Abstract
Objective
The purpose of this pilot study was to investigate the effect of short-term toe resistance training on toe pinch force and toe muscle quality in patients with type 2 diabetes mellitus.
Methods
A total of 12 patients with type 2 diabetes mellitus who were hospitalized to improve glycemic control (8 men and 4 women, duration of diabetes 12.2 ± 9.5 years) were enrolled in this pilot study. Exercise therapy was performed with conventional aerobic exercise and four newly developed toe resistance training exercises for 2 weeks. Changes in anthropometric parameters, blood pressure (BP), heart rate, and muscle parameters, i.e. muscle mass, toe pinch force and toe muscle quality were evaluated after the exercise program.
Results
There were no significant differences of body weight, body mass index, BP, heart rate, and upper/lower muscle mass after exercise performance. However, toe pinch force was significantly increased (pre: 2.92 ± 1.19 kg, post: 3.65 ± 1.58 kg, p = 0.007). Toe muscle quality (toe pinch force/lower leg muscle mass) were also significantly increased (pre: 2.15 ± 0.86 kg/kg, post: 2.72 ± 1.26 kg/kg, p = 0.009).
Conclusions
Two weeks of toe resistance training significantly increased toe pinch force and toe muscle quality in patients with type 2 diabetes mellitus. Toe resistance training is might be essential for treating patients with diabetes mellitus in clinical practice.
Electronic supplementary material
The online version of this article (doi:10.1007/s13340-017-0318-y) contains supplementary material, which is available to authorized users.
Keywords: Type 2 diabetes mellitus, Toe resistance training, Toe pinch force, Toe muscle quality
Introduction
It is well known that toe pinch force is critically involved in falling and gait speed, especially in elderly people [1, 2]. The use of Checker-kun (Nissin Sangyo Inc., Saitama, Japan) for measurement of toe pinch force was recently developed and established. We have also reported that the toe pinch force equipment has high reproducibility [3], and the measurements were significantly correlated with other muscle strength parameters such as handgrip strength, isometric knee extension force, and isometric ankle dorsiflexion force in patients with type 2 diabetes mellitus (DM) [4]. In addition, the toe pinch force in patients with type 2 DM was significantly lower than that in patients without type 2 DM, and diabetic polyneuropathy was fundamentally associated with lower toe pinch force in patients with type 2 DM [5]. Therefore, improvements in toe pinch force would be beneficial for patients with type 2 DM.
Muscle quality is expressed as the ratio of muscle strength to the muscle mass. Arm and leg muscle quality in patients with type 2 DM were significantly lower than that in control subjects [6]. Furthermore, a decrease in leg muscle quality has been reported to be closely associated with walking ability [7]. Although muscle quality as well as muscle strength thought to be important for patients with type 2 DM, the effect of toe resistance training on the reduction of toe pinch force and toe muscle quality remains to be investigated.
Therefore, the first purpose of this pilot study was to establish an exercise program for increasing toe pinch force as main outcome and toe muscle quality as second outcome in patients with type 2 DM. The second purpose was to estimate a proper sample size in future randomized controlled studies (RCT).
Methods
Study subjects
This study included 12 patients with type 2 DM who were treated for glycemic control at KKR Takamatsu Hospital, Kagawa, Japan (Table 1). Patients were excluded if they had severe cardiac or lung disease, acute or chronic musculoskeletal disorders, acute metabolic dysregulation, other neurological or endocrine disorders, a history of stroke, were implanted with metal such as bolts and metallic prosthetic joints, placement of a stent or pacemaker, previous or current asymmetric proximal lower leg weakness and toe deformity or atrophy of foot muscles.
Table 1.
