.681 | |
| None | 47.2 | 49.4 | 45.5 | 0.433 | |
| Family history of diabetes (%) | 389 (44.5 %) | 32.1 | 27.7 | 35.6 | 0.097 |
| Regular alcohol intake (%) | 393 (44.3 %) | 26.2 | 44.8 | 11.4 | <0.001 |
| Smoker (past/current) (%) | 393 (44.0 %) | 38.2 | 42.8 | 34.5 | 0.096 |
Data were examined for normality. Continuous variables were presented as mean ± SD and tested for significance by using Student’s t tests. The non-normally distributed variable (Age at onset) was presented as median (interquartile range) and tested for significance by using the Wilcoxon rank-sum test. Categorical variables are presented as percentages and tested for significance by using χ2 tests. P values are shown for sex differences, where a P value of 0.05 (two sided) is considered to indicate significance. Numbers of the samples out of a total of 394 were shown in (N)
Findings from physical/hematological examinations, as well as diabetes treatments implemented are summarized in Tables 2 and 3. The men/women had a mean body mass index (BMI) of 22.4 ± 2.6/21.8 ± 3.2 kg/m2 (P = 0.048) (BMI ≥ 25 kg/m2, 14.9 %/13.2 %), a mean waist circumference of 80.8 ± 7.6/76.1 ± 9.8 (P < 0.001), a mean systolic blood pressure (SBP) of 126.0 ± 15.4/124.9 ± 15.7 mmHg, and a mean diastolic blood pressure (DBP) of 73.5 ± 9.8/71.4 ± 9.6 mmHg (P = 0.030) with those achieving both the SBP and DBP targets accounting for the majority at 57.9 %/54.1 %.
Table 2.
Clinical variables at baseline
| N (men %) | All n = 394 |
Men n = 174 |
Women n = 220 | P values | |
|---|---|---|---|---|---|
| Body weight (kg) | 394 (44.2 %) | 56.8 ± 9.9 | 62.8 ± 8.9 | 52.0 ± 7.8 | <0.001 |
| Maximum BW (kg) | 390 (43.8 %) | 62.4 ± 10.5 | 68.0 ± 10.2 | 58.1 ± 8.5 | <0.001 |
| Maximum BW (Age) | 387 (43.7 %) | 41.9 ± 13.8 | 42.6 ± 12.6 | 41.3 ± 14.7 | 0.353 |
| BMI (kg/m2) | 393 (44.3 %) | 22.1 ± 2.9 | 22.4 ± 2.6 | 21.8 ± 3.2 | 0.048 |
| Waist circumference (cm) | 357 (42.6 %) | 78.1 ± 9.2 | 80.8 ± 7.6 | 76.1 ± 9.8 | <0.001 |
| HbA1c (%) | 392 (44.1 %) | 7.8 ± 1.4 | 7.9 ± 1.5 | 7.7 ± 1.3 | 0.172 |
| FPG (mg/dL) | 96 (44.8 %) | 134.9 ± 62.8 | 142.0 ± 63.4 | 129.2 ± 62.4 | 0.322 |
| PPPG (mg/dL) | 344 (44.8 %) | 174.2 ± 89.3 | 174.2 ± 91.6 | 174.2 ± 87.6 | 0.999 |
| SBP(mmHg) | 389 (44.0 %) | 125.3 ± 15.5 | 126.0 ± 15.4 | 124.9 ± 15.7 | 0.489 |
| DBP(mmHg) | 389 (44.0 %) | 72.3 ± 9.8 | 73.5 ± 9.8 | 71.4 ± 9.6 | 0.030 |
| Lipid profiles | |||||
| TC (mg/dL) | 364 (43.7 %) | 198.3 ± 29.7 | 193.8 ± 30.5 | 201.8 ± 28.6 | 0.011 |
| LDL-C (mg/dL) | 370 (43.2 %) | 107.0 ± 24.3 | 106.1 ± 26.3 | 107.7 ± 22.7 | 0.537 |
| HDL-C (mg/dL) | 388 (43.6 %) | 72.9 ± 18.3 | 68.0 ± 19.4 | 76.7 ± 16.5 | <0.001 |
| non-HDL-C (mg/dL) | 358 (43.0 %) | 125.3 ± 26.0 | 125.1 ± 27.6 | 125.4 ± 24.8 | 0.902 |
| TG (mg/dL) | 172 (40.7 %) | 73.0 (56.0–92.0) | 80.1 (63.0–115.0) | 67.5 (52.0–86.0) | 0.001 |
