Abstract
Aim
This study assessed the association between symptoms of bilateral numbness and/or paresthesia in the feet and postural instability in Japanese patients with diabetes.
Methods
This cross-sectional study included 303 patients with diabetes, aged 40–88 years, who were referred to an outpatient diabetic clinic between January and July 2013 at Shiga University of Medical Science Hospital. A posturography test was used to evaluate postural sway in patients. Indices of postural sway were the total length and the enveloped area. Analysis of covariance was used to estimate the multivariable-adjusted means of indices of postural sway according to the presence or absence of symptoms of bilateral numbness and/or paresthesia in the feet.
Results
Of 303 patients, 35 (11.6 %) had symptoms of bilateral numbness and/or paresthesia in the feet. After adjustment for age, sex, diabetic retinopathy, regular exercise, body mass index, hemoglobin A1c level, and quadriceps’ strength, patients with symptoms had higher levels of postural sway length and an enveloped area in the posturography test than those without symptoms. In addition, we observed similar results when we analyzed 234 patients aged ≥60 years.
Conclusions
Our findings suggest that patients who had symptoms of bilateral numbness and/or paresthesia in the feet may have more postural instability than those without symptoms.
Electronic supplementary material
The online version of this article (doi:10.1007/s13340-015-0214-2) contains supplementary material, which is available to authorized users.
Keywords: Numbness, Paresthesia, Feet, Postural instability, Diabetes
Introduction
Diabetes is associated with an increased risk of falls [1–4]. Recently, a prospective longitudinal study found that patients with diabetes were at greater risk of an injurious fall requiring hospitalization than those without diabetes [1]. Additionally, previous studies found that poor static balance was a major risk factor for falls, including injurious falls requiring hospitalization, among patients with diabetes [1, 5]. Therefore, it is important to prevent postural instability of patients with diabetes to prevent future falls.
Diabetic neuropathy is one of the most common complications of diabetes. In a previous study, patients with diabetes with peripheral neuropathy had more postural instability than those without peripheral neuropathy [6]. Therefore, it is necessary for patients with diabetes to be careful about peripheral neuropathy to prevent postural instability. However, because the clinical condition of diabetic peripheral neuropathy often varies, it is important to clarify the association between simpler parameters and postural instability among patients with diabetes.
It is thought that postural instability in patients with diabetic peripheral neuropathy is usually attributed to the lack of accurate peripheral sensory information in the feet. Patients with lower-extremity peripheral sensory neuropathy present with bilateral numbness or bilateral paresthesia in the feet as the main symptom [7]. These symptoms in the feet are thought to be associated with postural instability. However, there has been no study to investigate this association. In addition, little is known about the impact on the postural instability of these symptoms and other factors, such as diabetic retinopathy and regular exercise.
The aim of this study was to assess the association between symptoms of bilateral numbness and/or paresthesia in the feet and postural instability in Japanese patients with diabetes. Additionally, we assessed the association between the combination of presence or absence of these symptoms and the presence or absence of diabetic retinopathy/regular exercise and postural instability.
Materials and methods
Study participants
This cross-sectional study included 476 patients with diabetes who were referred to an outpatient diabetic clinic between January and July 2013 at Shiga University of Medical Science Hospital (Otsu, Japan). The exclusion criteria were those with dementia or those with gestational diabetes. Of 476 outpatients aged 40–88 years, 389 (81.7 %) agreed to participate in the survey. Of these patients, we excluded 58 based on the following criteria: patients with stroke, those with joint disease of the leg such as hip and knee osteoarthritis, those with spinal disease such as lumbar spinal canal stenosis, those who had broken their leg within the preceding 3 years, and those who used a stick or a wheelchair. Additionally, we excluded 28 patients with missing data. A total of 303 outpatients were included in the analysis.
Procedures
The presence or absence of symptoms of bilateral numbness and bilateral paresthesia in the feet was obtained using a self-administered questionnaire. Question items that we used referred to a recent previous study [7]. Question items regarding bilateral numbness and bilateral paresthesia were as follows. (1) Do you feel bilateral numbness in your toes and soles that persists? (bilateral numbness in the feet). (2) Do you feel dullness or abnormal sensation in your toes and soles that persists? (bilateral paresthesia in the feet). The self-administered questionnaire also included demographic characteristics, medical history, and health-related habits.
