Abstract
Background
Weight loss, which is an effective method for reducing visceral fat, may cause a concomitant loss of skeletal muscle mass. The aim of this study was to elucidate the changes in visceral fat and skeletal muscle mass in response to diabetes treatment including weight control.
Methods
For 6 months we observed the changes in the body compositions of 72 Japanese patients with type 2 diabetes who underwent multifaceted treatment including educational hospitalization. Visceral fat area (VFA) and appendicular skeletal muscle mass (ASM) were measured using a bioelectrical impedance method and dual-energy X-ray absorptiometry, respectively.
Results
During the follow-up period, VFA reduced significantly whereas the average ASM did not change. Changes in ASM were strongly positively associated with changes in body weight (r = 0.50). Additionally, in an analysis of covariance, an above-median BMI (27 kg/m2) and above-median VFA (110 cm2) at baseline were found to be independent predictors of ASM reduction prevention. Of the 55 patients who lost weight, those who had a baseline VFA of ≥110 cm2 had significantly greater reductions in VFA than those with a baseline VFA of <110 cm2 (p < 0.01). ASM reduced significantly in patients with a VFA of <110 cm2 (p < 0.01), but not in those with a VFA of ≥110 cm2 (p = 0.98).
Conclusions
Baseline accumulation of visceral fat may predict a preferential reduction of visceral fat rather than skeletal muscle during weight control programs in type 2 diabetes patients.
Keywords: Visceral fat, Skeletal muscle mass, Type 2 diabetes, Weight loss
Background
In overweight or obese patients with type 2 diabetes, modest and sustained weight loss has been found to improve glycemic control and reduce glucose-lowering medications [1–3]. The American Diabetes Association (ADA) has recommended that Asian patients categorized as overweight or obese, defined as having a body mass index (BMI) of ≥23 kg/m2, should attempt to lose 5% of their weight through lifestyle modification [4]. Weight reduction is a standard approach to improving visceral fat accumulation, which causes insulin resistance. However, there are serious concerns regarding concomitant loss of skeletal muscle during weight loss. Indeed, the Action for Health in Diabetes (Look AHEAD) trial has shown that intensive lifestyle intervention for 1 year reduces lean mass by 2.3 ± 0.1 kg and fat mass by 5.6 ± 0.2 kg [5]. Furthermore, although glucagon-like peptide-1 agonist, sodium-glucose cotransporter 2 inhibitor, and bariatric surgery are often used for weight control in obese patients with type 2 diabetes, they cause a considerable reduction in fat-free mass [6–9].
Loss of skeletal muscle decreases endurance and increases fatigability. These changes lead to physical inactivity and thus sarcopenia. Previously, Park et al. showed an association between type 2 diabetes and excessive loss of skeletal muscle [10]. Thus, weight reduction could increase the risk of sarcopenia in patients with type 2 diabetes. Furthermore, physical inactivity caused by loss of skeletal muscle may also lead to weight rebound and subsequent development of obesity along with sarcopenia. Decreased muscle mass and increased adiposity has also been associated with increased mortality [11]. Therefore, it is important to evaluate changes in skeletal muscle mass after hyperglycemia treatments including weight control in type 2 diabetes patients.
Additionally, some researchers have reported factors that can influence changes in skeletal muscle mass. Lee et al. reported that insulin sensitizers, such as biguanide and thiazolidinedione, are effective at preventing skeletal muscle loss in diabetes patients [12]. A Japanese cross-sectional study demonstrated that age, sex, weight, and visceral fat area are independent predictors of the skeletal muscle index (SMI) in healthy subjects [13]. However, there have been no studies of predictors of skeletal muscle mass changes in type 2 diabetic patients during weight loss intervention.
The primary aim of the present study was therefore to examine the changes in skeletal muscle mass concomitantly with those in visceral fat using bioelectrical impedance and dual-energy X-ray absorptiometry (DXA) in Japanese type 2 diabetes patients undergoing a multifaceted treatment consisting of diet, exercise, and hypoglycemic medication therapy, including educational hospitalization of diabetes. The secondary aim was to evaluate the associations between change in skeletal muscle mass and known factors that could affect skeletal muscle mass.