| Serum creatinine (mg/dL) | 383 (44.1 %) | 0.7 ± 0.2 | 0.8 ± 0.1 | 0.6 ± 0.1 | <0.001 |
| eGFR (ml/min/1.73 m2) | 383 (44.1 %) | 81.1 ± 17.4 | 81.6 ± 16.0 | 80.7 ± 18.4 | 0.634 |
| Serum albumin (mg/dL) | 349 (43.3 %) | 4.2 ± 0.3 | 4.2 ± 0.4 | 4.2 ± 0.3 | 0.812 |
Continuous variables were presented as mean ± SD and tested for significance by using Student’s t tests. The non-normally distributed variable (TG) was presented as median (interquartile range) and tested for significance by using the Wilcoxon rank-sum test. P values are given for sex differences, where a P value of 0.05 (two sided) was considered to indicate significance
BMI body mass index, FPG fasting plasma glucose, PPPG postprandial plasma glucose, IRI immunoreactive insulin, SBP systolic blood pressure, DBP diastolic blood pressure, TC total cholesterol, LDL-C low density lipoprotein cholesterol, HDL-C high density lipoprotein cholesterol, TG triglycerides, eGFR estimated glomerular filtration rate as calculated by the following formula: men (mL/min/1.73 m2) = 194 × Cr−1.094 × age−0.287; women (mL/min./1.73 m2) = 194 × Cr−1.094 × age−0.287 × 0.739
Table 3.
Proportion of patients who achieved the target values at baseline
| Target |
n
(Men %) |
All n = 394 (%) |
Men n = 174 (%) |
Women n = 220 (%) |
P values | |
|---|---|---|---|---|---|---|
| HbA1c (%) | <6 | 9 (55.6 %) | 2.3 | 2.9 | 1.8 | 0.485 |
| <7 | 99 (40.4 %) | 25.3 | 23.1 | 26.9 | 0.387 | |
| <8 | 254 (42.5 %) | 64.8 | 62.4 | 66.7 | 0.383 | |
| ≧8 | 138 (47.1 %) | 35.2 | 37.6 | 33.3 | 0.383 | |
| FPG (mg/dL) | <130 | 96 (44.8 %) | 59.4 | 46.5 | 69.8 | 0.021 |
| PPPG (mg/dL) | <180 | 344 (44.8 %) | 58.7 | 61.0 | 56.8 | 0.432 |
| BMI (kg/m2) | 18 to <25 | 393 (44.3 %) | 80.2 | 82.8 | 78.1 | 0.248 |
| Blood pressure (mmHg) | SBP < 130 and DBP < 80 | 389 (44.0 %) | 55.8 | 57.9 | 54.1 | 0.458 |
| LDL-C (mg/dL) | <120 | 370 (43.2 %) | 70.3 | 71.9 | 69.0 | 0.556 |
| HDL-C (mg/dL) | ≧40 | 388 (43.6 %) | 97.9 | 95.3 | 100.0 | 0.001 |
| non-HDL-C (mg/dL) | <150 | 358 (43.0 %) | 82.7 | 82.5 | 82.8 | 0.926 |
| TG (mg/dL) | <150 | 172 (40.7 %) | 91.3 | 87.1 | 94.1 | 0.111 |
FPG fasting plasma glucose, PPPG postprandial plasma glucose, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, LDL-C low density lipoprotein cholesterol, HDL-C high density lipoprotein cholesterol, TG triglycerides
Numbers of the samples out of a total of 394 were shown in (N). HbA1c was measured in a total of 5924 subjects. TG was measured in the fasting state. All differences in frequency were tested for significance using χ 2 test. P values are given for sex differences, where a P value of 0.05 (two-sided) was considered to indicate significance
Of the glycemic control indices evaluated, the men/women had a mean HbA1c of 7.9 ± 1.5 %/7.7 ± 1.3 % (HbA1c < 7 %, 62.4 %/66.7 %), with those with HbA1c < 7.0 % being as few as 23.1 %/26.9 % and those with HbA1c < 8 % being 62.4 %/66.7 % (Table 4)
Table 4.
