Abstract
Aim
We developed a novel estimation method for hemoglobin A1c (HbA1c) in type 2 diabetes (T2D) patients with end-stage renal disease (ESRD). This method is based on the glycated albumin (GA) level.
Methods
Of the 788 Japanese patients with T2D included in this study, 545 had normal renal function (NRF group) and 243 had ESRD. Oral glucose tolerance tests (OGTTs) were performed in 80 subjects. The variables GA, body mass index (BMI), hemoglobin (Hb), and estimated glomerular filtration rate (eGFR) were significantly associated with the GA-to-HbA1c ratio and were used to determine the estimated HbA1c (eHbA1c). One method of estimating HbA1c involved dividing GA by the GA-to-HbA1c ratio predicted from the estimated regression equation; the estimated HbA1c obtained in this manner was denoted eHbA1c-1.
Results
eHbA1c-1 (%) = GA × [4.688 − 18.833 × GA−1 − 0.015 × BMI − 0.037 × Hb (− 0.002 × eGFR for patients without ESRD)]−1; adjusted R2 = 0.676 for actual HbA1c. The sensitivity of eHbA1c-1 was better than that of GA for diabetes diagnosis using the 75-g OGTT. There were no differences in the slope of eHbA1c-1 versus GA and the variance of eHbA1c-1 between the ESRD and NRF groups. eHbA1c-1 was not associated with Hb, erythropoiesis-stimulating agent use, or ESRD concomitance.
Conclusions
eHbA1c-1 may be a useful parameter for estimating HbA1c in T2D patients with ESRD.
Keywords: Type 2 diabetes, End-stage renal disease, Hemoglobin A1c, Glycated albumin
Introduction
Hemoglobin A1c (HbA1c) is the gold standard marker for glycemic control in patients with diabetes mellitus (DM). However, several studies have suggested that HbA1c levels in end-stage renal disease (ESRD) patients, such as those receiving hemodialysis (HD), tend to be lower for their shorter red blood cell life span and use of erythropoiesis-stimulating agents (ESAs) [1–4]. Other studies have indicated that glycated albumin (GA), which is not affected by the red blood cell life span or ESA administration, could be used as an alternative marker to HbA1c for plasma glucose control in ESRD patients, including those receiving HD [1–3]. However, although GA levels are not influenced by the red blood cell life span or use of ESAs, they may fluctuate under the prolonged albumin (Alb) metabolism associated with common complications experienced by ESRD patients, such as hypothyroidism, decreased body mass index (BMI), chronic inflammation, and liver dysfunction [5–9]. Thus, there is an ongoing academic debate about whether HbA1c or GA is the more useful glycemic control index in ESRD patients [10–12].
In recent years, several studies have employed the GA-to-HbA1c ratio (GA/HbA1c ratio) to estimate HbA1c levels based on measured GA levels [13–15]. This method is useful for patients in pathophysiological states for which the HbA1c level may not accurately reflect the plasma glucose level. The equation used to predict the HbA1c level in these studies is a linear function in which the GA/HbA1c ratio is assumed to be constant. However, because the GA/HbA1c ratio is 30–40% higher in ESRD patients receiving HD than in DM patients without renal dysfunction [1–3], the conventional predictive equation for HbA1c that uses the measured GA is not suitable for ESRD patients.
To address these issues, we determined the independent factors that contribute to the GA/HbA1c ratio in order to establish a novel regression formula. We then used this formula as the coefficient in novel methods that provided an estimate of HbA1c (eHbA1c) based on the GA level. The purpose of this study was to investigate the utility of one such eHbA1c parameter, eHbA1c-1, derived using one of those novel methods, for type 2 diabetes (T2D) patients with ESRD, including those receiving HD.
Materials and methods
Study design and study subjects
In this cross-sectional study, the medical records of outpatients with and without T2D at the Osafune Clinic, Shin Kashiwa Clinic, Shin Kashiwa Clinic Ootaka No Mori, Kobayashi Medical Clinic, and Innoshima General Hospital were accessed to retrieve background characteristics and clinical data.
First, we developed novel equations for estimating the HbA1c using the GA/HbA1c ratio in T2D patients with normal renal function (NRF) (study design 1). We then investigated the association between the eHbA1c values given by each of two selected novel equations and the plasma glucose level using standard 75-g OGTT data, and assessed the utility of those two eHbA1c equations for diagnosing diabetes mellitus in patients with NRF (study design 2). Finally, we examined the utility of the two eHbA1c parameters (denoted eHbA1c-1 and eHbA1c-2) arising from the two selected novel equations as markers of glycemic control in T2D patients with ESRD (study design 3).
Study design 1
First, we developed novel eHbA1c equations based on the GA level in T2D patients. The clinical data for outpatients with T2D were analyzed and all patients that met the following criteria were considered for inclusion in study design 1: no episodes of ketoacidosis, initial diagnosis of diabetes at > 40 years of age, no demonstrable antibodies to glutamic acid decarboxylase, and no changes in T2D treatment during the 3 months prior to the measurement of HbA1c and GA levels. The GA/HbA1c ratio is influenced by several factors [1–9]. In order to develop a robust eHbA1c parameter, we investigated the independent factors that contribute to the GA/HbA1c ratio in T2D patients without severe anemia or severe hypoalbuminemia. Moreover, T2D patients with the following criteria were excluded from the analysis: diagnosed with DM less than 3 months previously, age < 20 years, signs of infection or treatment for malignancy at the time of specimen collection, and hemoglobin (Hb) level < 8.5 g/dL or serum Alb level < 3.0 g/dL. Subjects in the NRF group had an estimated glomerular filtration rate (eGFR) of ≥ 60 mL/min/1.73 m2, as calculated from the serum creatinine (Cr) level.
