Abstract
Objective
As a subanalysis of the Januvia Multicenter Prospective Trial in Type 2 Diabetes (JAMP study), we examined factors that decreased blood glucose control effect of sitagliptin after 3 months and patients requiring an addition or increase of diabetes treatment.
Methods
We selected patients in whom glycated hemoglobin (HbA1c) levels decreased by month 3 after initiation of sitagliptin treatment and conducted two analyses: (1) in patients who did not change drugs until month 12, we compared changes in HbA1c levels between concomitant drugs and examined factors that decreased blood glucose control effect of sitagliptin; (2) compared changes in HbA1c levels and backgrounds between patients who did and did not require an addition to or increased dose of the antidiabetic agent.
Results
Four hundred and ninety-eight patients were chosen. In 369 patients without drug change until month 12, changes in HbA1c levels during months 3–12 were not significantly different among concomitant drugs; factors causing rebound HbA1c were smoking and weight gain. Patient characteristics were compared between those who did and did not require an additional drug or a dose increase (n = 114) (n = 384). Drug changes were associated with longer disease duration, younger age, higher rate of smoking, and higher degree of insulin resistance but not with concomitantly administered drugs.
Conclusion
Smoking and weight gain were factors that decreased the effect of sitagliptin on reducing blood glucose levels. Differences in concomitant drugs did not affect sitagliptin’s effects on glycemic control. A dose increase or the addition of the antidiabetic drug was not associated with concomitant drugs.
Electronic supplementary material
The online version of this article (10.1007/s13340-017-0340-0) contains supplementary material, which is available to authorized users.
Keywords: DPP4-inhibitor, Type 2 diabetes mellitus, Sitagliptin, HbA1c rebound factor, Decreasing the therapeutic effect
Introduction
Sitagliptin controls blood glucose levels by increasing insulin secretion through inhibition of dipeptidyl peptidase (DPP)-4, an enzyme that degrades incretin, and by suppressing excess glucagon secretion. Hypoglycemia is unlikely to occur [1] because the action of the drug is dependent on blood glucose level; also, weight gain can be prevented [2]. Moreover, this effect lasts by administering sitagliptin once a day. Thus, sitagliptin is easy to use for both physicians and patients, and problems inherent to diabetes treatment, such as weight gain and dose frequency, can be solved by using sitagliptin. This drug is widely used in clinical settings.
Long-term studies of sitagliptin [3, 4] have described improvements after about 3–4 months of therapy, with the effect continuing thereafter. Fewer safety concerns were reported with sitagliptin therapy compared with placebo [3]. After long-term administration of sitagliptin, a reduction in the therapeutic effect was seen in a small number of patients. Kahn et al. reported that the effects of sulfonylurea, biguanide, and thiazolidinedione drugs on glycemic control are decreased when they are administered over the long term [5]. In the diabetes study, a certain number of patients had drugs added to their therapy or a dose increase to reduce glycemic levels. However, these patients are included in the full set or the intent-to-treat analysis. So, factors involved in reducing the efficacy of sitagliptin or patient background requiring a change in the administered drug must be analyzed separately.
Some factors that decrease long-term glycemic control by sitagliptin treatment have been reported [6–9]. A reduction in the effect 6 months after therapy initiation was caused by weight gain in some cases, which was generally a result of low adherence to diet/exercise therapy. However, those studies included discontinued antidiabetic drugs or drugs for which the dose was decreased at the start of sitagliptin treatment [6–8], and the influence of those other drugs could not be ruled out. Moreover, pretreatment drugs were not subdivided [9]. To analyze the factors that decrease the efficacy of sitagliptin, patients included in the analysis should be limited to those receiving sitagliptin in addition to other drugs that were neither discontinued nor reduced in dose at the start of the study. Additionally, it should involve patients undergoing monotherapy or multidrug therapy with different kinds of concomitant drugs, such as sulfonylureas, biguanides, and thiazolidinediones.
We conducted the JAMP study at multiple centers and obtained data for the 12-month period after additional administration of sitagliptin to the pre-existing therapy. As a subanalysis of the study, we examined factors that decreased sitaglintin’s effect on controlling blood glucose after 3 months of treatment and of patients requiring addition/increased treatment.
Methods
Study design
This open-label, central registration, multicenter, prospective observational study was conducted at Tokyo Women’s Medical University Hospital and 69 collaborating institutions in Japan. Patients were enrolled from 11 January 2011 to 30 June 2013 and followed up until 30 June 2014. This study was conducted with the approval of the ethics committee of Tokyo Women’s Medical University (UMIN000019154).
Study subjects
The study subjects were male or female outpatients ≥20 years with type 2 diabetes mellitus (DM) with inadequately controlled blood glucose levels [glycated hemoglobin (HbA1c)] level of ≥ 6.9% or a fasting blood glucose level of ≥ 130 mg/dl during the observation period) after at least 1 month of receiving diet/exercise therapy and/or antidiabetic drug therapy orally. At the start of the study, in 2011, HbA1c values were expressed according to The Japan Diabetes Society levels, the standard system in Japan, but were changed to National Glycohemoglobin Standardization Program (NGSP) system values at the end of the study in accordance with the “Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus (Revision for International Harmonization),” issued by The Japan Diabetes Society [10]. Pursuant to the above change, the lower limit of inadequate blood glucose control was also changed. Therefore, patients with an HbA1c level of ≥ 6.9% were enrolled at the start of the study.
