Abstract
Aims
To compare and evaluate effects of two DPP-4 inhibitors with different excretion routes on systemic and renal hemodynamics in Japanese patients with type 2 diabetes mellitus.
Methods
Seventy-three outpatients with type 2 diabetes who had been treated by 50 mg/day of sitagliptin (S) for at least 1 year were enrolled and prescribed 5 mg/day of linagliptin (L) instead of S for the next 1 year.
Results
After the initiation of S, the systolic and diastolic blood pressure decreased significantly. However, after switching to L for 1 year they increased significantly and returned to a comparable level as those before S treatment. The increase in serum creatinine or uric acid levels and the decrease in eGFR after S initiation were completely stopped or reversed after switching to L. The change in eGFR after the initiation of S was negatively correlated with the eGFR value at 1 year before switching.
Conclusions
The administration of S had an obvious effect on the systemic or renal hemodynamics in contrast to the fact that the administration of L had no effect on these parameters. It is thus important to use these agents with different excretion routes, properly taking the patients' renal function into account.
Keywords: Linagliptin, Sitagliptin, Blood pressure, eGFR, Type 2 diabetes
Introduction
A dipeptidyl peptidase-4 (DPP-4) inhibitor is an oral hypoglycemic agent (OHA) and was originally released in December 2009. Because in itself it is not expected to cause hypoglycemia through its glucose-dependent nature, especially in elderly patients, the percentage of prescription has been increasing since its approval and reached 70% among OHAs for the treatment of type 2 diabetes mellitus in Japan. Sitagliptin (S), the first approved DPP-4 inhibitor, is most frequently prescribed among this class of agents in Japan. DPP-4 itself is most abundantly expressed in the kidney [1], and it has been reported that DPP-4 inhibition possibly caused elevation of serum creatinine or uric acid in patients with type 2 diabetes [2–4]. Recent studies revealed that DPP-4 located in the renal proximal tubule modulates Na–H exchanger 3 (NHE3) and facilitates reabsorption of sodium [5, 6], and it is thus plausible that the DPP-4 inhibitor of renal excretion types such as S may increase natriuresis by an inactivation of NHE3 and possibly cause an elevation of the serum creatinine level. On the other hand, linagliptin (L) has a unique profile with a primarily non-renal but hepatobiliary route of elimination, requiring no dose adjustment in patients with chronic kidney disease [7]. Accordingly, the chance of L reaching the proximal tubules is quite low, and there might be a rare situation in which L modulates natriuresis through NHE3. The aim of the present study is to compare these two DPP-4 inhibitors with different excretion routes focusing on their effects on renal and systemic hemodynamics.
Patients and methods
Type 2 diabetic outpatients who had been treated by 50 mg/day of S for at least 1 year (duration of S treatment was 19.2 ± 4.8 months) in Shin Koga Hospital, Kurume University Hospital and other satellite hospitals or clinics were recruited between October 2011 and March 2012. This study was conducted according to the principles expressed in the Declaration of Helsinki. The protocol was approved by the ethical review committee at Kurume University School of Medicine (approved on 23 September 2011, approval number: 10305), and informed consent was obtained from each patients. This is an open-labeled observational study performed as a pilot study. In the results, 73 type 2 diabetic outpatients without severe renal dysfunction were enrolled and completed the study protocol. In addition to S, biganide (n = 55), sulfonylurea (n = 46), α-glucosidase inhibitor (n = 21), glinide (n = 7), thiazolidinedione (n = 5) and insulin (n = 22) were used for the management of blood glucose. As an anti-hypertension drug, an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) was used in 28 patients (38%), and a calcium channel blocker (CCB) was used in 23 patients (32%) (Table 1).
Table 1.