Clinical characteristics of type 2 diabtic patients
| n | 12 |
| Gender (male/female) | 8/4 |
| Age (years) | 59.0 ± 11.6 |
| Height (cm) | 166.2 ± 8.6 |
| Weight (kg) | 73.7 ± 18.3 |
| BMI (kg/m2) | 26.5 ± 5.6 |
| SBP (mmHg) | 124.7 ± 17.0 |
| DBP (mmHg) | 70.4 ± 9.7 |
| HR (beat/m) | 72.8 ± 8.7 |
| Duration of diabetes (years) | 12.2 ± 9.5 |
| HbA1c (%) | 10.1 ± 2.0 |
| FBG (mg/dL) | 195.5 ± 54.2 |
| Total cholesterol (mg/dL) | 207.6 ± 34.7 |
| Triglyceride (mg/dL) | 268.1 ± 231.0 |
| HDL-cholesterol (mg/dL) | 47.3 ± 19.0 |
| LDL-cholesterol (mg/dL) | 109.7 ± 37.1 |
| Serum creatine (mg/dL) | 0.79 ± 0.35 |
| IPAQ (kcal/day) | 339.8 ± 458.3 |
| ABI | 1.07 ± 0.06 |
| CAVI | 8.89 ± 1.67 |
| Skeletal muscle mass (kg) | |
| Upper extremity | 1.22 ± 0.4 |
| Brachial region | 0.72 ± 0.3 |
| Antebrachial region | 0.52 ± 0.1 |
| Lower extremity | 5.42 ± 1.3 |
| Femoral region | 4.03 ± 1.1 |
| Leg region | 1.38 ± 0.3 |
| Drinking habit (n) | 3 |
| Smoking habit | |
| Current smoker (n) | 2 |
| Previous smoker (n) | 6 |
| Non smoker (n) | 4 |
| Exercise habit (n) | 8 |
| Neuropathy (n) | 4 |
| Retinopathy | |
| None (n) | 8 |
| Simple (n) | 1 |
| Preproliferative (n) | 2 |
| Proliferative (n) | 1 |
| Nephropathy | |
| Stage 1 (n) | 8 |
| Stage 2 (n) | 2 |
| Stage 3 (n) | 2 |
| Drug theraphy | |
| Insulin (n) | 8 |
| OHA (n) | 2 |
| Insulin and OHA (n) | 1 |
Value are presented as the mean ± SD
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, HR heart rate, FBG fasting blood glucose, IPAQ international physical activity questionnaire, ABI ankle-brachial index, CAVI cardio-ankle vascular index, OHA oral hypoglycemic agent
Patients included in this study provided written informed consent. The research ethics committee of KKR Takamatsu Hospital approved this pilot study (Approval number: E73, approval date: September 19, 2014). The original study protocol was registered in the University Hospital Medical Information Network (UMIN000026081).
Exercise program
The exercise program included conventional aerobic exercise and four different toe resistance training exercises. The aerobic exercise was performed with a bicycle ergometer. The exercise intensity setting was calculated with the Karvonen method [8], exercise load was set at moderate level. Exercise was performed for 30 min 2 h after lunch.
The four toe resistance training exercises were as follows. (1) Patients gathered a towel with their toes (Fig. 1a), (2) patients bent their toes with a rubber tube (Fig. 1b), (3) patients bent their toes with a rubber ball (Fig. 1c), and (4) patients grasped a small stick with their toes (Fig. 1d). All procedures were performed 20 times in each side with 3 sets every day for 2 weeks.
Fig. 1.
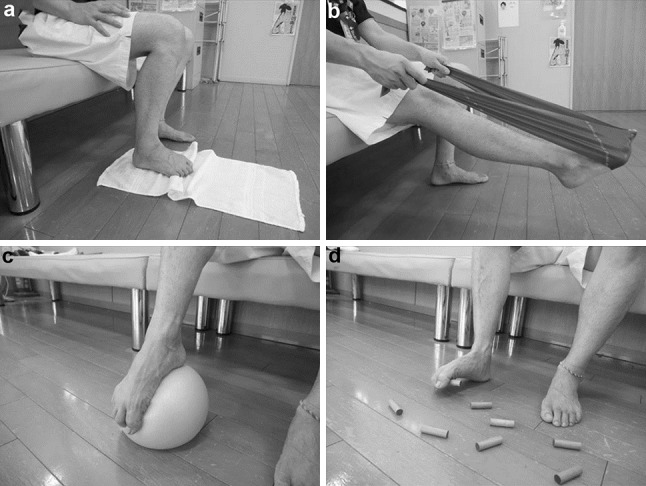
a Toe resistance training using a towel, b toe resistance training using a rubber tube, c toe resistance training using a rubber ball, d toe resistance training using a small stick
Measurement of toe pinch force and muscle mass
Toe pinch force was measured using Checker-kun as described in our previous studies [3–5]. Muscle mass was measured using bioelectrical impedance analysis (Physion MD, Physion Co., Ltd., Kyoto, Japan) [9]. The validity of this procedure has been reported in previous study [10]. The average values of the right and left upper muscle mass (brachial and antebrachial regions) and lower muscle mass (femoral and leg regions) were calculated. In addition, toe muscle quality was calculated as toe pinch force divided by lower leg muscle mass according to a previous report [11].