The GA/HbA1c ratio (HbA1c values in derived NGSP units) was calculated using data from T2D patients in the NRF group, and independent factors that contribute to this ratio were investigated. T2D patients with the following characteristics were excluded from this analysis: Hb concentration < 13.5 g/dL in males or < 11.5 g/dL in females, variation in HbA1c ≥ 1.0% (11 mmol/mol) or GA ≥ 2.5% over 3 months, BMI ≥ 30.0 kg/m2 or BMI < 18.5 kg/m2, and complication with disorders of the thyroid, liver, and blood, including renal anemia, treatment with ESAs, iron preparations, vitamins, and urinary protein concentration ≥ 0.5 g/gCr. NRF-group subjects with none of these exclusion criteria were included in the final analyses performed to develop equations for predicting HbA1c. A linear regression analysis was performed to identify the variables that influence the GA/HbA1c ratio. A stepwise multivariate linear regression analysis was then carried out to identify the independent variables associated with the GA/HbA1c ratio. A regression formula was derived. Finally, we developed two equations for predicting eHbA1c. In the first, GA was divided by the GA/HbA1c ratio predicted from the regression equation, and the eHbA1c parameter yielded by this equation was denoted eHbA1c-1. In the second equation, HbA1c was predicted from the regression equation, and the eHbA1c parameter yielded by this equation was denoted eHbA1c-2.
Study design 2
We examined the utility of the two eHbA1c equations for diabetes diagnosis. A standard 75-g OGTT was performed in patients with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), HbA1c ≥ 5.8% (40 mmol/mol), fasting plasma glucose (FPG) ≥ 110 mg/dL, or occasional plasma glucose (PG) ≥ 140 mg/dL, but not in those with a diagnosis of diabetes [16], FPG ≥ 126 mg/dL, eGFR < 60 mL/min/1.73 m2, age < 20 years, signs of infection or treatment for malignancy, Hb level < 8.5 g/dL, or serum Alb level < 3.0 g/dL at the time of specimen collection. The standard 75-g OGTT was performed after the patient had fasted for at least 10 h. We evaluated PG levels at 0, 30, 60, 90, and 120 min for the 75-g OGTT. We also evaluated C-peptide immunoreactivity (CPR) levels at 0 and 30 min, and calculated ΔCPR by subtracting the CPR at 30 min from the CPR at 0 min, for the 75-g OGTT. In addition, immunoreactive insulin (IRI) levels were evaluated at 0 min for the 75-g OGTT.
Study design 3
We examined the utility of the two eHbA1c equations as markers of glycemic control in T2D patients with ESRD. We investigated whether the average PG (aPG) exhibited any correlation with HbA1c, GA, eHbA1c-1, or eHbA1c-2, and identified the significant independent factors associated with those markers in all T2D patients. In the ESRD group, subjects included those with eGFR < 15 mL/min/1.73 m2 or those receiving HD. Renal anemia was treated according to Japanese guidelines [17] with ESAs and iron preparations.
Laboratory measures
Blood samples were collected from HD patients before HD therapy, ESRD patients who were not undergoing HD therapy, and NRF-group patients. HbA1c and GA were measured once a month for each subject, concomitant with the measurement of Hb, serum Alb, serum Cr, and urinary protein. aPG levels were measured on three occasions over the three months in the NRF and the ESRD groups. Anemia was defined as Hb < 13.5 g/dL in males and < 11.5 g/dL in females [17]. eGFR was calculated as follows: eGFR = 194 × Cr−1.094 × age−0.287 (age × 0.739 for females), in accordance with the 2012 Chronic Kidney Disease Guidelines [18]. Homeostasis model assessments (HOMAs) of insulin resistance (HOMA-IR) and HOMA β-cell function (HOMA-β) were carried out based on FPG and IRI levels using the formulae HOMA-IR = FPG (mg/dL) × IRI (μU/mL)/405 and HOMA-β = 360 × IRI (μU/mL)/(FPG (mg/dL) − 63), respectively [19]. PG levels were measured using the HK-G6PD method (DENKA SEIKEN, Tokyo, Japan), HbA1c levels were measured using high-performance liquid chromatography (ARKRAY, Kyoto, Japan), and GA levels were measured using an enzymatic method (Asahikasei Pharma Co., Tokyo, Japan). Plasma IRI levels were measured using CLEIA (Fujirebio Inc., Tokyo, Japan). Plasma CPR levels were measured using an immunoenzymometric assay (TOSOH Co., Tokyo, Japan).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation values and categorical variables as actual values or percentages. The Shapiro–Wilk test was used to test for Gaussian distributions of continuous variables. The variable urinary protein excretion rate was converted into a natural logarithm (ln). A multiple linear regression analysis was used to investigate the independent factors that contribute to the GA/HbA1c ratio. The multiple correlation coefficient adjusted for the degrees of freedom (adjusted R2) and the root mean square error (RMSE) were used to investigate the agreement between the calculated eHbA1c values and the actual HbA1c values. The two groups were compared using Student’s t test for continuous variables and the chi-square test for frequencies and ordinal variables. Analysis of variance (ANOVA) was used to investigate changes in aPG and HbA1c over three months. Pearson’s product-moment correlation coefficient (r) was used to assess correlations between continuous variables. Analysis of covariance (ANCOVA) was applied to examine the congruity of the slope and the variance between two groups. A binomial logistic regression analysis was used to investigate the HbA1c and GA levels in T2D patients in addition to the independent factors that contribute to the eHbA1c. Continuous variables were used in the binomial logistic regression analysis after the values of each variable had been categorized into values above and below its mean. Microsoft Excel® 2010 (Microsoft, Seattle, WA, USA) was used for the RMSE assessment and IBM SPSS® Statistics 19 software (IBM, Armonk, NY, USA) was used for all other analyses. P < 0.05 was considered to indicate statistical significance.