Patients who met any of the following criteria were excluded from the study: (i) history of severe ketosis, diabetic coma, or precoma within the past 6 months; (ii) severe infection before or after surgical treatment, or serious external injury; (iii) pregnancy, possible pregnancy, or lactation; (iv) moderate renal impairment (serum creatinine level ≥ 1.5 mg/dl in men and ≥ 1.3 mg/dl in women); (v) on insulin therapy; (vi) on treatment with rapid-acting insulin secretagogues; (vii) history of allergy to any ingredients in the study drug; and (viii) a medical reason that made the patient unsuitable for participation in the study, as judged by the investigator.
Treatment
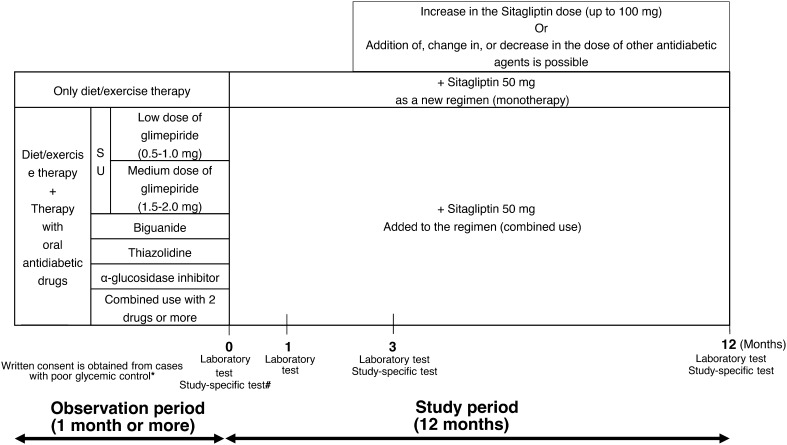
The pre-existing therapy for type 2 DM was not changed during the observation period or throughout the entire study period (for the first 3 months after add-on sitagliptin). Thereafter, sitagliptin (50 mg) was administered once daily as first-line treatment (single-drug therapy) or as additional treatment (combination therapy; Fig. 1). During the 3-month period after initiation (baseline), sitagliptin administration was continued without the addition of any other drugs or dose increases. At 3 months, sitagliptin dose was increased from 50 to 100 mg/day and other antidiabetic drugs were added, changed, or discontinued at the investigator’s discretion. No restrictions were imposed on the use of drugs for treating concurrent diseases, but dose changes or the addition of new drugs were avoided whenever possible during the study period.
Fig. 1.
Study design. *Criteria on poor glycemic control: HbA1c ≥6.9% or fasting blood glucose ≥130 mg/dl. #Study-specific test (arbitrary): GA, anhydroglucitol (1,5-AG), C-peptide, proinsulin/insulin ratio
Assessments
Data from patients in whom HbA1c levels decreased by month 3 after were extracted. Patients whose HbA1c levels were measured until 12 months after the start of therapy were chosen. Because the study allowed for drug changes 3 months after treatment initiation, it was difficult to uniformly assess influencing factors in patients with or without drug change. To address this issue, we analyzed the factors influencing continuation or reduction of the effect according to the two steps below (Fig. 2):
Analysis of patients who did not require a drug change (comparison of changes in HbA1c levels in groups based on the concomitant drug used, and multiple regression analysis of factors that decreased the effect); and
Comparison of changes in HbA1c levels and medical history between patients who did and did not require an addition to or increased dose of the antidiabetic agent (the latter included patients who had no drug change and those who had a drug discontinuation or dose reduction).
Fig. 2.
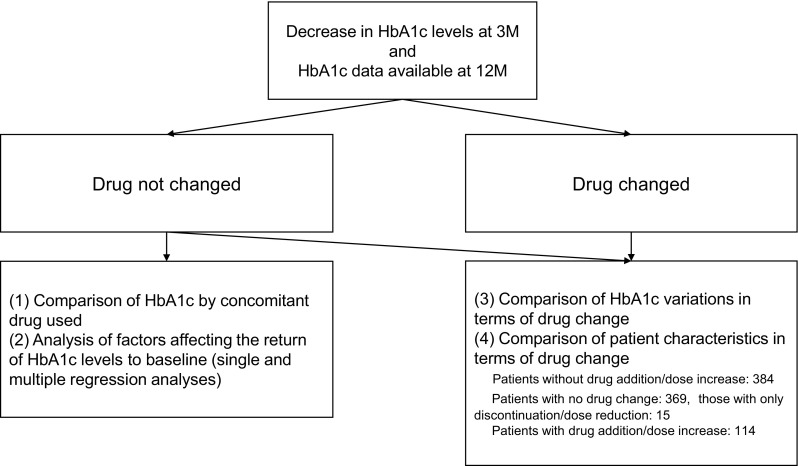
Analysis flow
Statistical analysis
The changes in the HbA1c levels by concomitant drugs in the group without drug change were compared using analysis of variance (ANOVA). To analyze factors reducing the glycemic control effect of sitagliptin, single regression analysis was first performed with changes in HbA1c levels from 3 to 12 months after starting therapy used as objective variables, and parameters such as age or sex were used as explanatory variables. Multiple regression analysis was then performed on factors with p < 0.25 in the single-regression analysis.