Methods
Patients
We recruited 72 Japanese patients with type 2 diabetes who were hospitalized at Tokyo Medical and Dental University Hospital to receive a diabetes educational program and treatment. Patients with severe renal impairment—defined as those with an estimated glomerular filtration rate (eGFR) of <15 mL/min/1.73 m2 or those undergoing renal replacement therapy—and those with a severe infection or serious trauma were excluded. The patients gave written informed consent to participate in this study and for the results of it to be published. Eligible participants (35–83 years old) were those with available data on measured visceral fat area (VFA), whole-body DXA, anthropometric parameters, and laboratory data at baseline and 6 months later. All patients received intensive medical therapy and underwent lifestyle modification, including caloric restriction and regular exercise. The components of the lifestyle guidance varied with the patient according to the plan discussed between the patient, dietician, and physician. Briefly, the patients were instructed to restrict their daily caloric intake to under 25–30 kcal per kg of ideal body weight, and to exercise for 30 min or more at least three times a week. After discharge, the participants continued to receive medical therapy and lifestyle guidance as an outpatient of the hospital. Type 2 diabetes was diagnosed according to the criteria of the Japan Diabetes Society (JDS) [14]. This study protocol was registered as a clinical trial in the University Hospital Medical Information Network (UMIN) system (UMINStudyID: UMIN000024401). The study complied with the principles laid down by the Declaration of Helsinki and was approved by the ethics committee of Tokyo Medical and Dental University (no. M2000-1573).
Clinical and biochemical analyses
Standardized questionnaires were used to obtain information on medication and past history. Hemoglobin A1c (HbA1c) levels were measured by the latex agglutination method and expressed in accordance with the National Glycohemoglobin Standardization Program recommended by the Japanese Diabetes Society [14]. Urinary C-peptide (UCP) levels were measured by chemiluminescent immunoassay. Glomerular filtration rate (GFR) was estimated using the following equation for Japanese people, as proposed by the Japanese Society of Nephrology [15]: GFR = 194 × SCr−1.094 × age−0.287 [×0.739 if female], where SCr stands for serum creatinine in mg/dL, measured by an enzymatic method. Body weight and height were measured. BMI was calculated as the weight divided by the square of the height (kg/m2) to determine obesity. These data were collected at baseline and 6 months later.
Measurement of body composition
A whole-body DXA scan (Lunar iDXA, GE Healthcare, Madison, WI, USA) was performed for each patient to evaluate total, trunk, and appendicular body composition. The Lunar iDXA is reported to provide excellent precision in body composition measurements, particularly for lean tissue mass; the precision error was CV 0.5% [16]. Appendicular skeletal muscle mass (ASM) (kg) was defined as the sum of the lean soft-tissue masses of the arms and legs, following the method of Heymsfield et al. [17]. SMI was calculated as the ASM divided by the square of the height (kg/m2). According to the criteria for sarcopenia in Asia [18], low muscle mass was defined as SMI <7.0 kg/m2 in men and SMI <5.4 kg/m2 in women. The VFA and subcutaneous fat area (SFA) were measured at the level of the umbilicus by a dual bioelectrical impedance analyzer (DUALSCAN, Omron Healthcare Co., Kyoto, Japan). There was good agreement between the DUALSCAN and computed tomography measurements of the intraabdominal fat area, with a correlation of 0.88 (p < 0.001) [19]. In addition, a high correlation was observed between changes in intraabdominal fat area (IAFA) observed by DUALSCAN and changes in IAFA seen on computed tomography during weight loss intervention [20]. These parameters were measured at baseline and 6 months later to permit a comparison of individual changes.
Statistical analysis
Statistical analysis was performed using programs available in the SPSS version 21.0 statistical software package (SPSS Inc., Chicago, IL, USA). Data are presented as values of mean ± SD or median with interquartile range (IQR) or as percentages, according to which is appropriate for the data distribution. Normality was tested using the Kolmogorov–Smirnov test. A paired-sample t test or Wilcoxon’s signed-rank test was performed, as appropriate, to compare baseline and follow-up levels of laboratory and body-composition parameters. Pearson’s correlation analysis was performed to investigate the relationships among change in body weight (Δbody weight), change in VFA (ΔVFA), and change in ASM (ΔASM). Analysis of covariance (ANCOVA) was used to investigate the association between ΔASM mass and the baseline characteristics of the participants (mean ± SE) in order to adjust Δbody weight accordingly. Differences were considered to be statistically significant when p was less than 0.05.
Results
Table 1 shows clinical and metabolic characteristics of the type 2 diabetic patients enrolled. They were 51 men and 21 women with a mean age of 62 ± 12 years. Most patients (90.3%) had BMI values of ≥23 kg/m2, meaning that they were categorized as overweight or obese according to the ADA guideline [4]. Twenty-two males and two females (33.3%) had SMI values lower than the cutoff points for low muscle mass, as defined by the consensus report of the Asian Working Group for Sarcopenia [18]. Insulin, biguanide, and incretin agents were commonly used during the follow-up period. A small number of patients were treated with sulfonylurea (n = 5), thiazolidinedione (n = 2), α-glucosidase inhibitor (n = 8), glinide (n = 6), and sodium-glucose cotransporter 2 inhibitor (n = 3). Two patients who were treated with thiazolidinedione also used biguanide.