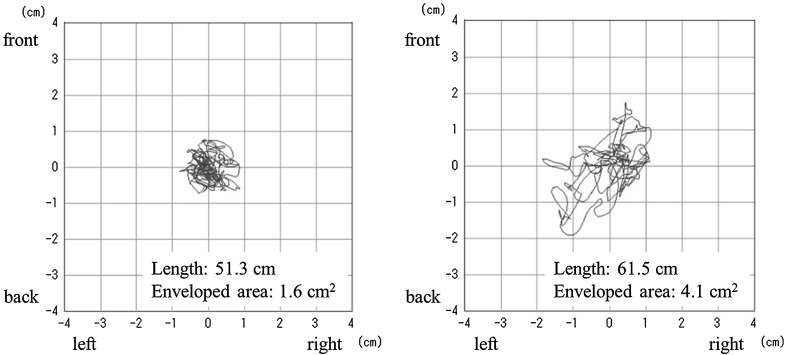
The posturography test was used to evaluate postural sway in patients. We used a stabilometer that conformed to the Japanese Industrial Standards (JIS. T1190–1987, Stabilometers) and was registered by the Japanese Society for Equilibrium Research [8]. The calibration of the stabilometer was conducted by the manufacturer before the survey. Additionally, the posturography test was administered by trained physical therapists and nurses according to the guidelines presented by the Japanese Society for Equilibrium Research [9, 10]. We used a Gravicorder GP-5000 (Anima Co., Ltd., Tokyo, Japan) (Fig. 1) consisting of an equilateral triangular footplate with built-in vertical force transducers to determine instantaneous fluctuations in the center of pressure (COP) with a sampling frequency of 20 Hz. Patients were instructed to maintain a static upright posture on the footplate with their feet together and eyes open (Fig. 2). They were also instructed to focus on a small red circle that was 1.5 m away from where they were standing in a quiet, well-lit room. Recording time was 30 s, beginning after the posture of patients had stabilized. A statokinesigram (sway path of the COP) was obtained from each patient (Fig. 3). Indices of postural sway were the ‘length’ (the total length in cm, determined from the statokinesigram of the COP movement over 30 s) and the ‘enveloped area’ (overall sway area in cm2, enveloped by the outermost perimeter of the statokinesigram as traced by the movement of the COP over 30 s) [11–13].
Fig. 1.
Gravicorder GP-5000 (Anima Co., Ltd., Tokyo, Japan)
Fig. 2.

Measurement condition
Fig. 3.
Two statokinesigram examples
Information on the glycemic control and treatment was collected by a review of the patient’s medical records. Additionally, the hemoglobin A1c (HbA1c) level, estimated glomerular filtration rate, and high-density lipoprotein cholesterol level were collected by a review of the patient’s most recent medical records. HbA1c (%) was estimated as a National Glycohemoglobin Standardization Program equivalent value (%) and calculated using the formula HbA1c (%) = 1.02 × HbA1c (Japan Diabetes Society, %) + 0.25 % [14].
The patients were weighed while wearing light clothing, and height was measured without shoes. Body mass index (BMI) was calculated as the weight (kg) divided by the height squared (m2). Blood pressure was measured using an automatic sphygmomanometer with the patients in the sitting position after resting for at least 5 min. Cognitive function was assessed using the Mini-Mental State Examination (MMSE) [15]. MMSE is a measure of global cognitive function consisting of 11 (mainly multipart) questions addressing orientation (time and place), immediate and delayed recall of three object names, understanding simple commands, naming, simple arithmetic or spelling, and constructional praxis. In the study, a total score out of 30 was used.
For assessment of leg muscle strength, quadriceps strength was measured by isometric contraction of the knee extensors with a handheld dynamometer (μTas F-1; Anima Co., Ltd., Tokyo, Japan). We measured quadriceps strength after the posturography test. With the patient in a seated position, the hip and the knee were positioned at 90° angles, and the force sensor was placed 10 cm above the lateral malleolus. The higher value of the two measurements in both legs was used as maximum muscle strength.