Changes in HbA1c levels in patients with or without drug addition/dose increase were compared using the Student’s t test. Patient characteristics were compared using the Student’s t test (continuous data) or the chi-square test (nominal data).
The significance threshold was set at a two-sided p value < 0.05. Multiplicity was considered. All statistical analyses were performed using the JMP software program, version 12.1.0 (SAS Institute Inc., Cary, NC, USA).
Results
Of the 651 patients in whom efficacy was assessed in the JAMP study, 498 were assessed in this subanalysis; these patients met requirements such as a decrease in HbA1c levels at month 3 of therapy and availability of HbA1c values until month 12 of therapy. Of these patients, 369 (74.1%) did not undergo any changes in their drug therapy, 15 (3.0%) had a dose discontinuation or reduction, and 114 (22.9%) had a drug added or a dose increase (Fig. 3).
Fig. 3.
Patient flow
In patients without drug change until month 12 of therapy, no significant differences in changes in HbA1c levels were found from months 3 to 12 of therapy, regardless of the kind of concomitant drug used (Table 1). Additionally, in the analysis of HbA1c rebound factor in cases without drug change until 12 months, smokers and patients who gained weight between months 3 and 12 were more likely to rebound HbA1c (Table 2). Factors affecting the change in HbA1c from 0 to 3 months for reference are shown in supplemental Table S1.
Table 1.
Changes in glycated hemoglobin (HbA1c) levels by pretreatment drug
| n | 0 months | 3 months | 12 months | 0–3 months | 3–12 months | |
|---|---|---|---|---|---|---|
| Diet/exercise therapy alone | 110 | 7.46 ± 0.76 | 6.67 ± 0.56 | 6.66 ± 0.62 | − 0.79 ± 0.54 | − 0.01 ± 0.56 |
| Low dose of glimepiride | 42 | 7.50 ± 0.52 | 6.67 ± 0.49 | 6.87 ± 0.77 | − 0.83 ± 0.40 | 0.20 ± 0.51 |
| Medium dose of glimepiride | 22 | 8.05 ± 0.85 | 7.37 ± 0.79 | 7.59 ± 0.78 | − 0.68 ± 0.45 | 0.21 ± 0.74 |
| BG | 51 | 7.74 ± 1.01 | 6.95 ± 0.92 | 6.99 ± 0.97 | − 0.78 ± 0.46 | 0.04 ± 0.58 |
| TZD | 22 | 7.37 ± 0.46 | 6.52 ± 0.42 | 6.56 ± 0.51 | − 0.85 ± 0.43 | 0.05 ± 0.34 |
| α-GI | 9 | 7.11 ± 0.43 | 6.47 ± 0.31 | 6.59 ± 0.42 | − 0.64 ± 0.23 | 0.12 ± 0.24 |
| Multidrug therapy | 113 | 7.88 ± 0.98 | 6.97 ± 0.68 | 7.11 ± 0.91 | − 0.91 ± 0.63 | 0.13 ± 0.6 |
| p (ANOVA) | 0.000 | 0.000 | 0.000 | 0.362 | 0.294 |
BG biguanide, TZD thizolidine, α-GI α-Glucosidase inhibitor, ANOVA analysis of variance
Table 2.