Results
Categorization of study subjects
A total of 885 outpatients with type 2 diabetes, IFG, or IGT were enrolled in this study (Fig. 1). The 788 type 2 diabetes patients enrolled in the present study were divided into NRF and ESRD groups (study design 3). Among the 545 NRF-group subjects, 156 selected subjects were examined to develop equations for estimating HbA1c (study design 1). Of the 94 patients that underwent a standard 75-g OGTT, 14 who met the exclusion criteria were eliminated from the analysis. Thus, 80 patients with IFG or IGT were enrolled in the analysis (study design 2).
Fig. 1.
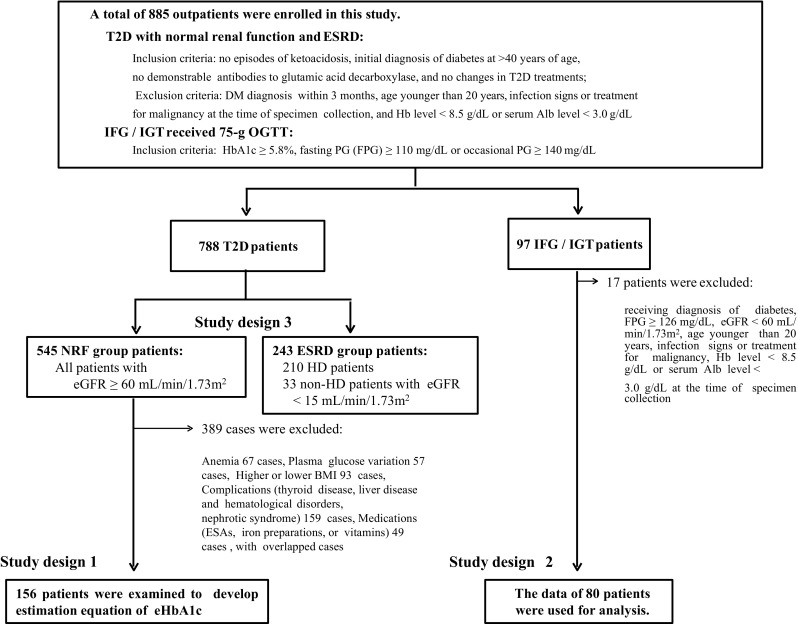
Flow diagram of the present study. A total of 885 outpatients with T2D, IFG, or IGT were enrolled in this study. The 788 T2D patients enrolled in the present study were divided into a NRF group and an ESRD group (study design 3). Among the 545 NRF-group subjects, 156 selected subjects were examined to develop equations for predicting HbA1c (study design 1). Of the 94 patients who underwent a standard 75-g OGTT, 14 who met the exclusion criteria were eliminated from the analysis. Eighty patients with IFG or IGT were ultimately enrolled for analysis (study design 2)
Development of two estimation methods for HbA1c (study design 1)
Of the 545 NRF group subjects, 389 (71.4%) had backgrounds that could possibly influence HbA1c levels and were therefore excluded from the analysis performed to develop the estimation equations. Those subjects had backgrounds that included at least one of the following: anemia (67 cases); plasma glucose variation within the past 3 months (57 cases); high or low BMI (93 cases); thyroid disease, liver disease, hematological disorders, or renal anemia (152 cases); treatment with ESA, iron preparations, or vitamins (49 cases); nephrotic syndrome (7 cases). The selected 156 subjects in the NRF group were examined to identify independent factors associated with the GA/HbA1c ratio. The mean values of HbA1c [% (mmol/mol)] and aPG (mg/dL) over the three consecutive months in the 156 NRF cases were 6.4 ± 0.7 (46 ± 8), 6.5 ± 0.7% (47 ± 8), 6.5 ± 0.7% (48 ± 8), and 6.4 ± 0.7% (46 ± 8) and 127.7 ± 44.2, 133.9 ± 53.1, 129.6 ± 43.8, 123.8 ± 44.5, respectively. Thus, the HbA1c or aPG values were stable before enrollment in the present study (HbA1c, P = 0.221; aPG, P = 0.181).
The slope of the correlation between the GA/HbA1c ratio and GA was found to be proportional to the inverse of HbA1c, so the GA/HbA1c ratio was nonlinearly correlated with GA. We therefore attempted to identify the GA-based formula that would best predict the GA/HbA1c ratio. We examined inverse (3.759 – 20.004 × GA−1, R2 = 0.573, P < 0.001), logarithmic (− 0.932 + 1.235 × ln(GA), R2 = 0.561, P < 0.001), power (0.624 × GA0.497, R2 = 0.551, P < 0.001), and exponential (1.555 × e0.029 × GA, R2 = 0.517, P < 0.001) models, all employing GA as an independent variable, as possible formulae to predict the GA/HbA1c ratio. We found that the inverse model of GA gave the best fit to the GA/HbA1c ratio among the four models. We thus employed GA−1 as an independent variable to estimate the GA/HbA1c ratio in a multiple linear regression analysis. In addition to GA−1, we also selected age, DM history, degree of proteinuria, serum Alb concentration, BMI, Hb, and eGFR as independent variables. In a stepwise multiple linear regression analysis, age, DM history, degree of proteinuria, and serum Alb concentration were excluded from the regression formula, and GA−1, BMI, Hb, and eGFR were identified as significant independent variables. We then defined eHbA1c-1 as the parameter obtained by dividing GA by the GA/HbA1c ratio predicted from the estimated regression equation, and we defined eHbA1c-2 as the parameter obtained from the estimated regression equation using GA, BMI, Hb, and eGFR.