Therapeutic measures at the baseline
| N (%) | |||
|---|---|---|---|
| Diabetes (n = 394) | |||
| Diet only | 0 (0.0) | ||
| OHA only | 3 (0.8) | ||
| Insulin only | 335 (85.0) | Once a day | 2.3 % |
| Insulin + OHA | 56 (14.2) | Twice a day | 7.9 % |
| 3–4 time a day | 13.0 % | ||
| 3–4 time a day | 61.1 % | ||
| >5 times a day | 12.8 % | ||
| CSII | 2.8 % | ||
| Insulin dose (U/day) | 33.0 ± 17.1 | ||
| (U/kg/day) | 0.58 ± 0.25 | ||
| (multiple choice) | |||
| Sulfonylurea | 1.3 % | ||
| Glinide | 0.3 % | ||
| Biguanides | 4.8 % | ||
| α-glucosidase inhibitors | 10.4 % | ||
| Thiazolidine derivatives | 0.8 % | ||
| SMBG (n = 381) | |||
| Yes | 365 (95.8) | ||
| Antihypertensive agents (n = 394) | |||
| yes | 96 (24.4) | (multiple choice) | |
| ARB | 18.8 % | ||
| ACEI | 3.8 % | ||
| CCB | 11.9 % | ||
| Diuretics | 1.3 % | ||
| β-Blocker | 0.8 % | ||
| α-Blocker | 1.5 % | ||
| Others | 0.0 % | ||
| Agents for dyslipidemia (n = 394) | |||
| yes | 94 (23.9) | (multiple choice) | |
| Statin | 22.6 % | ||
| Fibrate | 0.8 % | ||
| Ezetimibe | 0.0 % | ||
| Others | 0.5 % | ||
| Antiplatelet agents (n = 394) | |||
| yes | 23 (5.8) | (multiple choice) | |
| Aspirin | 4.6 % | ||
| Cilostazol | 0.5 % | ||
| Ticlopidine HCl | 0.5 % | ||
| Clopidogrel bisulfate | 0.5 % | ||
| Others | 0.3 % | ||
| Aldose reductase inhibitor (n = 394) | |||
| yes | 17 (5.1 %) | ||
| Others (n = 394) | |||
| yes | 33 (8.4 %) | (multiple choice) | |
| Activated charcoal | 0.0 % | ||
| Dipyridamole | 0.3 % | ||
| Ethyl icosapentate | 0.5 % | ||
OHA oral hypoglycemic agent, CSII continuous subcutaneous insulin infusion, SMBG self-monitoring of blood glucose, ARB angiotensin receptor blockers, ACEI angiotensin-converting enzyme inhibitors, CCB calcium channel blockers
The men/women had a mean fasting glucose level of 142.0 ± 63.4/129.2 ± 62.4 mg/dL, and a mean casual glucose level of 174.2 ± 91.6/174.2 ± 87.6 mg/dL, suggesting that while their mean glucose values were shown to be relatively close to those indicated in the JDS Treatment Guideline for Diabetes, the men/women achieving both the fasting glycemic target (<130 mg/dL) and the casual glycemic target (<180 mg/dL) accounted for 46.5 %/69.8 % (P = 0.021) and 61.0 %/56.8 %, respectively, with significantly more women achieving the fasting glycemic target.