Clinical and laboratory measurements
We collected data for age (years), height (cm), body weight (kg), body mass index (BMI) (kg/m2), duration of type 2 DM (years), systolic blood pressure (SBP) (mmHg), diastolic blood pressure (DBP) (mmHg), heart rate (beat/m), ankle-brachial index, cardio-ankle vascular index, medications and laboratory test results. The levels of fasting plasma glucose (mg/dL), HbA1c (%), total cholesterol (mg/dL), low-density lipoprotein cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dL), triglycerides (mg/dL), and serum creatinine (mg/dL) were measured using standard methods. Well-trained medical staff interviewed each patient to evaluate the International Physical Activity Questionnaire and drinking, smoking, and exercise habits. Blood pressure (BP) and heart rate were measured with an HBR-2070 (Omron Colin Co. Ltd., Tokyo, Japan). The ankle-brachial index and cardio-ankle vascular index were measured with a VS-1500 (Fukuda Denshi Co. Ltd., Tokyo, Japan). Body weight, BMI, BP, heart rate, muscle mass, toe pinch force and toe muscle quality were also evaluated after the exercise program.
Diagnosis of type 2 DM was based on the fasting plasma glucose level and HbA1c, and was performed according to the guidelines of the Japan Diabetes Society [12]. The definition of diabetic retinopathy, nephropathy, and neuropathy also were based on the diagnostic guidelines of the Japan Diabetes Society [12].
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Comparison between before and after toe resistance training was performed with the Wilcoxon signed-rank test, and p < 0.05 was considered to be statistically significant. All date were analyzed using JMP 12.1.0 software (SAS Institute, Cary, NC, USA).
Results
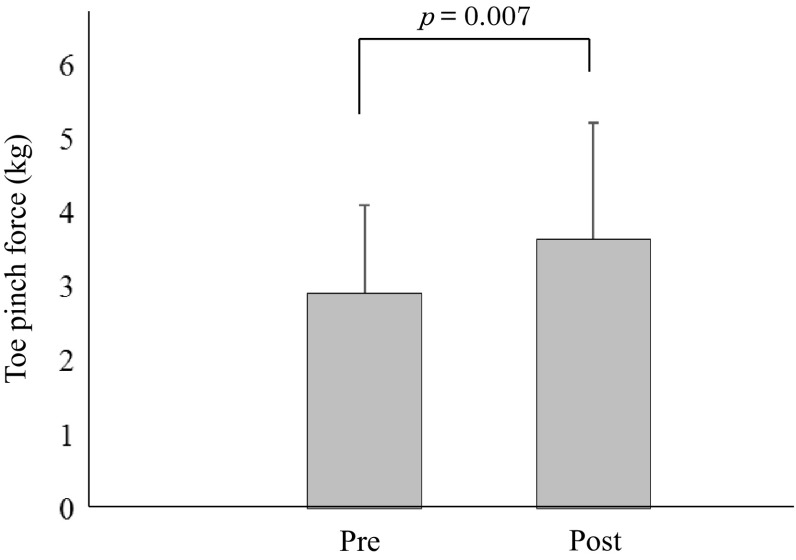
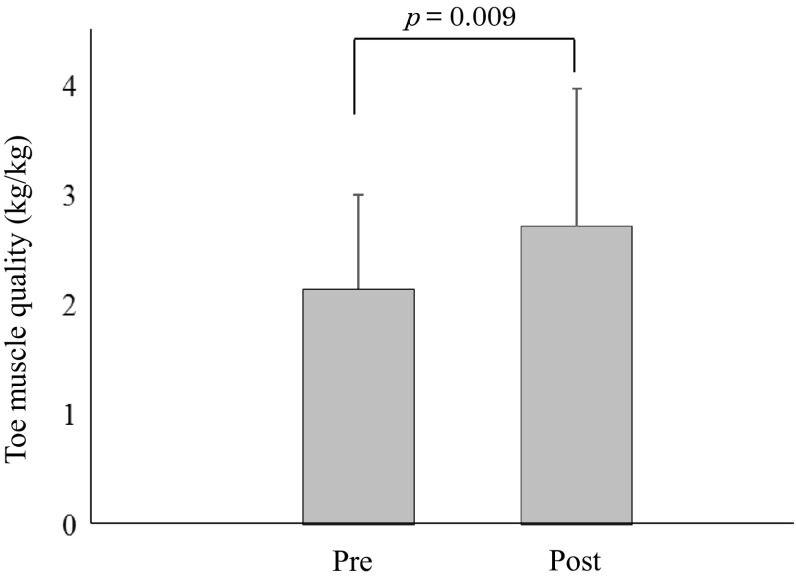
The clinical characteristics of the type 2 DM patients are summarized in Table 1. Overall, there were 8 men and 4 women aged 59.0 ± 11.6 years, height 166.2 ± 8.6 cm, weight 73.7 ± 18.3 kg, BMI 26.5 ± 5.6 kg/m2, and a duration of diabetes of 12.2 ± 9.5 years. The HbA1c was 10.1 ± 2.0%. The changes in parameters with the exercise program are also summarized in Table 2. There were no significant differences of body weight, BMI, SBP, DBP, heart rate, and upper/lower muscle mass between before and after the exercise program. However, after exercise, fasting blood glucose was significantly improved (Table 2), toe pinch force significantly increased from 2.92 ± 1.19 kg to 3.65 ± 1.58 kg (p = 0.007) (Fig. 2), and toe muscle quality also significantly increased from 2.15 ± 0.86 kg/kg to 2.72 ± 1.26 kg/kg (p = 0.009) (Fig. 3).