Statistical analysis
Participants in the study were classified into two groups according to the presence or absence of symptoms of bilateral numbness and/or paresthesia in the feet. Differences in the characteristics of 303 Japanese outpatients with diabetes among the two groups were determined by analysis of covariance (ANCOVA) for age with adjustments for sex, an ANCOVA with adjustments for age and sex for continuous data, and a χ 2 test/Fisher’s exact test for dichotomous and categorical data.
The length and enveloped area levels were normally distributed. ANCOVA was used to estimate the multivariable-adjusted means and 95 % confidence intervals (CIs) of indices of postural sway between the two groups. Age and sex were included in Model 1; all factors in model 1 plus diabetic retinopathy (presence or absence), regular exercise (presence or absence), BMI, and HbA1c level were included in model 2; all factors in model 2 plus quadriceps strength were included in model 3.
In addition, patients were classified into four groups according to the combination of the presence or absence of symptoms of bilateral numbness and/or paresthesia in the feet and presence or absence of diabetic retinopathy. Differences in indices of postural sway among the four groups were determined by ANCOVA with adjustments for age, sex, regular exercise, BMI, and HbA1c level. Moreover, patients were classified into four groups according to the combination of the presence or absence of symptoms of bilateral numbness and/or paresthesia in the feet and the presence or absence of regular exercise. Differences in indices of postural sway among the four groups were determined by ANCOVA with adjustments for age, sex, diabetic retinopathy, BMI, and HbA1c level.
All data were analyzed using SPSS statistical software version 21.0J (IBM SPSS Japan Inc., Tokyo, Japan). All reported p values are two-tailed; values <0.05 were considered statistically significant.
Results
Characteristics of study participants
The mean age of the 303 (198 men and 105 women) outpatients with diabetes was 66.7 years. Of these, 35 (11.6 %) had symptoms of bilateral numbness and/or paresthesia in the feet. Table 1 summarizes the characteristics of the 303 patients with diabetes according to the presence or absence of symptoms. Those with symptoms were significantly older than those without (p = 0.005). Except for age, there was no significant difference among the two groups for all variables.
Table 1.
Characteristics of 303 Japanese outpatients with diabetes according to the presence or absence of symptoms of bilateral numbness and/or paresthesia in the feet
| Symptoms of bilateral numbness and/or paresthesia in the feet | p value | ||
|---|---|---|---|
| Absence | Presence | ||
| N | 268 | 35 | |
| Age (years) | 66.0 (64.8–67.3) | 71.3 (67.8–74.8) | 0.005 |
| Males, n (%) | 177 (66.0) | 21 (60.0) | 0.480 |
| Smoking status, n (%) | 0.900 | ||
| Current smoker | 40 (14.9) | 6 (17.1) | |
| Ex-smoker | 117 (43.7) | 14 (40.0) | |
| Non-smoker | 111 (41.4) | 15 (42.9) | |
| Drinking status, n (%) | 0.446 | ||
| Current drinker | 98 (36.6) | 13 (37.1) | |
| Ex-drinker | 34 (12.7) | 7 (20.0) | |
| Non-drinker | 136 (50.7) | 15 (42.9) | |
| Regular exercise, at least twice a week (≥30 min each), n (%) | 143 (53.4) | 19 (54.2) | 0.961 |
| Diabetes duration (years) | 17.4 (16.1–18.6) | 19.0 (15.5–22.5) | 0.401 |
| Type 1 diabetes, n (%) | 24 (9.0) | 3 (8.6) | 0.980 |
| Treated with insulin, n (%) | 102 (38.1) | 15 (42.9) | 0.584 |
| Diabetic retinopathy, n (%) | 46 (17.2) | 8 (22.9) | 0.710 |
| PPDR + PDR, n (%) | 9 (3.4) | 3 (8.6) | 0.150 |