Factors decreasing glycated hemoglobin (HbA1c) in patients without drug change until month 12: analysis of factors causing changes in HbA1c levels (3–12 months)
| Variables | Single regression analysis | Multiple regression analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Regression coefficients | Lower limit of 95% CI | Upper limit of 95% CI | P value | Partial regression coefficient | Lower limit of 95% CI | Upper limit of 95% CI | P value | |
| Sex (male) | 0.041 | − 0.080 | 0.162 | 0.501 | ||||
| Age | − 0.004 | − 0.009 | 0.002 | 0.173 | − 0.003 | − 0.061 | 0.055 | 0.925 |
| Smoking | 0.226 | 0.079 | 0.373 | 0.003* | 0.192 | 0.042 | 0.342 | 0.013* |
| Alcohol consumption | 0.081 | − 0.035 | 0.197 | 0.169 | − 0.029 | − 0.145 | 0.088 | 0.627 |
| Duration of diabetes (year) | 0.007 | − 0.003 | 0.017 | 0.177 | 0.003 | − 0.006 | 0.013 | 0.495 |
| SBP (mmHg) | − 0.004 | − 0.007 | 0.000 | 0.048* | − 0.004 | − 0.008 | 0.000 | 0.060 |
| BMI (kg/m2) | 0.006 | − 0.008 | 0.021 | 0.366 | ||||
| HbA1c (%) | 0.001 | − 0.067 | 0.069 | 0.982 | ||||
| Weight variations (3–12 months) (kg) | 0.085 | 0.057 | 0.113 | < 0.001* | 0.081 | 0.054 | 0.109 | < 0.001* |
| Antihypertensive drug use | − 0.070 | − 0.188 | 0.047 | 0.238 | − 0.084 | − 0.203 | 0.036 | 0.169 |
| Diet/exercise therapy alone | − 0.136 | − 0.263 | − 0.009 | 0.036* | − 0.076 | − 0.214 | 0.063 | 0.283 |
| Low-dose glimepiride | 0.131 | − 0.053 | 0.315 | 0.161 | 0.148 | − 0.036 | 0.331 | 0.114 |
| Medium-dose glimepiride | 0.138 | − 0.109 | 0.385 | 0.272 | ||||
| BG | − 0.054 | − 0.223 | 0.115 | 0.532 | ||||
| TZD | − 0.041 | − 0.288 | 0.206 | 0.746 | ||||
| α-GI | 0.039 | − 0.340 | 0.419 | 0.838 | ||||
| Multidrug therapy | 0.072 | − 0.055 | 0.199 | 0.265 | ||||
BG biguanide, TZD thiazolidine, α-GI α-Glucosidase inhibitor, ANOVA analysis of variance, CI confidence interval
* P < 0.05 single or multiple regression analysis
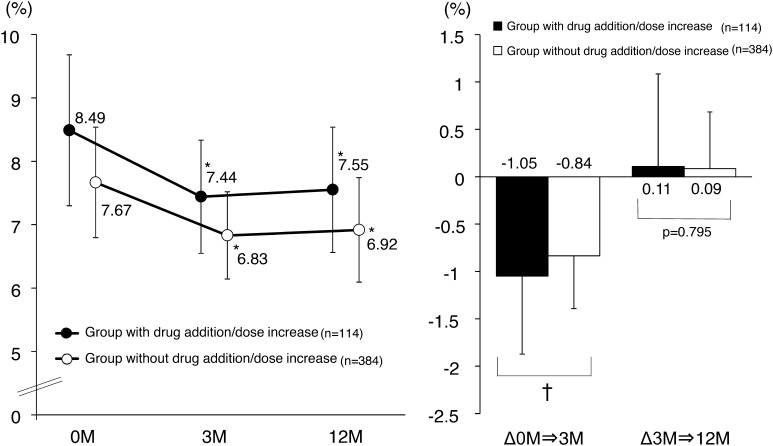
Patient characteristics of those who did and did not have drugs added or dose increased are shown in Table 3. Although changes in HbA1c levels were associated with many parameters such as age or smoking between groups, no differences were associated with concomitantly administered drugs (Table 3). HbA1c levels at the start of the study were significantly higher and changes in HbA1c levels 3 months later significantly larger in the group with added drugs or a dose increase (Fig. 4).
Table 3.
Comparison of patient characteristics
| Drug addition/dose increase (n = 114) | No drug addition/dose increase (n = 384) | P value | |
|---|---|---|---|
| Sex (male) | 81 (71.1) | 245 (63.8) | 0.153 |
| Smoking | 36 (32.4) | 69 (18.4) | 0.002* |
| Alcohol consumption | 56 (50.5) | 170 (45.6) | 0.366 |
| Hypertension | 66 (57.9) | 238 (62) | 0.432 |
| Dyslipidemia | 82 (71.9) | 246 (64.1) | 0.120 |
| Hyperuricemia | 9 (7.9) | 39 (10.2) | 0.472 |
| Retinopathy | 16 (14) | 21 (5.5) | 0.002* |
| Arteriosclerotic obliterans | 19 (16.7) | 25 (6.5) | 0.001* |
| Atrial fibrillation | 3 (2.6) | 10 (2.6) | 0.987 |
| Renal disease | 11 (9.6) | 25 (6.5) | 0.256 |
| Antihypertensive drug use | 57 (50) | 205 (53.4) | 0.525 |
| Diet/exercise therapy | 29 (25.4) | 110 (28.6) | 0.503 |
| Low dose of glimepiride | 11 (9.6) | 45 (11.7) | 0.539 |
| Medium dose of glimepiride | 9 (7.9) | 23 (6) | 0.466 |
| BG | 22 (19.3) | 53 (13.8) | 0.150 |
| TZD | 6 (5.3) | 23 (6) | 0.771 |
| α-GI | 3 (2.6) | 9 (2.3) | 0.860 |
| Multidrug therapy | 34 (29.8) | 121 (31.5) | 0.733 |
| Age (year) | 59.6 ± 10.8 | 65.6 ± 10.8 | < 0.001* |
| Duration of diabetes (year) | 10.1 ± 7.7 | 8.4 ± 6.2 | 0.017* |
| SBP (mmHg) | 128.4 ± 14.6 | 131 ± 15.2 | 0.109 |
| BMI (kg/m2) | 25.4 ± 3.5 | 25.1 ± 4.2 | 0.386 |
| HOMA-R | 8.49 ± 1.19 | 7.67 ± 0.87 | < 0.001* |
| HOMA-β (%) | 3.19 ± 1.99 | 2.7 ± 1.74 | 0.038* |
| Fasting C-peptide (ng/mL) | 23.9 ± 15.3 | 35.8 ± 30.1 | 0.001* |