Table 2.
Change in the clinical characteristics by toe resistance training
| Pre | Post | p value | |
|---|---|---|---|
| Weight (kg) | 73.7 ± 18.3 | 72.9 ± 17.6 | 0.074 |
| BMI (kg/m2) | 26.5 ± 5.6 | 26.2 ± 5.3 | 0.104 |
| FBG (mg/dL) | 195.5 ± 54.2 | 122.6 ± 29.6 | 0.001 |
| SBP (mmHg) | 124.7 ± 17.0 | 124.4 ± 14.3 | 1.000 |
| DBP (mmHg) | 70.4 ± 9.7 | 74.3 ± 10.6 | 0.380 |
| HR (beat/m) | 72.8 ± 8.7 | 74.4 ± 7.3 | 0.836 |
| Skeletal muscle mass (kg) | |||
| Upper extremity | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.802 |
| Brachial region | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.575 |
| Antebrachial region | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.681 |
| Lower extremity | 5.4 ± 1.3 | 5.4 ± 1.3 | 0.733 |
| Femoral region | 4.0 ± 1.1 | 4.0 ± 1.1 | 0.622 |
| Leg region | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.765 |
Value are presented as the mean ± SD
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, HR heart rate, FBG fasting blood glucose
Fig. 2.
change in toe pinch force after toe resistance training
Fig. 3.
change in toe muscle quality after toe resistance training
Discussion
In this pilot study, we explored whether a toe resistance training program improved toe pinch force and toe muscle quality. The exercise program, including toe pinch resistance training, significantly increased toe pinch force and toe muscle quality, although muscle mass was not significantly increased.
Aerobic exercise training has been advocated as most suitable for improving glycemic control in patients with type 2 DM [13]. Although high-intensity resistance training induces inverse effects on BP and diabetic complications, proper resistance training that targets the major muscles is also recommended to improve muscle strength and blood glucose [14, 15]. We have previously reported that the toe pinch force in patients with type 2 DM was significantly lower than in patients without DM [5]. However, there were no studies that investigated the effects of resistance training on toe pinch force. It is well known that toe pinch force stabilizes posture in standing and walking [16, 17], and lower toe pinch force is closely linked to falling [1]. Walking is used for exercise therapy in patients with type 2 DM. Taken together, toe resistance training would be essential for patients with type 2 DM in clinical practice.
Studies of resistance training in patients with type 2 DM found improvements in muscle strength at 6 months [14] and 4 months [15]. The positive effects of exercise therapy require time to be evident. However, in this pilot study, as improvements in toe pinch force were noted after only 2 weeks of exercise, this exercise program would also be effective in improving and maintaining motivation in patients with type 2 DM. In the initial stage of improving muscle strength, muscle hypertrophy was not generally observed [18], and central and/or peripheral neural factors typically have an important role in the absence of significant muscle hypertrophy, and muscle hypertrophy becomes a dominant factor after 4 weeks of training [19]. Moreover, although the possibility that improved glucose level enhanced muscle strength, there were few previous reports suggesting such possibility. Therefore, we may have to prove such link in longitudinal study. In addition, only 2 weeks training period and changes in diet and medication during hospitalization might affect these results.
Finally, we were able to estimate a proper sample size for future RCTs. It has been reported that the changes in muscle strength is hardly observed with aerobic exercise alone [20, 21]. Therefore, the changes in toe pinch force of our newly developed exercise program, including toe resistance training, was 0.73 kg and that of conventional exercise program, not including toe resistance training, was 0 kg with an SD of 0.37. To detect an effect size of magnitude with 80% power, assuming a two-sided test with an alpha of 0.05, it was estimated that 6 patients should be included in each group in an RCT. However, in exercise intervention in this pilot study was accompanied with other intervention such as diet therapy and medication. Thus, calculation of sample size may be affected in a limited case. For the future, RCTs should be performed to confirm this.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported in part by research grants form 114 Bank, Japan.