Table 1.
Clinical and metabolic characteristics of the study patients
| Sex | |
| Men | 51 (70.8) |
| Women | 21 (29.2) |
| Age (years) | 62 ± 12 |
| Duration of diabetes (years) | 9 [4, 16] |
| eGFR (ml/min/1.73 m2) | 75.2 ± 24.7 |
| UCP (μg/day) | 70.5 [37.3, 101.0] |
| Prevalence of obesity | |
| BMI ≥23 kg/m2 | 65 (90.3) |
| BMI ≥25 kg/m2 | 50 (69.4) |
| Prevalence of visceral obesity | 43 (59.7) |
| Prevalence of low muscle mass | 24 (33.3) |
| SMI <7.0 in men | 22 (43.1) |
| SMI <5.4 in women | 2 (9.5) |
| Prevalence of hypertension | 47 (65.3) |
| Prevalence of dyslipidemia | 51 (70.8) |
| History of CVD | 17 (23.6) |
| Medications during follow-up period | |
| DPP-4 inhibitor | 28 (38.9) |
| GLP-1 receptor agonist | 12 (16.7) |
| Biguanide | 39 (54.2) |
| Sulfonylurea | 5 (6.9) |
| Insulin | 40 (55.6) |
| Thiazolidinedione | 2 (2.8) |
| α-Glucosidase inhibitor | 8 (11.1) |
| Glinide | 6 (8.3) |
| SGLT2 inhibitor | 3 (4.2) |
Data are expressed as the mean ± standard deviation, the median [interquartile range], or the number (percentage)
eGFR estimated glomerular filtration rate, UCP urinary C-peptide, BMI body mass index, SMI skeletal muscle index, CVD cardiovascular disease, DPP-4 dipeptidyl peptidase-4 inhibitor, GLP-1 glucagon-like peptide-1, SGLT2 sodium-glucose cotransporter 2
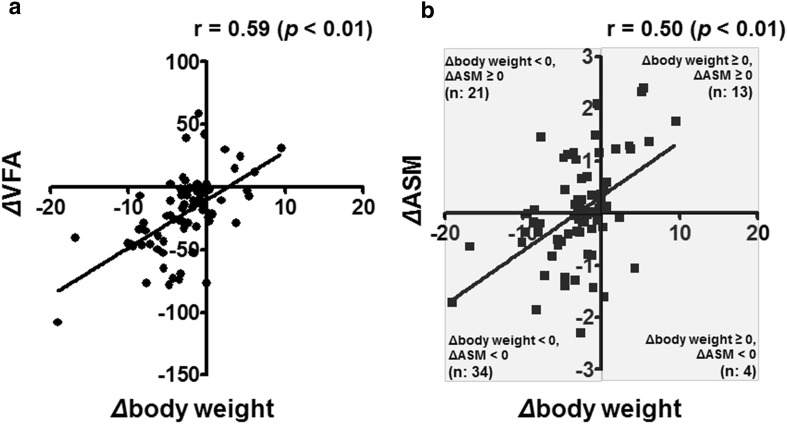
Table 2 shows the laboratory, anthropometric, and body-composition parameters at baseline and after 6 months. HbA1c, body weight, BMI, VFA, and SFA decreased significantly, whereas the average ASM did not change because a substantial proportion of the patients showed increased ASM during the follow-up period even though their body weights decreased a little. Among the 55 patients who showed reductions in body weight during the follow-up period, 21 (38%) had increased ASM (Fig. 1b). Correlation analysis revealed significant positive associations of ΔVFA with Δbody weight (r = 0.59) (Fig. 1a) and ΔASM with Δbody weight (r = 0.50) (Fig. 1b). There was no correlation between ΔVFA and ΔASM (p = 0.31).
Table 2.