| UACR (mg/g Cre) | 93.8 ± 369 | 51.8 ± 257.2 | 0.329 |
| eGFR (mL/min/1073m2) | 82.8 ± 20.2 | 76.3 ± 17.3 | 0.001* |
| HbA1c (0 months) (%) | 8.49 ± 1.19 | 7.67 ± 0.87 | < 0.001* |
| HbA1c (3 months) (%) | 7.44 ± 0.89 | 6.83 ± 0.69 | < 0.001* |
| HbA1c (12 months) (%) | 7.55 ± 0.99 | 6.92 ± 0.82 | < 0.001* |
| Body weight (0 months) (kg) | 69.5 ± 13.4 | 65.9 ± 14.3 | 0.018* |
| Body weight (3 months) (kg) | 69.4 ± 13.4 | 66 ± 14.5 | 0.027* |
| Body weight (12 months) (kg) | 69.1 ± 13.8 | 65.8 ± 14.2 | 0.031* |
| Body weight variation (3 months) (kg) | 0.2 ± 1.9 | − 0.1 ± 1.6 | 0.107 |
| Body weight variation (3 months → 12 months) (kg) | − 0.3 ± 2.4 | − 0.1 ± 2.1 | 0.606 |
BG biguanide, TZD thiazolidine, α-GI α-Glucosidase inhibitor, SPB systolic blood pressure, BMI body mass index, HOMA-β homeostasis model assessment–beta cell function, HOMA-R homeostasis model assessment–insulin resistance, UACR urine albumin-to-creatinine ratio, eGFR estimated glomerular filtration rate, HbA 1c glycated hemoglobin
* p < 0.05 chi-square test or Student's t-test, Data presented as n (%) or mean ± SD
Fig. 4.
Changes in glycated hemoglobin (HbA1c) levels by drug addition/dose increase. *P < 0.001 vs month 0; repeated measures analysis of variance (ANOVA) with Bonferroni correction. † P = 0.002; Student’s t test
Discussion
Many studies have reported that patients with higher baseline HbA1c levels are likely to show a larger decrease in HbA1c levels during therapy [11, 12]. Thus, the JAMP study first normalized changes in HbA1c levels to baseline values. Although the group received a medium dose of glimepiride, patients showed a smaller decrease in HbA1c levels at the start of the study [13]. No other factors significantly influenced these changes. In this study, analysis of factors that decreased the therapeutic effect showed there was a more significant reduction in the effect of sitagliptin on lowering HbA1c levels in the group without drug changes when patients were smokers or had gained weight from between months 3 and 12 of therapy. These results suggest that the glycemic control effect of sitagliptin would decrease over time in smokers or who gained weight. The effect noted in smokers can be attributed to the inhibition of endocrine pancreatic function due to smoking [14, 15]. In addition, the effect of sitagliptin is reduced in patients with high blood DPP-4 levels [16]; some reports indicate a correlation between blood DPP-4 levels and body mass index [17]. Weight gain was previously reported to weaken the glycemic control effect of sitagliptin [6, 8, 9], which was confirmed by the results of our study. Although sitagliptin is unlikely to cause increased appetite [18, 19], it is important to not rely exclusively on its characteristics but rather to continue diet/exercise therapy to prevent weight gain. Kubota et al. reported that a high dose of glibenclamide administered concomitantly weakened the glycemic control effect of sitagliptin [9]. In our study, there was no significant difference by agent for reduction of effect, which may be one reason that sulfonylurea (SU) drugs were limited to glimepiride.
We then investigated the characteristics of patients who did and did not require addition/dose increase of antidiabetic agents to improve glycemic control. In multiple differences between groups, an inverse relationship was noted between blood DPP-4 levels and age in patients requiring increased or additional medication [17], and our study supported this finding. We also found that group to comprise younger patients. The significantly high estimated glomerular filtration rate in that group was largely a result of age differences rather than differences in renal function. The need for drug addition/dose increase in patients with long-term diabetes and insulin resistance might be explained by exhaustion of the pancreas.