In the 156 selected T2D patients, eHbA1c-1 and eHbA1c-2 were found to correlate well with the actual HbA1c when equation model 4 and 8 were applied (Table 1), according to the adjusted R2 and RMSE values. This equation model included the independent variables GA−1, GA, BMI, Hb, and eGFR. It was not possible to evaluate the eGFR in HD patients, and eGFR had a minimal effect on eHbA1c-1 and eHbA1c-2 in the ESRD patients. In fact, the adjusted R2 values of the equation models with and without eGFR in the ESRD group were 1.000. We therefore eliminated eGFR as an independent variable for ESRD patients. The final equations for eHbA1c-1 and eHbA1c-2 were as follows:
Table 1.
Adjusted R2 values and RMSE values for comparisons of four equation models for eHbA1c-1 and eHbA1c-2 with actual HbA1c data for 156 type 2 diabetes patients
| Model | eHbA1c-1 | eHbA1c-2 | ||||
|---|---|---|---|---|---|---|
| Equation | Adjusted R2 | RMSE | Equation | Adjusted R2 | RMSE | |
| 1 | GA × (3.759 − 20.004 × GA−1)−1 | 0.601a | 0.454 | model 5 3.106 + 0.209 × GA | 0.590a | 0.458 |
| 2 | GA × (4.212 − 19.940 × GA−1 − 0.019 × BMI)−1 | 0.640a | 0.430 | model 6 1.869 + 0.207 × GA + 0.053 × BMI | 0.643a | 0.428 |
| 3 | GA × (4.512 − 19.146 × GA−1 − 0.015 × BMI-0.0032 × Hb)−1 | 0.662a | 0.415 | model 7 0.733 + 0.215 × GA + 0.041 × BMI + 0.049 × Hb | 0.664a | 0.415 |
| 4 | GA × (4.688 − 18.833 × GA−1 − 0.015 × BMI − 0.0037 × Hb − 0.002 × eGFR)−1 | 0.676a | 0.415 | model 8 0.099 + 0.218 × GA + 0.041 × BMI + 0.106 × Hb + 0.005 × eGFR | 0.677a | 0.406 |
The adjusted R2 is the multiple coefficient of determination
The adjusted R2 and RMSE values indicate that HbA1c is related to eHbA1c-1 or eHbA1c-2
RMSE root mean square error
aP < 0.001
In the 156 selected T2D patients, HbA1c, eHbA1c-1, and eHbA1c-2 yielded similar regression line slopes (aPG, P = 0.596; GA, P = 0.997) and variances (aPG, P = 0.340; GA, P = 0.061) when they were compared with aPG and GA.
Specificity and sensitivity of eHbA1c-1 and eHbA1c-2 for DM diagnosis (study design 2)
Eighty patients (33 males; mean age 59.5 ± 11.8 years old) with IFG or IGT were enrolled in the analysis. eHbA1c-1 and eHbA1c-2 showed significant positive correlations with the PG level at all time points after the 75-g OGTT, and with the peak and mean PG levels after the 75-g OGTT (Table 2). We examined the specificity and sensitivity of eHbA1c-1 and eHbA1c-2 for DM diagnosis based on 75-g OGTT and HbA1c data. Of the 80 patients who received a standard 75-g OGTT, 25 (31.3%) were diagnosed with DM with PG ≥ 200 mg/dL and HbA1c ≥ 6.5% (47 mmol/mol). In the 75-g OGTT, eHbA1c-1 ≥ 6.5% significantly predicted peak PG ≥ 200 mg/dL, while both GA ≥ 17.0% [20] (P = 1.000) and eHbA1c-2 ≥ 6.5% (P = 0.374) failed to predict peak PG ≥ 200 mg/dL. eHbA1c-1 showed superior sensitivity (68%) in the prediction of peak PG ≥ 200 mg/dL and HbA1c ≥ 6.5% (47 mmol/mol) compared with GA (28%) and eHbA1c-2 (40%). The specificity for PG ≥ 200 mg/dL and HbA1c ≥ 6.5% (47 mmol/mol) was similar for all the markers (Table 2).
Table 2.