Conflict of interest
All authors declare no financial support or relationships that could pose a conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
References
- 1.Mickle KJ, Munro BL, Lord SR, Menz HB, Steele JR. ISB Clinical Biomechanics Award 2009: toe weakness and deformity increase the risk of falls in older people. Clin Biomech. 2009;24:787–791. doi: 10.1016/j.clinbiomech.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Mann R, Inman VT. Phasic activity of intrinsic muscles of the foot. J Bone Joint Surg Am. 1964;46:469–481. doi: 10.2106/00004623-196446030-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kataoka H, Ishikawa I, Hayashino S, Yamanoto K, Miyazawa Y, Tanaka S, et al. Reproducibility of toe pinch dynamometer procedure. J Dist Environ Health Welf Res. 2015;18:39–44. [Google Scholar]
- 4.Kataoka H, Miyatake N, Kitayama N, Murao S, Kohi F, Tanaka S. Relationship of toe pinch force to other muscle strength parameters in men with type 2 diabetes. Environ Health Prev Med. 2016;21:179–185. doi: 10.1007/s12199-016-0512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kataoka H, Miyatake N, Kitayama N, Murao S, Tanaka S Toe pinch force in male type 2 diabetes mellitus patients. Acta Med Okayama (in press). [DOI] [PubMed]
- 6.Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813–1818. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 7.Volpato S, Bianchi L, Lauretani F, Bandinelli S, Guralnik JM, Zuliani G, et al. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35:1672–1679. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabet JY, Meurin P, Ben Driss A, Thabut G, Weber H, Renaud N, et al. Determination of exercise training heart rate in patients on β-blockers after myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2006;13:538–543. doi: 10.1097/01.hjr.0000209813.05573.4d. [DOI] [PubMed] [Google Scholar]
- 9.Yonei Y, Miwa Y, Hibino S, Takahashi Y, Miyazaki R, Yoshikawa T, et al. Japanese anthropometric reference data-Special emphasis on bioelectrical impedance analysis of muscle mass. Anti Aging Med. 2008;5:63–72. doi: 10.3793/jaam.5.63. [DOI] [Google Scholar]
- 10.Miyatani M, Kanehisa H, Masuo Y, Ito M, Fukunaga T. Validity of estimating limb muscle volume by bioelectrical impedance. J Appl Physiol. 2001;91:386–394. doi: 10.1152/jappl.2001.91.1.386. [DOI] [PubMed] [Google Scholar]
- 11.Senechal M, Johanssen NM, Swift DL, Earnest CP, Lavie CJ, Blair SN, et al. Association between changes in muscle quality with exercise training and changes in cardiorespiratory fitness measures in individuals with type 2 diabetes mellitus: results from the HART-D study. PLoS One. 2015;10:e0135057. doi: 10.1371/journal.pone.0135057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tajima N, Noda M, Irigasa H, Noda H, Yabe D, Fujita Y, et al. Evidence-based practice guideline for the treatment for diabetes in Japan 2013. Diabetol Int. 2015;6:151–187. doi: 10.1007/s13340-015-0206-2. [DOI] [Google Scholar]
- 13.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunstan DW, Daly RM, Owen H, Jolley D, DeCourten M, Shaw J, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25:1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 15.Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, et al. A randomized controlled trail of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 16.Endo M, Ashton-Miller JA, Alexander NB. Effects of age and gender on toe flexor muscle strength. J Gerontol A Biol Sci Med Sci. 2002;57A:392–397. doi: 10.1093/gerona/57.6.M392. [DOI] [PubMed] [Google Scholar]
- 17.Menz HB, Morris ME, Lord SR. Foot and ankle characteristics associated with impaired balance and functional ability in older people. J Gerontol A Biol Sci Med Sci. 2005;60A:1546–1552. doi: 10.1093/gerona/60.12.1546. [DOI] [PubMed] [Google Scholar]
- 18.Sale DG, Martin JE, Moroz DE. Hypertrophy without increased isometric strength after weight training. Eur J Appl Physiol Occup Physiol. 1992;64:51–55. doi: 10.1007/BF00376440. [DOI] [PubMed] [Google Scholar]
- 19.Moritani T, deVries HA. Potential for gross muscle hypertrophy in older men. J Gerontol. 1980;35:672–682. doi: 10.1093/geronj/35.5.672. [DOI] [PubMed] [Google Scholar]
- 20.Nemoto K, Genno H, Masuki S, Okazaki K, Nose H. Effect of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clin Proc. 2007;82:803–811. doi: 10.4065/82.7.803. [DOI] [PubMed] [Google Scholar]
- 21.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.