Laboratory, anthropometric, and body-composition parameters of the study patients at baseline and after 6 months
| Baseline | Follow-up | p value | |
|---|---|---|---|
| HbA1c (%) | 9.1 [8.3, 10.4] | 7.2 [6.6, 8.2] | <0.001 |
| Men | 9.1 [8.3, 10.5] | 7.2 [6.7, 8.4] | <0.001 |
| Women | 9.2 [8.2, 10.2] | 6.9 [6.3, 7.9] | <0.001 |
| Body weight (kg) | 73.1 ± 13.5 | 70.3 ± 12.9 | <0.001 |
| Men | 74.9 ± 12.3 | 72.4 ± 12.2 | <0.001 |
| Women | 68.6 ± 15.4 | 65.3 ± 13.5 | 0.003 |
| BMI (kg/m2) | 27.1 ± 4.2 | 26.0 ± 4.0 | <0.001 |
| Men | 26.6 ± 3.6 | 25.5 ± 3.6 | <0.001 |
| Women | 28.6 ± 5.3 | 27.2 ± 4.6 | 0.003 |
| VFA (cm2) | 113.4 ± 47.0 | 92.6 ± 40.5 | <0.001 |
| Men | 113.2 ± 47.2 | 91.1 ± 39.9 | <0.001 |
| Women | 114.0 ± 47.5 | 96.4 ± 42.8 | 0.001 |
| SFA (cm2) | 215.5 ± 75.6 | 190.8 ± 71.7 | <0.001 |
| Men | 204.5 ± 70.4 | 179.2 ± 60.0 | <0.001 |
| Women | 242.1 ± 82.9 | 219.1 ± 89.7 | 0.037 |
| ASM (kg) | 19.24 ± 3.82 | 19.27 ± 3.93 | 0.813 |
| Men | 20.64 ± 3.26 | 20.67 ± 3.41 | 0.826 |
| Women | 15.84 ± 2.87 | 15.85 ± 2.89 | 0.933 |
Data are presented as the mean ± SD or the median [interquartile range]. p values were obtained using the paired-sample t test or Wilcoxon’s signed-rank test
BMI body mass index, VFA visceral fat area, SFA subcutaneous fat area, ASM appendicular skeletal muscle mass
Fig. 1.
a Relationship between change in VFA (cm2) and change in body weight (kg). b Relationship between change in ASM (kg) and change in body weight (kg). VFA visceral fat area, ASM appendicular skeletal muscle mass
As Δbody weight was a strong determinant of ΔASM, ANCOVA analysis was performed to adjust Δbody weight so that the association between ΔASM and baseline characteristics which could affect the change in muscle mass could be assessed. Table 3 shows that patients with above-median BMI (27 kg/m2) and above-median VFA (110 cm2) at baseline were at significantly lower risk for a decrease in ASM after 6 months of treatment.
Table 3.
ANCOVA analysis with change in ASM after 6 months of intensive treatment as the dependent variable in type 2 diabetes patients
| Fixed factor | n | Estimated change | p value |
|---|---|---|---|
| Sex | |||
| Men | 51 | 0.012 ± 0.120 | 0.809 |
| Women | 21 | 0.065 ± 0.187 | |
| Median age (65 years) | |||
| Below median | 37 | 0.088 ± 0.141 | 0.541 |
| Above median | 35 | −0.037 ± 0.145 | |
| Median baseline BMI (27 kg/m2) | |||
| Below median | 36 | −0.212 ± 0.140 | 0.021 |
| Above median | 36 | 0.266 ± 0.140 | |
| Median baseline VFA (110 cm2) | |||
| Below median | 36 | −0.178 ± 0.140 | 0.045 |
| Above median | 36 | 0.232 ± 0.140 | |
| Median baseline HbA1c (9.2%) | |||
| Below median | 37 | −0.015 ± 0.144 | 0.678 |
| Above median | 35 | 0.072 ± 0.148 | |
| Median SMI of men (7.25 kg/m2) | |||
| Below median | 26 | 0.212 ± 0.178 | 0.157 |
| Above median | 25 | −0.154 ± 0.182 | |
| Median SMI of women (6.40 kg/m2) | |||
| Below median | 10 | 0.075 ± 0.218 | 0.702 |
| Above median | 11 | −0.043 ± 0.208 | |
| Utilization of insulin | |||
| No | 32 | −0.119 ± 0.150 | 0.196 |
| Yes | 40 | 0.144 ± 0.134 | |
| Utilization of biguanide | |||
| No | 33 | −0.095 ± 0.148 | 0.267 |
| Yes | 39 | 0.131 ± 0.136 | |
Covariate was Δbody weight
ASM appendicular skeletal muscle mass, BMI body mass index, VFA visceral fat area, SMI skeletal mass index
To evaluate the impact of baseline visceral fat on ΔASM in type 2 diabetes patients receiving multifaceted treatment, we classified the patients into four groups according to baseline VFA (below median vs. above median) and change in body weight (gain vs. reduction) (Table 4; Figs. 2, 3).
Table 4.