Of the (casual) lipid parameters evaluated, the men/women had a mean total cholesterol (TC) level of 193.8 ± 30.5/201.8 ± 28.6 mg/dL (P = 0.011) with the TC level significantly higher among women; a mean LDL-cholesterol (LDL-C) level of 106.1 ± 26.3/107.7 ± 22.7 mg/dL (LDL-C < 120 mg/dL, 71.9 %/69.0 %); a mean HDL-C level of 68.0 ± 19.4/76.7 ± 16.5 mg/dL (P < 0.001) (HDL-C ≥ 40 mg/dL, 95.3 %/100 %; P = 0.001) with the HDL-C level significantly higher among the women; a mean non-HDL-C level of 125.1 ± 27.6/125.4 ± 24.8 mg/dL (non-HDL-C < 150 mg/dL, 82.5 %/82.8 %); and a median triglyceride (TG) level of 80.1 mg/dL (63.0–115.0)/67.5 mg/dL (52.0–86.0) (P = 0.001) (TG < 150 mg/dL, 87.1 %/94.1 %) with the TG level significantly lower among the women.
The men/women had a mean eGFR of 81.6 ± 16.0/80.7 ± 18.4 mL/min/1.73 m2 (eGFR ≥ 60 mL/min/1.73 m2, 91.1 %/89.3 %).
The patients were treated with a mean insulin dose of 33.0 ± 17.0 U or 0.58 ± 0.25 U/kg body weight, with 10.2, 13.0, 61.1, 12.8, and 2.8 % of the patients using ≤2, 3, 4, ≥5 insulin injections, and continuous subcutaneous insulin infusion (CSII), respectively. Self-monitoring of blood glucose (SMBG) was used in 95.8 % of the patients.
Oral hypoglycemic agents (OHAs) were not being used in 85 % of the patients, but α-glucosidase inhibitors were used in 10.4 % of the patients.
The antihypertensive agents were used in 24.4 % of the patients; those receiving angiotensin receptor blockers (ARBs), angiotensin-converting enzyme (ACE) inhibitors, and calcium channel blockers (CCBs) accounted for 18.8, 3.8 and 11.9 %, respectively (multiple-choice responses).
Of the anti-dyslipidemic agents used, statins and fibrates accounted for 22.6 % and 0.8 %, respectively. Additionally, drugs with antiplatelet properties were used in 5.8 % of the patients.
All data available on nephropathy, retinopathy, neuropathy, and periodontal disease are currently being analyzed and assessed for reporting by the members in the respective working groups.
Discussion
The prognosis of Japanese patients diagnosed with childhood-onset type 1 diabetes was shown to be extremely poor but is now rapidly becoming comparable to that of their Western counterparts [6]. It is also shown that cardiovascular disease becomes a major cause of death with increasing attained age in Japanese patients with childhood-onset type 1 diabetes [5, 7, 9, 12].
However, adult-onset type 1 diabetes or type 1 diabetes in adults remains less well described, with very few studies conducted globally on the status of glycemic control and associated cardiovascular events, as well as on prognosis, in adult-onset type 1 diabetes. The current report thus represents the first to describe results from a large-scale observational study conducted in Japan.
The current report summarized the baseline clinical characteristics of 394 type 1 diabetic patients from 185 facilities, who accounted for 6.2 % of the 6338 patients with diabetes from 464 healthcare facilities specializing in diabetes care nationwide.
Their baseline characteristics demonstrated that the proportions of patients who had achieved the respective JDS targets at baseline were: HbA1c <7, 25.3 %; fasting glucose <130 mg/dL, 59.4 %; BMI < 25, 86.0 %; BP target (SBP < 130 mmHg and DBP < 80 mmHg), 55.8 %; LDL-C < 120 mg/dL, 70.3 %; HDL-C ≥ 40 mg/dL, 97.9 %; non-HDL-C < 150 mg/dL, 82.7 %; and TG (early morning fasting value) <150 mg/dL, 91.3 %. Additionally, while 60–90 % of the patients had achieved the lipid and body weight targets, the patients appeared to be having difficulty in achieving the glycemic and BP targets, suggesting the difficulty in achieving these targets, particularly the HbA1c target, in type 1 diabetic patients even at facilities specializing in diabetes care.
When compared to the Swedish cohort (n = 33,915; mean age, 35.8 ± 14.6 years; HbA1c, 65.8 ± 15.8 mmol/mol (NGSP value, 8.2 ± 3.6); BMI, 25.2 ± 4.0 kg/m2; SBP, 126.9 ± 17.0 mmHg) [5], the current cohort was shown to have better glycemic control and be much leaner as a population but have comparable BP control.