| History of ischemic heart disease, n (%) | 39 (14.6) | 6 (17.1) | 0.685 |
| Medication for hypertension, n (%) | 155 (57.8) | 24 (68.6) | 0.224 |
| Medication for dyslipidemia, n (%) | 143 (53.4) | 24 (68.6) | 0.105 |
| Body mass index (kg/m2) | 24.5 (24.1–25.0) | 24.4 (23.1–25.7) | 0.870 |
| Systolic blood pressure (mmHg) | 136.2 (133.9–138.6) | 136.6 (130.0–143.1) | 0.928 |
| Diastolic blood pressure (mmHg) | 74.3 (73.0–75.5) | 71.3 (67.8–74.8) | 0.118 |
| HDL-cholesterol (mmol/l) | 1.40 (1.35–1.45) | 1.47 (1.33–1.61) | 0.367 |
| HbA1c (%) | 7.4 (7.3–7.5) | 7.3 (7.0–7.7) | 0.775 |
| eGFR (ml/min/1.73 m2) | 71.1 (68.9–73.3) | 71.1 (64.9–77.3) | 0.998 |
| MMSE (score) | 28.5 (28.3–28.8) | 28.7 (28.1–29.3) | 0.620 |
| Quadriceps strength (kg)a | 31.0 (29.5–32.5) | 30.5 (26.6–34.4) | 0.817 |
Age was analyzed by analysis of covariance with adjustments for sex and is shown as sex-adjusted mean (95 % confidence interval)
Continuous, normally distributed data were analyzed by analysis of covariance with adjustments for age and sex and are shown as age- and sex-adjusted means (95 % confidence intervals)
Dichotomous and categorical data were analyzed by χ 2 test and Fisher’s exact test and are shown as number of patients (%)
PPDR pre-proliferative diabetic retinopathy, PDR proliferative diabetic retinopathy, HDL high-density lipoprotein, HbA1c hemoglobin A1c, eGFR estimated glomerular filtration rate, MMSE Mini-Mental State Examination
aThe number of patients who were measured for quadriceps strength was 218 outpatients, because we started measuring quadriceps strength in the middle of the study period
Postural instability according to presence or absence of symptoms
Patients who had symptoms of bilateral numbness and/or paresthesia in the feet had higher postural sway length and enveloped area levels than those without symptoms after adjustment for age, sex, diabetic retinopathy, regular exercise, BMI, and HbA1c level (Table 2; model 2). These results were the same as when we adjusted for quadriceps strength (Table 2; model 3). Although the statistical power decreased because the sample size of those with symptoms was low, the tendency of these results did not change with the sex-specific analysis. Additionally, we observed similar results when we analyzed 234 outpatients aged ≥60 years (Supplementary Table 1). The multivariable-adjusted means of indices of postural sway according to each symptom are shown in Supplementary Figures 1 and 2. These results were the same as when we adjusted for quadriceps strength.
Table 2.
Multivariable-adjusted means of indices of postural sway according to the presence or absence of symptoms of bilateral numbness and/or paresthesia in the feet
| Symptoms of bilateral numbness and/or paresthesia in the feet | p value | ||
|---|---|---|---|
| Absence | Presence | ||
| N | 268 | 35 | |
| Length (cm) | |||
| Model 1 | 52.4 (50.1–54.7) | 59.3 (52.9–65.6) | 0.027 |
| Model 2 | 52.3 (50.1–54.6) | 59.5 (53.1–65.9) | 0.018 |
| Model 3a | 51.5 (48.8–54.2) | 58.9 (51.9–65.8) | 0.042 |
| Enveloped area (cm2) | |||
| Model 1 | 2.7 (2.5–2.9) | 3.5 (2.9–4.0) | 0.011 |
| Model 2 | 2.7 (2.5–2.9) | 3.5 (2.9–4.0) | 0.010 |
| Model 3a | 2.6 (2.3–2.8) | 3.3 (2.7–3.9) | 0.017 |
Length and enveloped area levels were analyzed by analysis of covariance, and are shown as adjusted means (95 % confidence intervals)
Model 1: Adjusted for age and sex
Model 2: Adjusted for all factors in model 1 plus diabetic retinopathy, regular exercise, body mass index, and hemoglobin A1c level