Characteristics of the patients in each subgroup classified according to the changes in body weight and baseline VFA
| Weight loss | Weight gain | |||||
|---|---|---|---|---|---|---|
| VFA <110 cm2 (n = 27) | VFA >110 cm2 (n = 28) | p value | VFA <110 cm2 (n = 9) | VFA >110 cm2 (n = 8) | p value | |
| Sex (%men) | 60 | 75 | 0.214 | 89 | 75 | 0.453 |
| Age (years) | 64 ± 12 | 61 ± 11 | 0.340 | 60 ± 14 | 59 ± 9 | 0.896 |
| Baseline HbA1c (%) | 8.7 [8.2, 9.7] | 8.6 [8.1, 9.7] | 0.966 | 11.6 [9.3, 12.8] | 10.5 [9.9, 10.7] | 0.531 |
| ΔHbA1c | −1.6 [−2.5, −0.5] | −1.4 [−2.7, −0.2] | 0.966 | −3.9 [−5.3, −2.7] | −2.4 [−3.1, −0.6] | 0.114 |
| BMI (kg/m2) | 24.9 ± 2.4 | 30.0 ± 4.0 | <0.001 | 23.5 ± 3.7 | 28.6 ± 2.5 | 0.005 |
| Baseline BW (kg) | 64.3 ± 8.0 | 81.9 ± 11.6 | <0.001 | 65.0 ± 13.7 | 81.0 ± 10.5 | 0.018 |
| ΔBW | −3.6 ± 2.7 | −5.3 ± 4.4 | 0.092 | 3.6 ± 3.1 | 1.6 ± 2.0 | 0.142 |
| Baseline VFA (cm2) | 77.4 ± 24.7 | 149.7 ± 30.6 | <0.001 | 75.1 ± 25.0 | 151.3 ± 44.5 | <0.001 |
| ΔVFA | −12.4 ± 23.0 | −36.9 ± 32.8 | 0.002 | 9.8 ± 17.5 | −27.1 ± 21.4 | 0.001 |
| Baseline SFA (cm2) | 166.6 ± 45.4 | 268.2 ± 72.6 | <0.001 | 161.8 ± 52.4 | 256.0 ± 31.6 | 0.001 |
| ΔSFA | −23.6 ± 38.6 | −47.5 ± 43.6 | 0.036 | 30.5 ± 41.7 | −9.9 ± 27.9 | 0.036 |
| Baseline ASM (kg) | 17.43 ± 3.29 | 20.50 ± 3.38 | 0.001 | 18.97 ± 4.40 | 21.23 ± 4.27 | 0.302 |
| ΔASM | −0.33 ± 0.65 | 0.01 ± 1.02 | 0.151 | 0.69 ± 0.94 | 0.53 ± 1.34 | 0.707 |
| Target energy intake (kcal/IBW) | 27.4 ± 1.7 | 26.6 ± 1.7 | 0.070 | 26.9 ± 1.4 | 26.3 ± 1.6 | 0.417 |
| Medications (%) | ||||||
| DPP-4 inhibitor | 44 | 43 | 0.906 | 11 | 38 | 0.200 |
| GLP-1 receptor agonist | 11 | 18 | 0.478 | 22 | 25 | 0.893 |
| Insulin | 48 | 54 | 0.688 | 56 | 88 | 0.149 |
| Biguanide | 37 | 71 | 0.005 | 44 | 50 | 0.819 |
BMI body mass index, BW body weight, BMI body mass index, VFA visceral fat area, SFA subcutaneous fat area, ASM appendicular skeletal muscle mass, IBW ideal body weight
Fig. 2a–d.
Differences in changes in VFA and ASM among the patients showing weight loss. The patients were divided into two groups according to whether they were below or above median baseline VFA: a, c VFA <110 cm2 and b, d VFA ≥110 cm2. VFA visceral fat area, ASM appendicular skeletal muscle mass
Fig. 3a–d.
Differences in changes in VFA and ASM among the patients showing weight gain. The patients were divided into two groups according to whether they were below or above median baseline VFA: a, c VFA <110 cm2 and b, d VFA ≥110 cm2. VFA visceral fat area, ASM appendicular skeletal muscle mass
Among the 55 patients who showed reductions in body weight during the follow-up period, there was no statistical difference in mean Δbody weight between patients with VFA <110 cm2 and those with VFA ≥110 cm2 (−3.6 ± 2.7 vs. −5.3 ± 4.4, p = 0.09), whereas ΔVFA was significantly smaller in VFA <110 cm2 patients compared with VFA ≥110 cm2 patients (−12.4 ± 23.0 vs. −36.9 ± 32.8, p < 0.01) (Table 4). In patients with VFA <110 cm2, mean VFA significantly decreased (77.4 ± 24.7 vs. 65.0 ± 28.2, p < 0.01), as did mean ASM (17.43 ± 3.29 vs. 17.10 ± 3.10, p = 0.01) (Fig. 2a, c). In patients with VFA ≥110 cm2, although mean VFA also significantly decreased (149.7 ± 30.6 vs. 112.8 ± 39.9, p < 0.01), there was no significant change in mean ASM (20.50 ± 3.38 vs. 20.50 ± 3.46, p = 0.98) (Fig. 2b, d). In other words, the change in ASM largely depended on the baseline VFA in patients who showed reductions in body weight.