Changes in HbA1c levels after 3 months were greater in the group requiring addition/dose increase after 3 months, indicating that the probable subsequent return of HbA1c levels to baseline or the need for addition/dose increase is difficult to anticipate based only on changes in HbA1c levels after 3 months. An analysis by pretreatment drug showed no differences between groups, which also indicates the difficulty in detecting a future reduction in effect when drugs are administered concomitantly. However, smoking is considered a factor in HbA1c levels returning to baseline in the group not requiring drug change and a significantly higher rate in the group requiring addition/dose increase. Moreover, weight gain decreased blood glucose control among patients without drug change. Therefore, improving lifestyle is important to achieve a permanent effect. Our results showed that in the group that did not require addition/dose increase, HbA1c remained at almost the same level, ranging from 6.83% at 3 months to 6.92% at 12 months, and each value was <7% on average. This group included some patients whose values returned to baseline but who had favorable glycemic control in general; thus, glycemic control seemed to be generally maintained by sitagliptin alone. Of patients in whom lower HbA1c levels were noted at 3 months, 77.1% had a continuous effect when patients with a discontinuation/dose reduction were included. Some reports suggest that an increase in blood glucose levels in patients with type 2 diabetes mellitus, despite intervention with diet/exercise therapy or treatment, is caused by a decrease in the number of pancreatic beta cells [20, 21] or a decline in their function [22]. This data has not been reportef for humans, but sitagliptin [23] and other DPP-4 inhibitors [24] seem to have a protective or proliferative effect on pancreatic beta cells. These pharmacological mechanisms can explain the lasting effect of sitagliptin, even with long-term administration. Given the safety and efficacy demonstrated by long-term studies, sitagliptin is considered a useful option for long-term diabetes treatment.
Limitations
This was an exploratory subanalysis performed under actual treatment conditions in clinics. Patients with no efficacy information at month 3 after starting therapy or with discontinuation/withdrawal before month 12 of therapy were excluded from the analysis. Thus, patient selection was likely biased. In addition, because there was a long interval between data collection (i.e., between months 3 and 12), it was impossible to assess action efficacy against HbA1c levels returning to baseline when the event occurred. Because glimepiride was the only SU drug used by patients in this study, results may not be applicable to other SU drugs.
Conclusion
Smoking and weight gain are factors that decreased the effect of sitagliptin on reducing blood glucose levels. Importantly, differences in concomitant drugs did not affect its effects on glycemic control. Patient characteristics that warrant an addition/dose increase of the antidiabetic drug included long disease duration, younger age, smoking, and high insulin resistance. On the other hand, if a patient shows an improvement in HbA1c levels after 3 months of therapy, glycemic control with sitagliptin can be continued without changing the drug. Additional administration of sitagliptin in patients with poorly controlled blood glucose levels indicates the possibility of long-term glycemic management. However, by considering factors that decrease the effect of sitagliptin, treatment for patients who have poorly controlled blood glucose levels may be more effective.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We express our sincere gratitude to Mr. Shogo Shishikura (MSD K.K.,) (Teikyo University Graduate School of Public Health, MPH) for scientific advice, including references, and for revising the manuscript. We also thank Nouvelle Place Inc. for conducting data analyses.
JAMP Study Investigators: Akiko Sato (Maruyama Internal Medicine Clinic); Akira Miyashita (Miyashita Surgery Clinic); Asako Kokubo (Kokubo Clinic); Atsuro Tsuchiya (Tsuchiya Clinic); Dai Hirohara (Hanazono Clinic); Daiji Kogure (Kogure Clinic); Daijo Kasahara (Kasahara Clinic); Hideki Tanaka (Internal Medicine, Seiwa Clinic); Hideki Tanaka (Internal Medicine, Nishiarai Hospital); Hideo Tezuka (Tezuka Clinic); Hiroyuki Kuroki (Internal Medicine, Johsai Hospital); Jun Ogino (Department of Diabetes, Endocrine and Metabolic Diseases, Tokyo Women’s Medical University Yachiyo Medical Center); Kanu Kin (Internal Medicine, Nishiarai Lifestyle-related diseases Clinic); Kanu Kin (Internal Medicine, Nishiarai Hospital); Kazuko Muto (Tokyo Women’s Medical University); Kazuo Suzuki (Kenkokan Suzuki Clinic of Internal Medicine); Keiko Iseki (Iseki Clinic); Keita Watanabe (Watanabe Clinic); Kenshi Higami (Higami Hospital); Kenzo Matsumura (Matsumura Gastroenterological Clinic); Kiyotaka Nakajima (Ebisu Clinic); Koki Shin (Shin Clinic); Kuniya Koizumi (Kuniya Clinic); Maki Saneshige (Mugishima Medical Clinic); Makio Sekine (Sekine Clinic); Makoto Yaida (Urban Heights Clinic); Mari Kiuchi (Physician, Kanauchi Medical Clinic); Mari Mugishima (Mugishima Medical Clinic); Mari Osawa (Department of Diabetes Mellitus, Institute of Geriatrics, Tokyo Women’s Medical University); Masae Banno (Banno Medical Clinic); Masahiro Yamamoto (Internal Medicine 1, Shimane University Faculty of Medicine); Masatake Hiratsuka (Higashishinagawa Clinic); Masumi Hosoya (Yasui Clinic); Michika Atsuta (Internal Medicine, Nishiarai Lifestyle-related diseases Clinic); Mitsutoshi Kato (Kato Clinic of Internal Medicine); Miwa Morita (Internal Medicine 1, Shimane University Faculty of Medicine); Munehiro Miyamae (Johsai Hospital); Mutsumi Iijima (Abe Hospital); Naomi Okuyama (Shinjuku Mitsui Building Clinic); Nobuo Hisano (Mejiro Medical Clinic); Norihiro Tsuchiya (Omotesando Naika Ganka); Rie Wada (Kanauchi Medical Clinic); Rie Wada (Nerimasakuradai Clinic); Ryuji Momose (Momo Medical Clinic); Sachiko Otake (Tokyo Women’s Medical University); Satoko Maruyama (Shinjuku Mitsui Building Clinic); Satoru Takada (Internal Medicine, Social welfare corporation Shineikai Takinogawa Hospital); Shigeki Dan (Ube Internal Medicine and Pediatrics Hospital); Shigeki Nishizawa (Nishizawa Medical Clinic); Shigeo Yamashita (Department of Diabetes and Endocrinology, JR Tokyo General Hospital); Shingo Saneshige (Internal Medicine, Kamiochiai Shin Clinic); Shinichi Teno (Teno Clinic); Shinji Tsuruta (Diabetic Medicine, Itabashi Chuo Medical Center); Shinobu Kumakura (Kumakura Medical Clinic); Sumiko Kijima (Abe Hospital); Takashi Kondo (Kondo Clinic); Takeo Onishi (Internal Medicine, Onishi Clinic); Taku Kudo (Internal Medicine, Social welfare corporation Shineikai Takinogawa Hospital); Tatsushi Sugiura (Internal Medicine, Seiwa Clinic); Toshihiko Ishiguro (Kaname Clinic); Yasue Suzuki (Suzuki Medical Clinic); Yasuhiro Tomita (Nakanobu Clinic); Yasuko Takano (Department of Diabetes, Shiseikai Daini Hospital); Yoshihisa Akimoto (Akimoto Yoshi Medical Clinic); Yoshiko Odanaka (Ito Internal Medicine Pediatrics Clinic); Yoshimasa Tasaka (Tokyo Women’s Medical University); Yoshitaka Aiso (Internal Medicine, Diabetes, Aiso Clinic); Yukiko Inoue (Inoue Medical Clinic); Yukinobu Kobayashi (Kobayashi Clinic).
Funding
This study was funded by Japan Diabetes Foundation.
Conflict of interest
Hiroshi Sakura received honoraria from Mitsubishi Tanabe Pharma Corporation and research grant from Ono Pharmaceutical Co., Ltd. Other authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The ethics committee at the Tokyo Women’s Medical University approved the study (Approval Number: 2064) on 11 January 2011. Informed consent or substitute was obtained from all patients included in the study.
Contributor Information
Hideo Nunome, Phone: +81-3-5879-7121, Email: mps_research@yahoo.co.jp.
for the JAMP Study Investigators:
Akiko Sato, Akira Miyashita, Asako Kokubo, Atsuro Tsuchiya, Dai Hirohara, Daiji Kogure, Daijo Kasahara, Hideki Tanaka, Hideki Tanaka, Hideo Tezuka, Hiroyuki Kuroki, Jun Ogino, Kanu Kin, Kanu Kin, Kazuko Muto, Kazuo Suzuki, Keiko Iseki, Keita Watanabe, Kenshi Higami, Kenzo Matsumura, Kiyotaka Nakajima, Koki Shin, Kuniya Koizumi, Maki Saneshige, Makio Sekine, Makoto Yaida, Mari Kiuchi, Mari Mugishima, Mari Osawa, Masae Banno, Masahiro Yamamoto, Masatake Hiratsuka, Masumi Hosoya, Michika Atsuta, Mitsutoshi Kato, Miwa Morita, Munehiro Miyamae, Mutsumi Iijima, Naomi Okuyama, Nobuo Hisano, Norihiro Tsuchiya, Rie Wada, Rie Wada, Ryuji Momose, Sachiko Otake, Satoko Maruyama, Satoru Takada, Shigeki Dan, Shigeki Nishizawa, Shigeo Yamashita, Shingo Saneshige, Shinichi Teno, Shinji Tsuruta, Shinobu Kumakura, Sumiko Kijima, Takashi Kondo, Takeo Onishi, Taku Kudo, Tatsushi Sugiura, Toshihiko Ishiguro, Yasue Suzuki, Yasuhiro Tomita, Yasuko Takano, Yoshihisa Akimoto, Yoshiko Odanaka, Yoshimasa Tasaka, Yoshitaka Aiso, Yukiko Inoue, and Yukinobu Kobayashi
References
- 1.Iwamoto Y, Tajima N, Kadowaki T, et al. Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes. A randomized, double-blind trial. Diabetes Obes Metab. 2010;12:613–622. doi: 10.1111/j.1463-1326.2010.01197.x. [DOI] [PubMed] [Google Scholar]
- 2.Nonaka K, Kakikawa T, Sato A, Okuyama K, Fujimoto G, Kato N, Suzuki H, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79(2):291–298. doi: 10.1016/j.diabres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Green JB, Bethel A, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 4.Ku EJ, Jung KY, Kim YJ, Kim KM, Moon JH, Choi SH, Cho YM, Park KS, Jang HC, Lim S, Ahrén B. Four-year durability of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes in clinical practice; COSMIC study. PLoS ONE. 2015 doi: 10.1371/journal.pone.0129477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G, ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 6.Tajiri Y, Kato N, Kudo T, Hasuo R, Yoshinobu S, Kono S, Nakayama H, Yamada K. Long-term efficacy and safety of sitagliptin in Japanese patients with type 2 diabetes. J Jpn Diabetes Soc. 2014;57(7):513–519. [Google Scholar]