Model 3: Adjusted for all factors in model 2 plus quadriceps strength among 218 outpatients who were measured for quadriceps strength
aThe number of patients who were measured for quadriceps strength was 218 outpatients because we started measuring quadriceps strength in the middle of the study period
Postural instability according to the combination of presence or absence of feet symptoms and the presence or absence of diabetic retinopathy or regular exercise
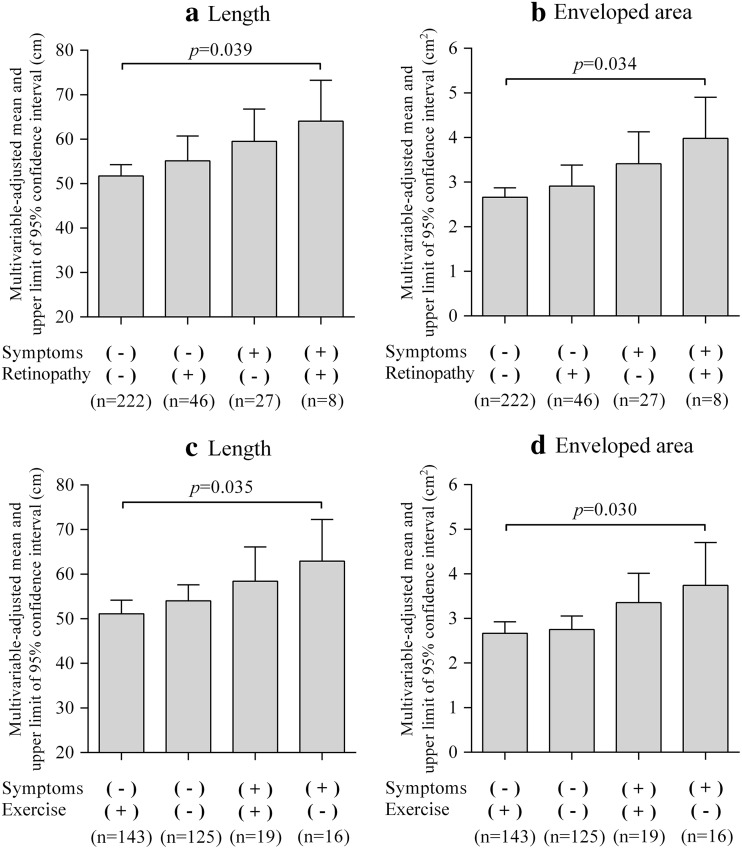
Figure 4 shows multivariable-adjusted means of the length and enveloped area levels according to the combination of the presence or absence of feet symptoms and the presence or absence of either diabetic retinopathy or regular exercise. After adjustment for confounding factors, postural sway length and enveloped area levels for both diabetic retinopathy (p = 0.039 and p = 0.034, respectively) and regular exercise (p = 0.035 and p = 0.030, respectively) differed significantly among the four groups. Patients who had feet symptoms and diabetic retinopathy had the highest postural sway length and enveloped area levels among the four groups. Patients who had feet symptoms and who did not have regular exercise had the highest postural sway length and enveloped area levels among the four groups. These results were the same as when we adjusted for quadriceps strength.
Fig. 4.
Multivariable-adjusted means of length and enveloped area levels according to the combination of the presence or absence of symptoms of bilateral numbness and/or paresthesia in the feet and the presence or absence of diabetic retinopathy/regular exercise; a length level according to feet symptoms and diabetic retinopathy, b enveloped area level according to feet symptoms and diabetic retinopathy, c length level according to feet symptoms and regular exercise, and d enveloped area level according to feet symptoms and regular exercise. Length and enveloped area levels were analyzed by analysis of covariance and are shown as adjusted means and upper limit of 95 % confidence intervals. Length and enveloped area levels according to feet symptoms and diabetic retinopathy were adjusted for age, sex, regular exercise, body mass index, and hemoglobin A1c level. Length and enveloped area levels according to feet symptoms and regular exercise were adjusted for age, sex, diabetic retinopathy, body mass index, and hemoglobin A1c level
Discussion
The main findings of the present study indicated that after adjustment for confounding factors, patients who had symptoms of bilateral numbness and/or paresthesia in the feet had higher postural sway length and enveloped area levels in the posturography test than those without symptoms. In addition, postural sway length and enveloped area levels differed according to the combination of the presence or absence of feet symptoms and the presence or absence of diabetic retinopathy or regular exercise.
Our findings suggest that patients who had symptoms of bilateral numbness and/or paresthesia in the feet may have more postural instability than those without symptoms. Somatosensory information, together with visual and vestibular information, plays an important role in postural control [16]. In particular, it has been reported that somatosensory information in the part of the feet that touches the ground is an important component of the human postural control system [17]. Because somatosensory deficit in the feet is thought to compromise functional postural stability, it is thought that symptoms of bilateral numbness and paresthesia in the feet are associated with postural instability in patients with diabetes.