Associations of various characteristics with HbA1c, GA, eHbA1c-1, and eHbA1c-2 in a glucose tolerance test
| Number of patients (n): 80 | |
| Age (years): 59.5 ± 11.8 | |
| Gender: male/female (n): 33/47 |
| Association of characteristics in 75 g-OGTT | HbA1c | GA | eHbA1c-1 | eHbA1c-2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | ||
| HbA1c (% (mmol/mol)) | 6.3 (45) ± 0.4 (4) | – | – | – | – | – | – | – | – |
| GA (%) | 15.0 ± 1.8 | 0.141 | 0.213 | – | – | – | – | – | – |
| eHbA1c-1 (%) | 6.4 ± 0.3 | 0.555 | < 0.001 | 0.410 | < 0.001 | – | – | – | – |
| eHbA1c-2 (%) | 6.3 ± 0.4 | 0.508 | < 0.001 | 0.581 | < 0.001 | 0.959 | < 0.001 | – | – |
| PG (mg/dL) | |||||||||
| 0 min | 108.3 ± 11.0 | 0.388 | < 0.001 | 0.181 | 0.108 | 0.515 | < 0.001 | 0.507 | < 0.001 |
| 30 min | 186.3 ± 31.5 | 0.332 | 0.003 | 0.162 | 0.152 | 0.312 | 0.005 | 0.331 | 0.003 |
| 60 min | 213.0 ± 47.5 | 0.339 | 0.002 | 0.073 | 0.522 | 0.354 | 0.001 | 0.329 | 0.003 |
| 90 min | 196.4 ± 55.8 | 0.294 | 0.008 | 0.018 | 0.877 | 0.320 | 0.004 | 0.284 | 0.011 |
| 120 min | 164.1 ± 53.7 | 0.289 | 0.009 | −0.014 | 0.899 | 0.330 | 0.003 | 0.298 | 0.007 |
| Peak | 224.5 ± 42.1 | 0.418 | < 0.001 | 0.149 | 0.188 | 0.405 | < 0.001 | 0.400 | < 0.001 |
| Mean | 173.6 ± 33.4 | 0.375 | 0.001 | 0.065 | 0.569 | 0.406 | < 0.001 | 0.379 | 0.001 |
| Serum CPR (ng/mL) | |||||||||
| 0 min | 2.0 ± 1.0 | 0.214 | 0.056 | − 0.526 | < 0.001 | 0.248 | 0.027 | 0.131 | 0.248 |
| ΔCPR | 3.0 ± 1.6 | − 0.247 | 0.027 | − 0.371 | 0.001 | − 0.349 | 0.002 | − 0.368 | 0.001 |
| HOMA-IR | 2.6 ± 1.7 | − 0.003 | 0.989 | − 0.693 | < 0.001 | 0.086 | 0.677 | − 0.060 | 0.771 |
| HOMA-β (%) | 69.6 ± 62.6 | − 0.172 | 0.400 | − 0.704 | < 0.001 | − 0.103 | 0.617 | − 0.245 | 0.228 |
| BMI (kg/m2) | 25.0 ± 5.9 | 0.313 | 0.005 | − 0.533 | < 0.001 | 0.409 | < 0.001 | 0.265 | 0.018 |
| Hb (g/dL) | 14.2 ± 1.5 | 0.257 | 0.022 | − 0.285 | 0.010 | 0.464 | < 0.001 | 0.388 | < 0.001 |
| eGFR (mL/min/1.73 m2) | 80.8 ± 14.9 | 0.142 | 0.209 | − 0.236 | 0.035 | 0.063 | 0.576 | − 0.023 | 0.841 |
| Specificities and sensitivities of HbA1c, GA, eHbA1c-1, and eHbA1c-2 for DM diagnosis | HbA1c ≥ 6.5% (48 mmol/mol) | GA ≥ 17.0% | eHbA1c-1 ≥ 6.5% | eHbA1c-2 ≥ 6.5% | ||||
|---|---|---|---|---|---|---|---|---|
| % | P a | % | P a | % | P a | % | P a | |
| Peak PG ≥ 200 mg/dL | ||||||||
| Sensitivity | 43.1 | 0.004 | 15.5 | 1.000 | 44.8 | 0.038 | 24.1 | 0.374 |
| Specificity | 90.9 | 86.4 | 81.1 | 86.4 | ||||
| DM diagnosisb | ||||||||
| Sensitivity | 100.0 | < 0.001 | 28.0 | 0.042 | 68.0 | < 0.001 | 40.0 | 0.009 |
| Specificity | 96.4 | 90.9 | 76.4 | 87.3 | ||||
Values are mean ± SD values for continuous variables and numbers for categorical variables
OGTT oral glucose tolerance test, CPR C-peptide response, HOMA-IR homeostatic model assessment—insulin resistance, HOMA-β homeostatic model assessment of beta cells, ΔCPR difference between CPR at 30 min and CPR at 0 min, r Pearson’s correlation coefficient
aP values were obtained using the chi-square test
bDiabetes was diagnosed when peak PG ≥ 200 mg/dL and HbA1c ≥ 6.5% (48 mmol/mol)
Correlations of aPG with HbA1c, GA, eHbA1c-1, and eHbA1c-2 (study design 3)
BMI, serum Alb concentration, and serum Hb concentration were significantly lower in the ESRD group (n = 243) than in the NRF group (n = 545) (Table 3). Although HbA1c values were significantly lower in the ESRD group than in the NRF group, aPG, GA, and eHbA1c-1 values were significantly higher in the ESRD group. The GA/HbA1c ratio was significantly higher (by 30.7%) in the ESRD group than in the NRF group. There were no significant differences between the ESRD and NRF groups in the slopes of the correlations of aPG with HbA1c (P = 0.065), GA (P = 0.137), eHbA1c-1 (P = 0.918), and eHbA1c-2 (P = 0.733) (Fig. 2). However, the variances of HbA1c (P < 0.001) and eHbA1c-2 (P = 0.002) were significantly lower and the variance of GA (P < 0.001) was significantly higher in the ESRD group. Conversely, there was no significant difference between the groups (P = 0.066) in the variance of eHbA1c-1 correlated with aPG.
Table 3.