Among the 17 patients who gained body weight during the follow-up period, no statistical difference in mean Δbody weight was observed between the two groups (3.6 ± 3.1 vs. 1.6 ± 2.0, p = 0.14) (Table 4). In patients with VFA <110 cm2, both mean VFA (75.1 ± 25.0 vs. 84.8 ± 25.9, p = 0.13) and mean ASM (18.97 ± 4.40 vs. 19.70 ± 3.88, p = 0.07) tended to increase, but not statistically significantly (Fig. 3a, c). In patients with VFA ≥110 cm2, mean VFA decreased significantly (151.3 ± 44.5 vs. 124.2 ± 31.5, p < 0.01) whereas mean ASM tended to increase but not statistically significantly (21.23 ± 4.27 vs. 21.75 ± 5.06, p = 0.25) (Fig. 3b, d). Despite gaining body weight, VFA reduced in the patients with VFA ≥110 cm2 under the multifaceted intervention of this study (Fig. 3b).
The subgroups showed similar data for target energy intake and medications during the follow-up period. However, among the patients who showed a reduced body weight, biguanide was used more frequently in patients with VFA ≥110 cm2 than in those with VFA <110 cm2 (Table 4).
Conclusions
In this study, we observed significant reductions in HbA1c, body weight, and body fat accumulation, but not in the amount of skeletal muscle mass lost. However, Δbody weight had strong positive associations with ΔASM and ΔVFA. Notably, after adjusting Δbody weight appropriately, higher BMI or VFA were found to be independent positive predictors of prevention of skeletal muscle mass loss. These observations could help to identify patients at a high risk of losing skeletal muscle mass after multifaceted diabetes treatment including lifestyle modification and weight control.
Visceral fat tends to decrease more rapidly than subcutaneous fat in response to diet therapy [21]. In this study, during weight reduction, there was a significant reduction in VFA without any change in ASM among patients with above-median baseline VFA (VFA ≥110 cm2), whereas ASM significantly decreased in parallel with a reduction in VFA among patients with below-median baseline VFA (VFA <110 cm2). Collectively, these observations suggest that visceral fat is reduced more readily than skeletal muscle mass during weight loss in type 2 diabetes patients with excess visceral fat accumulation.
Among patients who showed reductions in body weight, ASM significantly reduced in patients with a low baseline VFA. In contrast, patients with a high baseline VFA were able to maintain their ASM, while their VFA decreased markedly compared with the VFA in the low-VFA group. These differences may be caused by the potential effect of body fat on the change in muscle mass. Several recent observational studies without weight loss intervention showed that body fat mass has a negative impact on skeletal muscle mass [13, 22–24]. In prospective studies, Kim et al. showed that baseline VFA is an independent negative predictor of ΔASM, whereas the association between baseline ASM and ΔVFA is not statistically significant in nondiabetic subjects [22]. The authors proposed that chronic subclinical inflammation induced by adipokines may contribute to the loss of skeletal muscle mass. Body fat accumulation could accelerate the reduction of muscle mass through the release of proinflammatory adipokines—such as tumor necrosis factor-α and interleukin-6, which have catabolic effects on skeletal muscle—from visceral adipose tissue [25]. This being the case, the maintenance of ASM observed in patients with high VFA may have been caused by the considerable reduction in visceral fat induced by clinical intervention. In our case, a significant negative correlation between baseline VFA and ΔVFA was observed in our patients (r = −0.52, p < 0.01; data not shown). This correlation indicates that a large baseline VFA is associated with a significant decrease in VFA during a weight loss intervention program. It is therefore conceivable that those with visceral obesity are able to prevent muscle loss by attenuating the catabolic effect of proinflammatory adipokines produced in visceral fat during the process of decreasing the VFA. Further analysis is required to examine this hypothesis, as serum inflammatory cytokine levels were not evaluated in this study.
Insulin resistance is considered to be closely associated with sarcopenia [26]. Lee et al. reported that insulin sensitizers such as biguanide and thiazolidinedione are effective at attenuating skeletal muscle loss in diabetes patients [12]. In our study, ANCOVA analysis showed no significant association between the utilization of biguanide and ΔASM. However, among the patients who reduced body weight in our study, patients with VFA ≥110 cm2 were administered biguanide more frequently than those with VFA <110 cm2. Therefore, there is a possibility that potential effects of biguanide contributed to the maintenance of skeletal muscle.