While the mean HbA1c is 0.1–0.3 % higher in the current cohort than that (7.60 %) reported in 2013 for the 3263 type 1 diabetic patients from the Japan Diabetes Clinical Data Management (JDDM) study, [11] their HbA1c values may have improved by 2013 due to the advent of novel insulin preparations in the years since their entry in the study, and thus outcomes are awaited with interest.
Of note, the Swedish report [4] revealed that the hazard ratio (HR) for death and cardiovascular death in those patients was increased to 3.5 and 4.6, respectively, compared to that in the general population, after follow-up of about 8 years. Similarly, follow-up of 22,000 patients 20 years old or older in the UK demonstrated that the HR for death due to cardiovascular disease was 3.0 in women and 2.3 in men, compared to that in the general population [12]. Thus, it is one of the major issues to addressed in the JDCP study to investigate how the JDCP cohort may offer comparisons during the course of their follow-up.
Again, while the American Diabetes Association’s Approaches to Glycemic Treatment [13] recommends proactive use of frequent insulin injections or CSII in type 1 diabetes, the current report showed that type 1 diabetic patients using ≥2 insulin injections accounted for no more than <10 %, while those using CSII accounted for no more than 2.8 %, suggesting the need to address this discrepancy in practice and to establish an approach to insulin therapy in Japanese type 1 diabetic patients that applies across the board.
Additionally, one major finding from the current study is that smokers accounted for as many as 38.2 % of the patients with type 1 diabetes with no difference shown in number between the men and women, suggesting the rationale for intervention in these patients with smoking in mind.
As of the end of November 2014, when the JDCP study completed its full 5-year follow-up since the last patient was enrolled in the study, the follow-up rates 1, 2, 3, 4, and 5 years after the start of the study (as of February 2015) were 91.3, 81.3, 75.3, 67.0, and 58.7 %, respectively. While every effort was made to maintain a high follow-up rate during the course of the study, consideration may need to be given to how best to follow up the JDCP cohort in the years to come.
The JDCP study investigators thus intend to follow up its cohort over the long term, while maintaining a high follow-up rate, to offer relevant recommendations for the JDS Treatment Guideline for Diabetes, in light of study data becoming available on risk factors for the onset and progression of diabetic complications in type 1 diabetic patients.
Acknowledgments
The JDCP study is a Japan Diabetes Society (JDS)-originated research project. The study was supported by Ministry of Health, Labour and Welfare grants-in-aid during the 2009–2010 period and then by JDS grants-in-aid from 2011 onwards. The project also received research grants from the Manpei Suzuki Diabetes Foundation to provide support in registry configuration that had to do with data collection. The JDCP study investigators would like to thank all physicians and their staff at the participating institutions for their cooperation and assistance in the conduct of the study. Their heartfelt thanks are also due to all diabetic patients for their participation in the study from all parts of Japan.
Conflict of interest
Rimei Nishimura received a speaker’s fee from Astellas Pharma Inc., Takeda Pharmaceutical Company Ltd., Eli Lily Japan K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., and Sanofi K.K. Mitsuhiko Noda received a speaker’s fee from Mitsubishi Tanabe Pharma Corporation, Taisho Toyama Pharmaceutical Co., Ltd., and received research grants from Takeda Pharmaceutical Company Ltd. Kohjiro Ueki received a speaker’s fee from Takeda Pharmaceutical Company Ltd., Astellas Pharma Inc., MSD K.K., AstraZeneca, Kyowa Hakko Kirin Co., Ltd., Mitsubishi Tanabe Phama Corporation, Nippon Boehringer Ingelheim Co., Ltd., and Novo Nordisk Pharma Ltd., received research grants from AstraZeneca and Sanofi K.K., and was endowed lectures by Novo Nordisk Pharma Ltd., MSD K.K., and Nippon Boehringer Ingelheim Co., Ltd. Naoko Tajima received a speaker’s fee from Astellas Pharma Inc., MSD K.K., Kissei Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Ltd., Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co., Ltd., and Novo Nordisk Pharma Ltd.