The present study assessed the association between simple parameters (i.e., symptoms in the feet) and postural instability among patients with diabetes. Even when patients have not yet been diagnosed with diabetic peripheral neuropathy using nerve conduction testing, medical workers need to be aware of postural instability, which is a major risk factor for falls, in patients with these symptoms. Fortunately, most patients can recognize these symptoms, which is thought to be helpful in preventing falls.
Patients in the study who had feet symptoms and diabetic retinopathy had increased postural instability. Diabetic retinopathy is one of the most serious complications of diabetes and is a leading cause of reduced visual acuity and acquired blindness. Visual perception is closely related to postural control and provides afferent feedback regarding postural sway to the cerebellum [18, 19]. Therefore, it is thought that the concurrent effect of symptoms in the feet and diabetic retinopathy is associated with increased postural instability among patients with both factors. Additionally, it has been reported that visual impairment is associated with postural instability [20]. It is thought that patients with both factors had more postural instability, because three of eight (37.5 %) patients with both factors had proliferative diabetic retinopathy or pre-proliferative diabetic retinopathy.
Patients in the study who had feet symptoms and who did not have regular exercise had increased postural instability. In a systematic review and meta-analysis, regular exercise was reported to improve postural instability among older people [21]. Recently, it has been reported that elderly patients with diabetes who exercised by walking and balance-keeping for 12 weeks (twice a week for 60 min) showed a decrease in levels of postural sway length of 31 % [22]. Though exercise as a therapeutic intervention for patients with diabetic peripheral neuropathy has not yet been adequately investigated, several studies have demonstrated that regular exercise improves clinical measures of balance among these patients [23–25]. In our study, the proportion of patients participating in regular exercise (at least twice a week) was approximately 50 % in those with feet symptoms. Therefore, it is important to promote exercise and to support continuation of exercise for patients with symptoms, unless exercise is contraindicated.
There are several limitations to the present study. First, the cross-sectional design cannot prove causality. Therefore, a further investigation in a prospective study is necessary. Second, the sample size of patients with feet symptoms was low. Hence, a further investigation with a larger sample size of patients with feet symptoms is necessary to confirm these results. Third, the subjects were limited to the patients of one university hospital. Fourth, we did not evaluate whether symptoms of bilateral numbness and paresthesia in the feet were due to diabetic peripheral neuropathy. However, we excluded patients with stroke, those with joint disease of the leg such as hip and knee osteoarthritis, those with spinal disease such as lumbar spinal canal stenosis, and those who had broken their leg within the preceding 3 years. Fifth, we did not evaluate the severity of bilateral numbness and paresthesia in the study. Finally, a further investigation that evaluates both feet symptoms and ankle jerk and vibration sense is necessary to confirm our hypothesis.
In conclusion, this is believed to be the first report to suggest the possibility that the simple parameters of symptoms of bilateral numbness and/or paresthesia in the feet are associated with postural instability among Japanese patients with diabetes. Increased postural instability is associated with an increased risk of falls among patients with diabetes. Therefore, patients with diabetes who have symptoms of bilateral numbness and/or paresthesia in the feet should take precautions against increased postural instability.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the Fund for Care Prevention from NPO Biwako Health and Welfare Consortium and Shiga Prefecture. This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Young Scientists (B) (grant no. 25862144). The authors sincerely thank the researchers and medical staff at Shiga University of Medical Science Hospital for their examinations.
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Shiga University of Medical Science, an Ethical Committee) and with the Helsinki Declaration of 1964 and later revisions. Informed consent was obtained from all patients included in the study.