Characteristics of diabetic patients
| Number of patients | 73 |
| Gender (male/female) | 41/32 |
| Age (years old) | 66 ± 13 |
| Duration of diabetes (years) | 15.3 ± 7.8 |
| BMI | 24.6 ± 4.1 |
| HbA1c (%) | 7.3 ± 1.1 |
| Use of ACEI or ARB | 28 (38) |
| Use of CCB | 23 (32) |
| Systolic blood pressure (mmHg) | 128 ± 17 |
| Diastolic blood pressure (mmHg) | 68 ± 12 |
| Serum creatinine concentration (mg/dl) | 0.83 ± 0.32 |
| eGFR (ml/min/1.73 m2) | 71.8 ± 23.3 |
| Serum uric acid concentration (mg/dl) | 5.3 ± 1.24 |
| Serum Na concentration (mEq/l) | 139.2 ± 2.4 |
| Serum K concentration (mEq/l) | 4.5 ± 0.6 |
Abbreviations are shown in the text. Data are expressed as mean ± SD or numbers of patients (percentage)
Patients were prescribed 5 mg/day of L instead of S for the next year. Each measurement was performed at a switch to L and every 3 months after a switch up to 1 year in a prospective manner. Data when S was started and at 1 year, 9, 6 and 3 months before switching were retrieved from the medical records, retrospectively. Changes in parameters for 1 year before switching from S to L are expressed as Δvalue 1 = (a value at switching) − (a value at 1 year before switching). Changes in parameters from the initiation of S to switching to L are expressed as Δvalue 1 ext. = (a value at switching) − (a value at the initiation of S). Changes in parameters for 1 year after switching from S to L are Δvalue 2 = (a value at 1 year after switching) − (a value at switching).
All tests were performed using JMP Pro version 12 (SAS Institute Inc., USA). Data are presented as the mean ± SD. Changes in parameters were analyzed by repeated measures ANOVA with Bonferroni correction. Relationships between ΔeGFR1, ΔeGFR1 ext. or ΔeGFR2 as an outcome variable and other parameters as predictive variables were analyzed by Pearson’s correlation coefficient and by multiple regression analysis. P values less than 0.05 were considered statistically significant.
Results
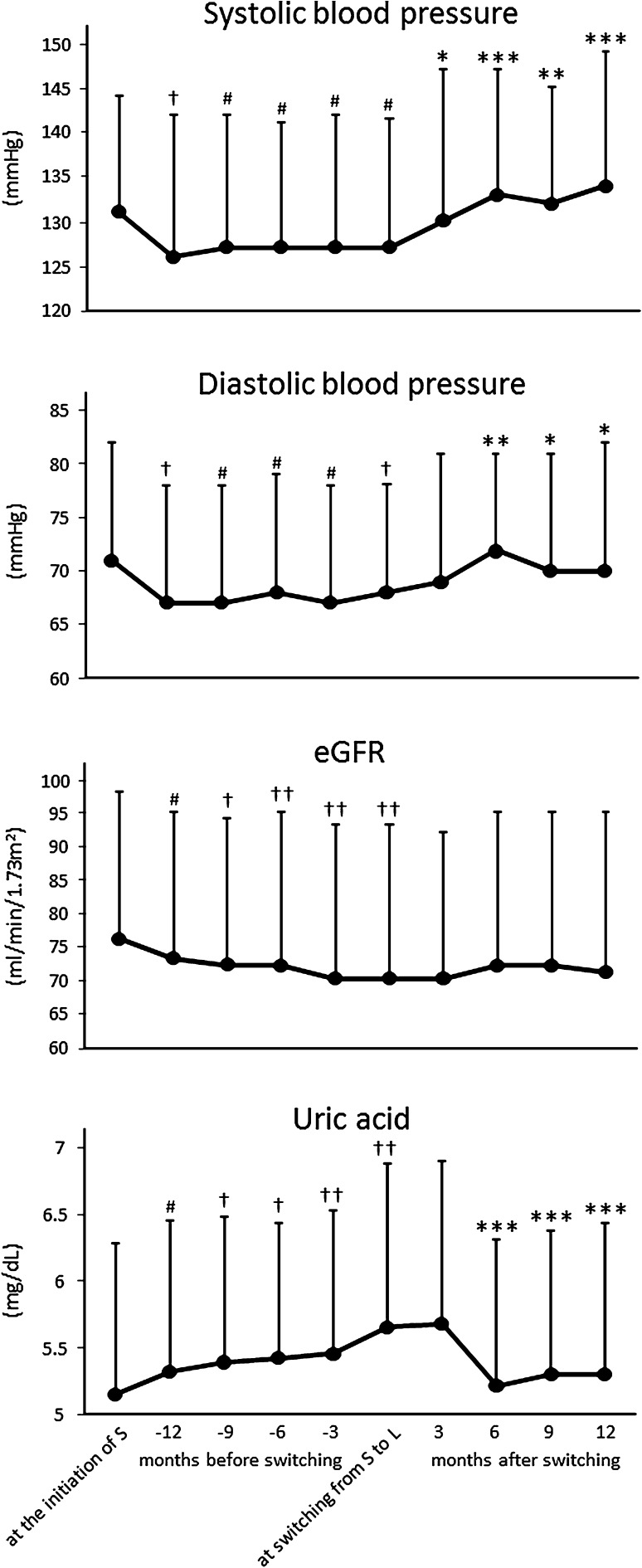
HbA1c significantly decreased after S initiation and did not change after switching to L. Systolic and diastolic blood pressure significantly decreased after the initiation of S, and after switching to L they significantly increased to a comparable level as that at the initiation of S. After the initiation of S, a significant increase in serum creatinine and decrease in eGFR were observed up