Introduction
It is well known that exercise in itself has several benefits in terms of health and fitness [1, 2], as well as neural and cognitive effects [3], in both humans and laboratory animals. Food restriction and regular exercise are the two major established strategies for the prevention and treatment of obesity, which is currently recognized as a serious burden worldwide. However, obesity is often associated with physical inactivity and disrupted life rhythms, including binge and night eating [4], which makes the treatment of obesity more complicated and weight reduction less attainable. Although exercise is recommended for the purpose of weight reduction through the increment of energy expenditure, it is generally difficult for most obese subjects to continue regular exercise for long periods of time. Thus, it is important to explore the putative mechanisms for producing the motivation to perform and adhere to exercise, especially in obese subjects.
Ghrelin, originally identified as a growth hormone secretagogue (GHS), is an orexigenic gut hormone. It is a 28-amino-acid peptide produced by the X/A-like endocrine cells in the oxyntic glands of the gastric fundus [5, 6]. Ghrelin functions primarily as an orexigen [7] and as a GH-releasing hormone [5]. Various other physiologic roles have been reported, including modulation of energy metabolism [8] and regulation of the autonomic nervous system [9, 10] and cardiovascular system [11]. In contrast to a significant decrease after a single bout of exercise [12], the long-term outcomes of periodic exercise on the ghrelin dynamics are still controversial and have not been clarified. Here we demonstrate beneficial effects of long-term voluntary exercise on disrupted rhythms of daily activity, which are often observed in obese subjects, in relation to the amelioration of extraordinary ghrelin production in an obese model. Furthermore, because ghrelin is relevant to higher motivation and hyperactivity [13, 14], it is plausible that ghrelin plays an essential role in the formation of motivation and in the adherence to exercise.
Effects of exercise on abnormal feeding behavior and disrupted ghrelin production in an obese model
As mentioned above, human obese subjects are often associated with abnormal eating behavior including binge and night eating [4] and physical inactivity. In our recent work [15], a disrupted daily rhythm of locomotor activity including hyperactivity during light phase was observed in high-fat-diet (HFD)-induced obese rats. Marked increase of food intake during light phase was observed in these obese models as well. Voluntary exercise was performed in metabolic chambers equipped with a running wheel apparatus for 3 days of every other week from 6 to 16 weeks old. The induction of voluntary exercise to HFD-obese rats brought about an effective reduction of weight and fat amount together with a significant decrease of locomotor activity and feeding during light phase, leading to a normal daily rhythm observed in rats fed control diet (CD). The HFD-obese rats exhibited a deterioration of ghrelin production and a lower serum ghrelin concentration compared to that in CD-rats. After the induction of voluntary exercise, however, the dysregulated ghrelin production was restored to the level comparable to CD rats. Because ghrelin is related to food anticipatory activity, it is plausible that ghrelin participates in the circadian rhythmicity of daily activity including eating behavior. A beneficial effect of voluntary exercise, even only 3 days of 2 weeks, for the treatment of obesity has now been confirmed in terms of the amelioration of the daily rhythms in eating behavior and physical activity probably controlled by endogenous ghrelin production.
Ghrelin accelerates the motivation for voluntary exercise via an enhancement of reward circuit
To provide an adequate energy balance, the food intake is regulated by a rewarding force as well as the sensation of hunger through the activation of other brain regions, such as the cerebral cortex and nucleus accumbens (NAc) [16]. Recent studies have shown that mesolimbic dopamine neurons in the ventral tegmental area (VTA) that project to the NAc represent a critical site for ghrelin to trigger food consumption behavior, since high levels of GHS receptor expressions are recognized in the dopamine neurons in the VTA [17]. It is suggested that ghrelin is involved in the brain reward circuits that are related to motivational properties as well as hedonic feeding. In the previous report [15], we demonstrate a possible relationship between exercise and this appetite-regulating hormone. Because ghrelin is relevant to higher motivation and hyperactivity [13, 14], we hypothesized that it plays an essential role as an initiator of voluntary exercise as well as feeding behavior. To this end, we used ghrelin knockout (GKO) mice for further experiments.
From our unpublished results, the plasma ghrelin concentration showed a bimodal diurnal rhythm with peaks at the beginning and at the end of the dark phase in the wild-type (WT) mice housed under a 12-h light-dark cycle (light off 1900–0700). Although predominant increases in wheel-running activity were observed accordant with both peaks of plasma ghrelin in the WT mice, those were severely attenuated in the GKO mice. A single injection of ghrelin receptor agonist into GKO mice at the beginning of dark phase brought about a marked enhancement of wheel-running activity to the level comparable to WT mice in contrast to no effect by the continuous administration of the same amount of this agonist. The brain dopamine response was severely attenuated in the GKO mice compared to that observed in the WT mice. These findings emphasize that the surge in ghrelin, which coincides with light being turned off, plays a crucial role in the motivation for voluntary exercise via the central hedonic dopamine system.