Clinical characteristics of the subjects
| Characteristics | NRF group (n = 545) | ESRD group (n = 243) | P |
|---|---|---|---|
| Age (years) | 62.6 ± 11.2 | 66.5 ± 11.1 | < 0.001 |
| Gender: male/female (n) | 272/273 | 171/72 | < 0.001 |
| Duration of diabetes (years) | 6.3 ± 6.4 | 21.6 ± 15.4 | < 0.001 |
| Duration of dialysis (years) | – | 5.1 ± 4.8 | – |
| BMI (kg/m2) | 24.4 ± 4.3 | 23.9 ± 4.2 | 0.122 |
| < 18.5 kg/m2 (%) | 6.1 | 7.8 | 0.355 |
| ≥ 30.0 kg/m2 (%) | 11.1 | 8.6 | 0.602 |
| Serum albumin (g/dL) | 4.2 ± 0.3 | 3.7 ± 0.3 | < 0.001 |
| Hemoglobin (g/dL) | 13.8 ± 1.6 | 10.8 ± 0.9 | < 0.001 |
| Anemia (%) | 11.9 | 92.0 | < 0.001 |
| eGFR (mL/min/1.73 m2) | 83.8 ± 16.6 | – | – |
| Average plasma glucose (mg/dL) | 133.6 ± 41.5 | 156.5 ± 47.0 | < 0.001 |
| HbA1c [% (mmol/mol)] | 6.7 (49) ± 1.0 (10) | 6.3 (45) ± 1.0 (11) | < 0.001 |
| GA (%) | 16.7 ± 3.0 | 20.4 ± 4.0 | < 0.001 |
| GA/HbA1c ratioa | 2.51 ± 0.29 | 3.28 ± 0.45 | < 0.001 |
| eHbA1c-1 (%) | 6.7 ± 0.7 | 6.8 ± 0.9 | 0.020 |
| eHbA1c-2 (%) | 6.6 ± 0.7 | 6.7 ± 0.9 | 0.208 |
| Urinary protein (g/gCr) | 0.18 ± 0.37 | – | – |
| Medications (%) | |||
| Oral antihyperglycemic agents | 76.1 | 57.2 | < 0.001 |
| Insulin therapy | 9.0 | 20.6 | < 0.001 |
| Erythropoietin injection | 0.0 | 90.5 | < 0.001 |
| Complications and histories (%) | |||
| Liver disease | 19.4 | 9.1 | < 0.001 |
| Thyroid disease | 6.1 | 7.0 | 0.636 |
Values are mean ± SD values for continuous variables and numbers or percentages for categorical variables
P values for comparisons between two groups were obtained using the t test for parametric continuous variables or the corresponding chi-square test for categorical variables
aHbA1c values in derived NGSP units
Fig. 2.
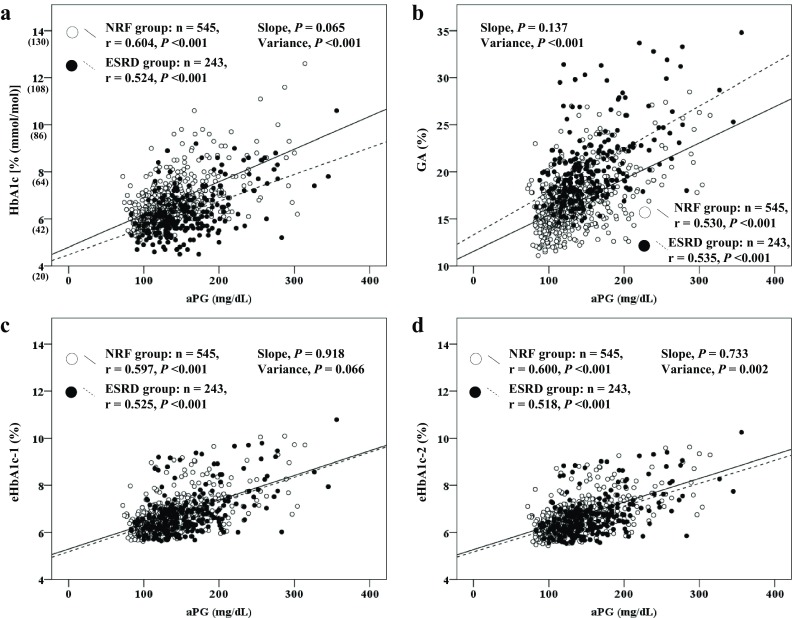
Relationships of aPG with HbA1c (a), GA (b), eHbA1c-1 (c), and eHbA1c-2 (d) in each group. aPG exhibited significantly positive correlations with HbA1c (a), GA (b), eHbA1c-1 (c), and HbA1c 2 (d) in the NRF and ESRD groups. No significant differences in slope were noted between the two groups in terms of the relationships of aPG with HbA1c, GA, eHbA1c-1, and eHbA1c-2. However, significant differences were noted between the groups in terms of the variances of HbA1c and eHbA1c-2, which were significantly smaller in the ESRD group than in the NRF group. Moreover, a significant difference was noted between the groups in the variance of GA, which was significantly higher in the ESRD group than in the NRF group. No significant difference in the variance for eHbA1c-1 in relation to aPG was noted between the two groups
Independent factors that contribute to HbA1c, GA, eHbA1c-1, and eHbA1c-2 (study design 3)
To identify the factors that contribute to HbA1c, GA, eHbA1c-1, and eHbA1c-2, a binomial logistic regression analysis was performed based on the data from all T2D patients (Table 4). The independent variables included aPG, BMI, Alb, presence of anemia, concomitant ESRD, and use of ESAs. aPG and BMI were positive contributing factors to eHbA1c-1, while Hb, use of ESAs, and ESRD concomitance were excluded. In contrast, significant positive contributing factors to eHbA1c-2 were aPG, BMI, and absence of anemia.
Table 4.