- 7.Kanamori A, Matsuba I. Factors associated with reduced efficacy of sitagliptin therapy: analysis of 93 patients with type 2 diabetes treated for 1.5 years or longer. J Clin Med Res. 2013;5(3):217–221. doi: 10.4021/jocmr1256w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tajiri Y, Tsuruta M, Ohki T, Kato T, Sasaki Y, Tanaka K, Kono S, Tojikubo M, Yamada K. Long-term efficacy of sitagliptin for the treatment of type 2 diabetic patients in Japan. Endocr J. 2012;59:197–204. doi: 10.1507/endocrj.EJ11-0248. [DOI] [PubMed] [Google Scholar]
- 9.Kubota A, Yabe D, Kanamori A, Kuroe A, Takahashi N, Saito T, Matsuba I, Nabe K, Kurose T, Seino Y. Factors influencing the durability of the glucose-lowering effect of sitagliptin combined with a sulfonylurea. J Diabetes Investig. 2014;5:445–448. doi: 10.1111/jdi.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakura H, Hashimoto N, Sasamoto K, Ohashi H, Hasumi S, Ujihara N, Kasahara T, Tomonaga O, Nunome H, Honda M, Iwamoto Y, JAMP Study Investigators Effect of sitagliptin on blood glucose control in patients with type 2 diabetes mellitus who are treatment naive or poorly responsive to existing antidiabetic drugs: the JAMP study. BMC Endocr Disord. 2016;16(1):70. doi: 10.1186/s12902-016-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota A, Maeda H, Kanamori A, et al. Efficacy and safety of sitagliptin monotherapy and combination therapy in Japanese type 2 diabetes patients. J Diabetes Investig. 2012;3(6):503–509. doi: 10.1111/j.2040-1124.2012.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda H, Kubota A, Tanaka Y, et al. The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes. Diabetes Res Clin Pract. 2012;95(1):e20–e22. doi: 10.1016/j.diabres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Jpn Diabetes Soc. 2012;55:485–504. doi: 10.11213/tonyobyo.55.485. [DOI] [Google Scholar]
- 14.Murthy SN, Dinoso VP, Jr, Clearfield HR, Chey WY. Simultaneous measurement of basal pancreatic, gastric acid secretion, plasma gastrin, and secretin during smoking. Gastroenterology. 1977;73:758–761. [PubMed] [Google Scholar]
- 15.Taminato T, Seino Y, Goto Y, Inoue Y, Matsukura S, Imura H. Cigarettes smoking inhibits arginine-induced insulin release in man. Horm Metab Res. 1978;10:78–80. doi: 10.1055/s-0028-1095814. [DOI] [PubMed] [Google Scholar]
- 16.Aso Y, Ozeki N, Terasawa T, Naruse R, Hara K, Suetsugu M, Takebayashi K, Shibazaki M, Haruki K, Morita K, Inukai T. Serum level of soluble CD26/dipeptidyl peptidase-4 (DPP-4) predicts the response to sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes controlled inadequately by metformin and/or sulfonylurea. Transl Res. 2012;159(1):25–31. doi: 10.1016/j.trsl.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, Eckardt K, Kaufman JM, Ryden M, Muller S, Hanisch FG, Ruige J, Arner P, Sell H, Eckel J. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917–1925. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gül OO, Kıyıcı S, Ersoy C, Cander S, Yorulmaz H, Gül CB, Unal OK, Sarandol E, Kırhan E, Sigirli D, Ertürk E, Tuncel E, Imamoğlu S. Effect of sitagliptin monotherapy on serum total ghrelin levels in patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;94(2):212–216. doi: 10.1016/j.diabres.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Huang CL, Hsu CH, Huang KC, et al. Preprandial single oral dose of sitagliptin does not affect circulating ghrelin and gastrin levels in normal subjects. Pharmacology. 2010;85:131–135. doi: 10.1159/000280583. [DOI] [PubMed] [Google Scholar]
- 20.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 21.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese type II diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 22.UK Prospective Diabetes Study Group UK prospective diabetes 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. doi: 10.2337/diab.44.11.1249. [DOI] [PubMed] [Google Scholar]
- 23.Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, Feng Y, Zhu L, Li C, Howard AD, Moller DE, Thornberry NA, Zhang BB. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic β-cell mass and function in a rodent model of type 2 diabetes. Diabetes. 2006;55:1695–1704. doi: 10.2337/db05-1602. [DOI] [PubMed] [Google Scholar]
- 24.Moritoh Y, Takeuchi K, Asakawa T, Kataoka O, Odaka H. Chronic administration of alogliptin, a novel, potent, and highly selective dipeptidyl peptidase-4 inhibitor, improves glycemic control and beta-cell function in obese diabetic ob/ob mice. Eur J Pharmacol. 2008;588:325–332. doi: 10.1016/j.ejphar.2008.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.