Skeletal muscle mass loss is one of the components of frailty. Recently, frailty has been recognized as a risk factor for mortality and hypoglycemia in older people with type 2 diabetes [27]. On the other hand, weight loss is effective at improving glycemic control, and ADA has recommended that Asian diabetes patients with a BMI of ≥23 kg/m2 should attempt to lose 5% of their weight [4]. Therefore, evaluating the changes in muscle mass during weight loss intervention is an important tool for improving total clinical outcomes for aging patients with type 2 diabetes who are at high risk for frailty.
In our study, body weight reduced significantly after multifaceted diabetes treatment, whereas ASM did not change. These findings are different from the results of previous reports on the changes in skeletal muscle mass during various diabetes treatments, including weight loss [5–9]. As our research was a single-arm, small-scale study, it is unclear which factor in our multifaceted intervention had a decisive impact on the maintenance of ASM. However, our findings may imply that multifaceted treatment including educational hospitalization has unexpected effects on preventing skeletal muscle loss for patients with severe type 2 diabetes. Furthermore, our results suggest that changes in muscle mass differ greatly among individuals and that baseline VFA may predict muscle mass loss in patients receiving diabetes treatments that include weight loss. These findings may help to identify the target weight for diabetes treatment, especially in aging patients.
Some limitations of our study should be considered. First, physical activity during the follow-up period was not evaluated. Castaneda et al. reported positive effects of resistance training in that it increased lean mass in older adults with type 2 diabetes [28]. Although our patients were instructed to perform only moderate-intensity aerobic exercises, the level of resistance training performed by the patient may be a confounding factor. Second, data about nutrition intake were not available. Considering the effects of nutrients such as vitamin D and amino acids on muscle mass [29, 30], differences among the patients in diet may have affected the outcome. Third, we were unable to determine the prevalence of sarcopenia because we had no information on muscle strength and physical performance. Fourth, the number of study patients was not large enough to allow multiple regression analyses to be performed in order to eliminate the influence of confounding factors. Therefore, more important predictors for ΔASM could exist. Finally, the participants in this study were all hospitalized patients at baseline. Therefore, the ability to generalize our findings might be limited.
In summary, no significant reduction in the amount of skeletal muscle mass was observed in Japanese type 2 diabetes patients receiving multifaceted treatment. Additionally, change in body weight and high visceral fat accumulation at baseline were associated with the change in skeletal muscle mass. These results suggest that in type 2 diabetes patients with visceral obesity, visceral fat may be preferentially reduced relative to skeletal muscle mass during weight control programs. For type 2 diabetes patients without visceral fat accumulation, especially the elderly group, intensive lifestyle intervention that includes a resistance training program may be recommended to prevent loss of muscle mass and subsequent development of frailty when considerable weight loss has been observed.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan [Grant Number 25860403].
Abbreviations
- VFA
Visceral fat area
- ASM
Appendicular skeletal muscle mass
- ADA
American Diabetes Association
- BMI
Body mass index
- DXA
Dual-energy X-ray absorptiometry
- JDS
Japan Diabetes Society
- HbA1c
Hemoglobin A1c
- UCP
Urinary C-peptide
- eGFR
Estimated glomerular filtration rate
- SMI
Skeletal muscle index
- SFA
Subcutaneous fat area
Conflict of interest
None of the authors have any conflicts of interest associated with this research.
Ethical standards
This study was carried out in accordance with the Declaration of Helsinki of 1964 and later versions and approved by the ethics committee of Tokyo Medical and Dental University (approval no. M2000-1573, approval date: 23 July 2013).
Informed consent
The patients gave their written informed consent to participate in this study and for its results to be published.