Ethical considerations
In this study, the Declaration of Helsinki and the “Ethical Guidelines in Epidemiological Research” compiled by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare of Japan (as applicable in the study period) [3] were followed to obtain informed consent from study participants, to protect their rights and welfare, and to protect them against any potential harm and risk associated with the conduct of the study. The informed consent form as part of the study protocol clearly stated that ophthalmologic and dental examinations were to be conducted as appropriate in routine care settings to ensure no additional time or financial burden on the part of the patient being examined. This study, currently registered with the University Hospital Medical Information Network Center (UMIN000016519), was approved by the JDS Ethics Review Committee for Scientific Surveys and Studies and by the ethics committee of each participating institution. An ad hoc ethics committee was convened at the request of the principal investigator if the required review process was not feasible at any participating institution. Care was also taken to ensure that all data obtained from the study was anonymized in a linkable fashion to protect the privacy of all study participants.
Footnotes
The Japan Diabetes Society launched the Diabetes Registry Configuration Committee to conduct the Japan Diabetes Complication and its Prevention prospective (JDCP) study, which reported, in Japanese, the results of a large-scale observational study to investigate the current status of diabetes complications and their prevention in Japan [1]. This is an English version of that report.
References
- 1.Nishimura R, Izumi K, Hayashino Y, et al. A large-scale observational study to investigate the current status of diabetic complications and their prevention in Japan: research outline and baseline data for type 1 diabetes—JDCP study 2. J Jpn Diabet Soc. 2015;58:426–436. doi: 10.1007/s13340-015-0248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CC, Gyürüs E, Rosenbauer J, Cinek O, Neu A, Schober E, Parslow RC, Jone G, Svenson J, Catell C, Bingley PJ, Schoenle E, Jarosz-Chobot P, Urbonaité B, Rothe U, Krzisnik C, Ionescu-Tirgoviste C, Weets I, Kocova M, Stipancic G, Samardzic M, de Beaufort CE, Green A, Dahlquist GG, Soltész G. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55:2142–2147. doi: 10.1007/s00125-012-2571-8. [DOI] [PubMed] [Google Scholar]
- 3.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 4.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 5.Lind M, Svensson AM, Kosiborod M, Gudbjörnsdottir S, Pivodic A, Wedel H, Dahlqvist S, Clements M, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 6.Asao K, Sarti C, Forsen T, Hyttinen V, Nishimura R, Matsushima M, Reunanen A, Tuomilehto J, Tajima N, Diabetes Epidemiology Research International Mortality Study Group Long-term mortality in nationwide cohorts of childhood-onset type 1 diabetes in Japan and Finland. Diabetes Care. 2003;26:2037–2042. doi: 10.2337/diacare.26.7.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the pittsburgh epidemiology of diabetes complications study experience. Diabetes. 2006;44:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 8.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. 2006;49:298–305. doi: 10.1007/s00125-005-0082-6. [DOI] [PubMed] [Google Scholar]
- 9.Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny county type 1 diabetes registry. Diabetes Care. 2010;33:2573–2579. doi: 10.2337/dc10-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tajima N, Nishimura R, Izumi K, Hayashino Y, Origasa H, Noda M, Ueki K. A large-scale, prospective observational study to investigate the current state of diabetic complications and their prevention in Japan: a research outline and baseline data for type 2 diabetes: JDCP study 1. Diabetol Int. 2015;6:243–251. doi: 10.1007/s13340-015-0223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Japan Diabetes Clinical Data Management (JDDM) study. http://jddm.jp/what/index.html.
- 12.Livingstone SJ, Looker HC, Hothersall EJ, Wild SH, Lindsay RS, Chalmers J, Cleland S, Leese GP, McKnight J, Morris AD, Pearson DW, Peden NR, Petrie JR, Philip S, Sattar N, Sullivan F, Colhoun HM. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: scottish registry linkage study. PLos Med. 2012;9:e1001321. doi: 10.1371/journal.pmed.1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association Approaches to glycemic treatment. Diabetes Care. 2015;38:S41–S48. doi: 10.2337/dc15-S010. [DOI] [PubMed] [Google Scholar]