Bivariate logistic regression analysis (stepwise variable selection) of the relationships of HbA1c, GA, eHbA1c-1, and eHbA1c-2 with various characteristics of type 2 diabetes
| Variables | HbA1c | GA | eHbA1c-1 | eHbA1c-2 | ||||
|---|---|---|---|---|---|---|---|---|
| ORs (95% CIs) | P | ORs (95% CIs) | P | ORs (95% CIs) | P | ORs (95% CIs) | P | |
| aPG | 7.40 (5.02–10.89) | < 0.001 | 5.73 (3.90–8.43) | < 0.001 | 5.82 (4.09–8.28) | < 0.001 | 5.82 (4.05–8.35) | < 0.001 |
| BMI | – | – | 0.59 (0.40–0.87) | 0.007 | 1.60 (1.13–2.27) | 0.008 | 1.82 (1.29–2.57) | 0.001 |
| Alb | – | – | – | – | – | – | – | – |
| Presence of anemia | 0.43 (0.24–0.76) | 0.004 | – | – | – | – | 0.65 (0.43–0.99) | 0.044 |
| Presence of ESRD | – | – | 9.75 (5.51–17.27) | < 0.001 | – | – | – | – |
| Use of ESAs | 0.43 (0.21–0.89) | 0.024 | – | – | – | – | – | – |
Values are odds ratios (ORs) and 95% confidence intervals (CIs)
Values of continuous variables were separated into two groups (0: below the mean value of the variable, 1: above the mean)
Discussion
We propose eHbA1c-1 as a novel parameter for estimating HbA1c values based on GA, BMI, Hb, and eGFR. eHbA1c-1 showed a significant positive correlation with the PG level at all time points, and with the peak and mean PG values during the 75-g OGTT. We found that eHbA1c-1 was closely associated with aPG level but not with anemia, use of ESAs, serum Alb, or ESRD concomitance in T2D. Our results suggest that eHbA1c-1 may be a useful novel parameter for estimating HbA1c using GA in T2D patients with ESRD. In our study, the GA/HbA1c ratio was significantly increased by 30.7% in the patients in the ESRD group, as previously reported [1–3]. Furthermore, a significant negative correlation was found between eGFR and the GA/HbA1c ratio. In recent years, several studies have investigated whether HbA1c levels can be estimated from GA levels in patients without ESRD [13–15]. However, eHbA1c-2 and the equations employed in previous studies assume a linear correlation between HbA1c and GA with a constant slope. The formulae used in previous reports [1–3] and eHbA1c-2 are not applicable to ESRD patients, as the GA/HbA1c ratio is large. Instead, eHbA1c-1, which is calculated based on the regression equation for the GA/HbA1c ratio, may be a useful parameter for monitoring glycemic control in T2D patients with fluctuating GA/HbA1c ratios, such as ESRD patients.
Both Hb and BMI demonstrated significant negative correlations with the GA/HbA1c ratio. Hb was positively associated with HbA1c and negatively associated with GA. Chronic inflammation and malnutrition, which are commonly observed in ESRD patients [21, 22], may enhance Alb catabolism and prolong the survival of erythrocytes. It was shown that both HOMA-β and BMI are negatively correlated with the GA/HbA1c ratio and positively correlated with HbA1c [22, 23]. In another study, GA values were found to be elevated in patients with a low BMI and a low HOMA-IR [5]. Thus, patients with a high BMI may be mistakenly diagnosed as nondiabetic based on their GA values [20]. eHbA1c-1 may be a useful marker not only for T2D patients with ESRD but also in other patients in whom the HbA1c level may not exactly reflect the actual PG level, such as those suffering from anemia, obesity, or malnutrition.
Previous reports [1–3] showed that there was no difference in blood glucose levels and GA values between diabetic patients with ESRD and those without nephropathy. However, in our study, significantly higher GA variance was observed in the ESRD group, and concomitant ESRD was a significant independent contributing factor to higher GA levels. One reason for this discrepancy between studies may be that T2D patients were classified by Cr value in previous reports but by eGFR value in this study. However, further studies are necessary to clarify this discrepancy.
There are some limitations of this study. Firstly, the blood samples from patients who were not receiving HD were obtained in an outpatient clinic and were collected before dialysis sessions from HD patients. A similar approach to sample collection was also used in previous studies examining the interrelationships between PG, HbA1c, and GA in T2D patients [1–3, 24]. Further investigation based on data provided by continuous glucose monitoring is needed to resolve this limitation [25]. Moreover, the utility of eHbA1c for diagnosing diabetes mellitus in T2D patients with ESRD has not been demonstrated. Further clinical studies are required to address this issue. Secondly, the number of patients enrolled in this study was rather small. An analysis using a large database of diabetic patients encompassing a variety of races and ethnicities is needed to develop more accurate eHbA1c-1 equation models. Finally, eHbA1c-1, which is calculated using a GA-based equation, may reflect faster changes in plasma glucose levels than in HbA1c.
In conclusion, we have proposed a novel method of estimating HbA1c from GA using a GA/HbA1c ratio regression formula. The resulting parameter, eHbA1c-1, may be useful for diagnosing DM and for monitoring glycemic control in T2D patients with ESRD. Further studies are need to confirm the usefulness of this parameter for ESRD patients with diabetes.
Acknowledgements
We would like to extend our deepest gratitude to Dr. Kanji Kobayashi of Kobayashi Medical Clinic, Dr. Keita Kimura of Shin Kashiwa Clinic, and Drs. Kiichi Koumoto and Kazuhiro Ujike of Innoshima General Hospital, who helped to provide clinical data for this study. We would also like to extend our sincerest gratitude to Dr. Hiromi Kouguchi, the chief clinical technologist at Osafune Clinic, who played a central role in data input and management in this study, and Professor Kiyoshi Ichihara of the Division of Clinical Laboratory, Faculty of Health Sciences, Yamaguchi University, who provided us with valuable advice on statistical analysis for this study. Finally, we would like to express our heartfelt gratitude to staff at Osafune Clinic, Shin Kashiwa Clinic, Shin Kashiwa Clinic Ootaka No Mori, Kobayashi Medical Clinic, and Innoshima General Hospital, who aided specimen collection for this study.