References
- 1.UK Prospective Diabetes Study 7. Response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–12. [PubMed]
- 2.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- 3.Pastors JG, Warshaw H, Daly A, Franz M, Kulkarni K. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613. doi: 10.2337/diacare.25.3.608. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association 6. Obesity management for the treatment of type 2 diabetes. Diabetes Care. 2016;39(Suppl 1):S47–S51. doi: 10.2337/dc16-S009. [DOI] [PubMed] [Google Scholar]
- 5.Pownall HJ, Bray GA, Wagenknecht LE, Walkup MP, Heshka S, Hubbard VS, Hill J, Kahn SE, Nathan DM, Schwartz AV, Johnson KC. Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring). 2015;23:565–72. [DOI] [PMC free article] [PubMed]
- 6.Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, During M, Zdravkovic M, Strauss BJ, Garber AJ. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11:1163–1172. doi: 10.1111/j.1463-1326.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 7.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 8.Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 9.Garciacaballero M, Reyes-Ortiz A, Garcia M, Martinez-Moreno JM, Toval JA, Garcia A, Minguez A, Osorio D, Mata JM, Miralles F. Changes of body composition in patients with BMI 23–50 after tailored one anastomosis gastric bypass (BAGUA): influence of diabetes and metabolic syndrome. Obes Surg. 2014;24:2040–2047. doi: 10.1007/s11695-014-1288-9. [DOI] [PubMed] [Google Scholar]
- 10.Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA, Nevitt M, Cho YW, Newman AB. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr. 2007;86:1339–1346. doi: 10.1093/ajcn/86.5.1339. [DOI] [PubMed] [Google Scholar]
- 12.Lee CG, Boyko EJ, Barrett-Connor E, Miljkovic I, Hoffman AR, Everson-Rose SA, Lewis CE, Cawthon PM, Strotmeyer ES, Orwoll ES. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34:2381–2386. doi: 10.2337/dc11-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada M, Moriguch Y, Mitani T, Aoyama T, Arai H. Age-dependent changes in skeletal muscle mass and visceral fat area in Japanese adults from 40 to 79 years-of-age. Geriatr Gerontol Int. 2014;14(Suppl 1):8–14. doi: 10.1111/ggi.12209. [DOI] [PubMed] [Google Scholar]
- 14.Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M, Ueki K. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Investig. 2010;1:212–28. [DOI] [PMC free article] [PubMed]
- 15.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Hind K, Oldroyd B, Truscott JG. In vivo precision of the GE Lunar iDXA densitometer for the measurement of total body composition and fat distribution in adults. Eur J Clin Nutr. 2011;65:140–142. doi: 10.1038/ejcn.2010.190. [DOI] [PubMed] [Google Scholar]
- 17.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN., Jr Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 18.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Ryo M, Maeda K, Onda T, Katashima M, Okumiya A, Nishida M, Yamaguchi T, Funahashi T, Matsuzawa Y, Nakamura T, Shimomura I. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care. 2005;28:451–453. doi: 10.2337/diacare.28.2.451. [DOI] [PubMed] [Google Scholar]
- 20.Yamakage H, Ito R, Tochiya M, Muranaka K, Tanaka M, Matsuo Y, Odori S, Kono S, Shimatsu A, Satoh-Asahara N. The utility of dual bioelectrical impedance analysis in detecting intra-abdominal fat area in obese patients during weight reduction therapy in comparison with waist circumference and abdominal CT. Endocr J. 2014;61:807–819. doi: 10.1507/endocrj.EJ14-0092. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Bujo H, Takahashi K, Shibasaki M, Zhu Y, Yoshida Y, Otsuka Y, Hashimoto N, Saito Y. Visceral fat: higher responsiveness of fat mass and gene expression to calorie restriction than subcutaneous fat. Exp Biol Med (Maywood). 2003;228:1118–1123. doi: 10.1177/153537020322801004. [DOI] [PubMed] [Google Scholar]
- 22.Kim TN, Park MS, Ryu JY, Choi HY, Hong HC, Yoo HJ, Kang HJ, Song W, Park SW, Baik SH, Newman AB, Choi KM. Impact of visceral fat on skeletal muscle mass and vice versa in a prospective cohort study: the Korean Sarcopenic Obesity Study (KSOS) PLoS One. 2014;9:e115407. doi: 10.1371/journal.pone.0115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, You T, Lee JS, Visser M, Newman AB, Schwartz AV, Cauley JA, Tylavsky FA, Goodpaster BH, Kritchevsky SB, Harris TB. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66:888–895. doi: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yagi S, Kadota M, Aihara K, Nishikawa K, Hara T, Ise T, Ueda Y, Iwase T, Akaike M, Shimabukuro M, Katoh S, Sata M. Association of lower limb muscle mass and energy expenditure with visceral fat mass in healthy men. Diabetol Metab Syndr. 2014;6:27. doi: 10.1186/1758-5996-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–888. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 27.Abdelhafiz AH, Koay L, Sinclair AJ. The effect of frailty should be considered in the management plan of older people with type 2 diabetes. Future Sci OA. 2016;2:FSO102. [DOI] [PMC free article] [PubMed]
- 28.Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 29.Ito S, Harada A, Kasai T, Sakai Y, Takemura M, Matsui Y, Hida T, Ishiguro N. Use of alfacalcidol in osteoporotic patients with low muscle mass might increase muscle mass: an investigation using a patient database. Geriatr Gerontol Int. 2014;14(Suppl 1):122–128. doi: 10.1111/ggi.12222. [DOI] [PubMed] [Google Scholar]
- 30.Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, Katayama M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc. 2012;60:16–23. doi: 10.1111/j.1532-5415.2011.03776.x. [DOI] [PubMed] [Google Scholar]