References
- 1.Yau RK, Strotmeyer ES, Resnick HE, et al. Diabetes and risk of hospitalized fall injury among older adults. Diabetes Care. 2013;36:3985–3991. doi: 10.2337/dc13-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volpato S, Leveille SG, Blaum C, et al. Risk factors for falls in older disabled women with diabetes: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:1539–1545. doi: 10.1093/gerona/60.12.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25:1749–1754. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Beckle G, Williams DF, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23:1272–1277. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–396. doi: 10.2337/dc07-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto R, Kinoshita T, Momoki T, et al. Postural sway and diabetic peripheral neuropathy. Diabetes Res Clin Pract. 2001;52:213–221. doi: 10.1016/S0168-8227(01)00236-4. [DOI] [PubMed] [Google Scholar]
- 7.Nakatani M, Sasaki H, Kurisu S, et al. Numbness and paresthesia in bilateral toes and soles, and disproportional sweating restricted to face and trunk are suitable symptoms useful for the diagnosis of diabetic symmetric polyneuropathy. J Diabetes Investig. 2011;2:464–473. doi: 10.1111/j.2040-1124.2011.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diagnosis Standardization Committee and Insurance Medical Care Committee in Japanese Society for Equilibrium Research Precision of the stabilometer for medical use. Equilibr Res. 2015;74:44–50. doi: 10.3757/jser.74.44. [DOI] [Google Scholar]
- 9.Diagnosis Standardization Committee in Japanese Society for Equilibrium Research Materials for the normalization of equilibrium function test methods. Equilibr Res. 2006;65:468–503. doi: 10.3757/jser.65.468. [DOI] [Google Scholar]
- 10.The Japanese Society for Equilibrium Research . Illustrated neurotology. 2. Tokyo: Shindan-to-Chiryo Sha; 2009. [Google Scholar]
- 11.Fujimoto C, Murofushi T, Chihara Y, et al. Assessment of diagnostic accuracy of foam posturography for peripheral vestibular disorders: analysis of parameters related to visual and somatosensory dependence. Clin Neurophysiol. 2009;120:1408–1414. doi: 10.1016/j.clinph.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto C, Murofushi T, Chihara Y, et al. Effects of unilateral dysfunction of the inferior vestibular nerve system on postural stability. Clin Neurophysiol. 2010;121:1279–1284. doi: 10.1016/j.clinph.2010.02.149. [DOI] [PubMed] [Google Scholar]
- 13.Chihara Y, Sato A, Ohtani M, et al. The effect of a first-generation H1-antihistamine on postural control: a preliminary study in healthy volunteers. Exp Brain Res. 2013;231:257–266. doi: 10.1007/s00221-013-3675-1. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Massion J, Alexandrov A, Frolov A. Why and how are posture and movement coordinated? Prog Brain Res. 2004;143:13–27. doi: 10.1016/S0079-6123(03)43002-1. [DOI] [PubMed] [Google Scholar]
- 17.Meyer PF, Oddsson LI, De Luca CJ. Reduced plantar sensitivity alters postural responses to lateral perturbations of balance. Exp Brain Res. 2004;157:526–536. doi: 10.1007/s00221-004-1868-3. [DOI] [PubMed] [Google Scholar]
- 18.Loughlin PJ, Redfern MS. Spectral characteristics of visually induced postural sway in healthy elderly and healthy young subjects. IEEE Trans Neural Syst Rehabil Eng. 2001;9:24–30. doi: 10.1109/7333.918273. [DOI] [PubMed] [Google Scholar]
- 19.Loughlin PJ, Redfern MS, Furman JM. Time-varying characteristics of visually induced postural sway. IEEE Trans Rehabil Eng. 1996;4:416–424. doi: 10.1109/86.547944. [DOI] [PubMed] [Google Scholar]
- 20.Black AA, Wood JM, Lovie-Kitchin JE, et al. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci. 2008;85:489–497. doi: 10.1097/OPX.0b013e31817882db. [DOI] [PubMed] [Google Scholar]
- 21.Sherrington C, Whitney JC, Lord SR, et al. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc. 2008;56:2234–2243. doi: 10.1111/j.1532-5415.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 22.Allet L, Armand S, de Bie RA, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53:458–466. doi: 10.1007/s00125-009-1592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kluding PM, Pasnoor M, Singh R, et al. Safety of aerobic exercise in people with diabetic peripheral neuropathy. Phys Ther. 2014. doi:10.2522/ptj.20140108. [DOI] [PMC free article] [PubMed]
- 24.Song C, Petrofsky J, Lee S, et al. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol Ther. 2011;13:803–811. doi: 10.1089/dia.2011.0036. [DOI] [PubMed] [Google Scholar]
- 25.Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82:205–209. doi: 10.1053/apmr.2001.19742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.