Conflict of interest
AN, RK, and HU declare that they have no conflict of interest. NS received honoraria for lectures from Astellas, AstraZeneca, Eli Lilly Japan, Kyowa Hakko Kirin, Tanabe Mitsubishi, MSD, Novartis Pharma, Novo Nordisk, Ono, Sanofi Aventis, Shionogi, and Takeda; honoraria for manuscripts from Kyowa Hakko Kirin; and grant support from AstraZeneca, Novartis Pharma, Shionogi, and Takeda. JW received honoraria for lectures from Astellas, Boehringer Ingelheim, Daiichi Sankyo, Novartis, and Tanabe Mitsubishi, and grant support from Astellas, Bayer, Baxter, Chugai, Daiichi Sankyo, Kissei, Kyowa Hakko Kirin, MSD, Novartis Pharma, Novo Nordisk, Ono, Otsuka, Pfizer, Takeda, Teijin, and Torii. KS received honoraria for lectures from Astellas, Boehringer Ingelheim, Eli Lilly Japan, MSD, Novartis Pharma, Novo Nordisk, Ono, Sanofi Aventis, and Tanabe Mitsubishi, and grant support from Eli Lilly Japan, Takeda, and Tanabe Mitsubishi. HM is a consultant for AbbVie and Teijin, and received honoraria for lectures from Astellas, Boehringer Ingelheim, MSD, and Tanabe Mitsubishi.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Innoshima General Hospital, Ethics Committee, date of approval: 24 June 2013, approval number: 2013-010; and Osafune Clinic, Institutional Review Board, date of approval: 25 August 2016, approval number: 2016-16) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
The information of this study was disclosed to potential research participants.
References
- 1.Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 2.Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73:1062–1068. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 3.Freedman BI, Shenoy RN, Planer JA, et al. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Perit Dial Int. 2010;30:72–79. doi: 10.3747/pdi.2008.00243. [DOI] [PubMed] [Google Scholar]
- 4.Hoshino J, Molnar MZ, Yamagata K, et al. Developing an HbA(1c)-based equation to estimate blood glucose in maintenance hemodialysis patients. Diabetes Care. 2013;36:922–927. doi: 10.2337/dc12-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751–762. doi: 10.1507/endocrj.K10E-138. [DOI] [PubMed] [Google Scholar]
- 6.Kim MK, Kwon HS, Baek KH, et al. Effects of thyroid hormone on A1C and glycated albumin levels in nondiabetic subjects with overt hypothyroidism. Diabetes Care. 2010;33:2546–2548. doi: 10.2337/dc10-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koga M, Matsumoto S, Saito H, et al. Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr J. 2006;53:387–391. doi: 10.1507/endocrj.K05-137. [DOI] [PubMed] [Google Scholar]
- 8.Koga M, Otsuki M, Matsumoto S, et al. Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta. 2007;378:48–52. doi: 10.1016/j.cca.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Koga M, Kasayama S, Kanehara H, et al. CLD (chronic liver diseases)-HbA1C as a suitable indicator for estimation of mean plasma glucose in patients with chronic liver diseases. Diabetes Res Clin Pract. 2008;81:258–262. doi: 10.1016/j.diabres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death. A1C remains the gold standard outcome predictor in diabetic dialysis patients. Diabetes Care. 2012;35:1625–8. [DOI] [PMC free article] [PubMed]
- 11.Freedman BI. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: time to dispense with the hemoglobin A1C in end-stage kidney disease. Diabetes Care. 2012;35:1621–1624. doi: 10.2337/dc12-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrotra R, Kalantar-Zadeh K, Adler S. Assessment of glycemic control in dialysis patients with diabetes: glycosylated hemoglobin or glycated albumin? Clin J Am Soc Nephrol. 2011;6:1520–1522. doi: 10.2215/CJN.04210511. [DOI] [PubMed] [Google Scholar]
- 13.Tahara Y. Analysis of the method for conversion between levels of HbA1c and glycated albumin by linear regression analysis using a measurement error model. Diabetes Res Clin Pract. 2009;84:224–229. doi: 10.1016/j.diabres.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K, Tsujimoto T, Yamamoto-Honda R, et al. A newer conversion equation for the correlation between HbA1c and glycated albumin. Endocr J. 2014;61:553–560. doi: 10.1507/endocrj.EJ13-0450. [DOI] [PubMed] [Google Scholar]
- 15.Jung CH, Hwang YC, Kim KJ, et al. Development of an HbA1c-based conversion equation for estimating glycated albumin in a Korean population with a wide range of glucose intolerance. PLoS One. 2014;9:e95729. doi: 10.1371/journal.pone.0095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. [DOI] [PubMed]
- 17.Tsubakihara Y, Nishi S, Akiba T, et al. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ther Apher Dial. 2010;14:240–275. doi: 10.1111/j.1744-9987.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Wu WC, Ma WY, Wei JN, et al. Serum glycated albumin to guide the diagnosis of diabetes mellitus. PLoS One. 2016;11:e0146780. [DOI] [PMC free article] [PubMed]
- 21.Ramos LF, Shintani A, Ikizler TA, et al. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol. 2008;19:593–599. doi: 10.1681/ASN.2007030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi R, Ito Y, Takahashi H, et al. Combined values of serum albumin, C-reactive protein and body mass index at dialysis initiation accurately predicts long-term mortality. Am J Nephrol. 2012;36:136–143. doi: 10.1159/000339940. [DOI] [PubMed] [Google Scholar]
- 23.Fukui M, Tanaka M, Hasegawa G, et al. Association between serum bioavailable testosterone concentration and the ratio of glycated albumin to glycated hemoglobin in men with type 2 diabetes. Diabetes Care. 2008;31:397–401. doi: 10.2337/dc07-1898. [DOI] [PubMed] [Google Scholar]
- 24.Koga M, Murai J, Saito H, et al. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care. 2010;33:270–272. doi: 10.2337/dc09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazempour-Ardebili S, Lecamwasam VL, Dassanyake T, et al. Assessing glycemic control in maintenance hemodialysis patients with type 2 diabetes. Diabetes Care. 2009;32:1137–1142. doi: 10.2337/dc08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]