Conclusion
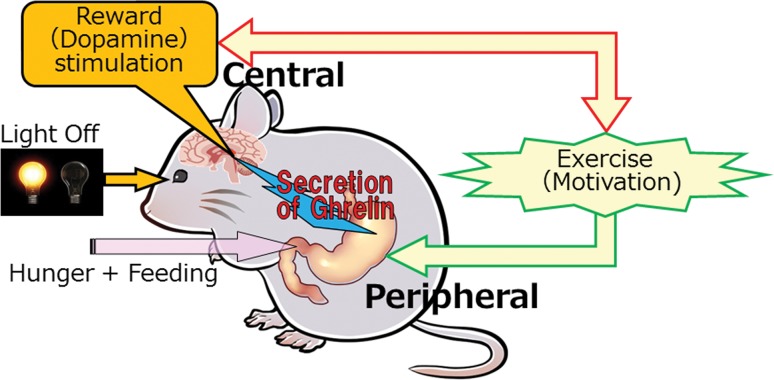
A close and essential relationship between exercise and appetite-regulating hormone has now been demonstrated. Because voluntary exercise in itself brought about a significant increase of brain dopamine (our unpublished data), an interactive relationship between exercise and the central reward circuit might exist. The production of ghrelin in the stomach was mainly accelerated at the beginning of the dark phase because of some unknown mechanisms related to hunger, feeding or light-off in this period. An enhanced ghrelin signal was transmitted to the central mesolimbic area, and the reward circuit was activated, which then motivated voluntary exercise, making a virtuous circle (Fig. 1). Because of ghrelin’s nature as a peptide hormone, it is expected that the peripheral administration of ghrelin or some analogs may be clinically applied for the treatment of obesity in the future.
Fig. 1.
Putative mechanisms for producing exercise motivation via peripheral ghrelin production and central reward circuit
Acknowledgements
The authors thank Miss Yuri Okabe for her technical support. This work was supported in part by Grant-in-Aid for Scientific Research (C) (Nos. 25504019 and 25350914) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
The author declares no conflict of interest.
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Haskell-Luevano C, Schaub JW, Andreasen A, et al. Voluntary exercise prevents the obese and diabetic metabolic syndrome of the melanocortin-4 receptor knockout mouse. FASEB J. 2009;23:642–655. doi: 10.1096/fj.08-109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CM, Levin BE. Role of exercise in the central regulation of energy homeostasis and in the prevention of obesity. Neuroendocrinology. 2008;87:65–70. doi: 10.1159/000100982. [DOI] [PubMed] [Google Scholar]
- 3.Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev. 2002;30:75–79. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Marcus MD, Wildes JE. Disordered eating in obese individuals. Curr Opin Psychiatry. 2014;27:443–447. doi: 10.1097/YCO.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 5.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 6.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 7.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 8.De Vriese C, Perret J, Delporte C. Focus on the short- and long-term effects of ghrelin on energy homeostasis. Nutrition. 2010;26:579–584. doi: 10.1016/j.nut.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Date Y, Shimbara T, Koda S, et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006;4:323–331. doi: 10.1016/j.cmet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura K, Tsuchihashi T, Fujii K, et al. Central ghrelin modulates sympathetic activity in conscious rabbits. Hypertension. 2002;40:694–699. doi: 10.1161/01.HYP.0000035395.51441.10. [DOI] [PubMed] [Google Scholar]
- 11.Tesauro M, Schinzari F, Caramanti M, et al. Cardiovascular and metabolic effects of ghrelin. Curr Diabetes Rev. 2010;6:228–235. doi: 10.2174/157339910791658871. [DOI] [PubMed] [Google Scholar]
- 12.Tiryaki-Sonmez G, Ozen S, Bugdayci G, et al. Effect of exercise on appetite-regulating hormones in overweight women. Biol Sport. 2013;30:75–80. doi: 10.5604/20831862.1044220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum ID, Patterson Z, Khazall R, et al. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164:351–359. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menzies JR, Skibicka KP, Leng G, et al. Ghrelin, reward and motivation. Endocr Dev. 2013;25:101–111. doi: 10.1159/000346058. [DOI] [PubMed] [Google Scholar]
- 15.Mifune H, Tajiri Y, Nishi Y, et al. Voluntary exercise contributed to an amelioration of abnormal feeding behavior, locomotor activity and ghrelin production concomitantly with a weight reduction in high fat diet-induced obese rats. Peptides. 2015;71:49–55. doi: 10.1016/j.peptides.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology. 2007;191:483–495. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- 17.Zigman JM, Jones JE, Lee CE, et al. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]