to the switching. Although the decline of eGFR at this period (ΔeGFR1 ext.) in ACEI or ARB users was not significantly higher than that in non-users (−8.17 ± 1.32 and −3.07 ± 10.49 ml/min/1.73 m2/year, respectively, P = 0.111), a significant decline in eGFR still remained in non-users (75.4 ± 22.2 → 72.3 ± 23.0 ml/min/1.73 m2, P < 0.05, n = 45). The use of CCB did not affect the change in eGFR (data not shown). However, these changes in serum creatinine and eGFR completely ceased after switching to L. The increment of uric acid after S initiation completely reversed to the initial level after switching to L. BMI and serum electrolyte concentrations did not change significantly during the treatment term of either S or L (Table 2; Fig. 1).
Table 2.
Changes in parameters before and after switching from sitagliptin to linagliptin
| At the initiation of sitagliptin | At 1 year before switching | At swiching | At 6 months after switching | At 1 year after switching | |
|---|---|---|---|---|---|
| BMI | 24.6 ± 5.0 | 24.6 ± 5.2 | 24.6 ± 4.1 | 23.8 ± 5.7 | 24.6 ± 4.1 |
| HbA1c (%) | 8.19 ± 1.07 | 7.39 ± 1.04†† | 7.32 ± 1.05†† | 7.44 ± 1.25†† | 7.46 ± 1.02†† |
| Systolic blood pressure (mmHg) | 132 ± 14 | 129 ± 17# | 128 ± 17# | 134 ± 15*** | 134 ± 15*** |
| Diastolic blood pressure (mmHg) | 72 ± 12 | 69 ± 12# | 68 ± 12† | 73 ± 10** | 71 ± 12* |
| Serum creatinine concentration (mg/dl) | 0.75 ± 0.25 | 0.79 ± 0.27# | 0.83 ± 0.32†† | 0.82 ± 0.32 | 0.84 ± 0.37 |
| eGFR (mi/min/1.73 m2) | 78.0 ± 24.4 | 74.2 ± 24.2# | 71.8 ± 23.3†† | 72.8 ± 23.9 | 72.3 ± 25.6 |
| Serum uric acid concentration (mg/dl) | 5.10 ± 1.13 | 5.34 ± 1.14# | 5.63 ± 1.24†† | 5.17 ± 1.04*** | 5.24 ± 1.10*** |
| Serum Na concentration (mEq/l) | 139.3 ± 2.4 | 139.8 ± 2.5 | 139.2 ± 2.4 | 140.3 ± 2.6 | 140.1 ± 2.8 |
| Serum K concentration (mEq/l) | 4.52 ± 0.35 | 4.47 ± 0.41 | 4.48 ± 0.56 | 4.54 ± 0.4 | 4.46 ± 0.4 |
Abbreviations are shown in the text. Repeated measures ANOVA with post hoc Bonferroni test. Data are expressed as mean ± SD
* P < 0.05, ** P < 0.01, *** P < 0.001 versus the value at switching from sitagliptin to linagliptin. # P < 0.05, † P < 0.01, †† P < 0.001 versus the value at the initiation of sitagliptin
Fig. 1.
Changes in blood pressure, eGFR and serum uric acid concentration before and after switching from sitagliptin to linagliptin. Abbreviations are shown in the text. Data are expressed as mean ± SD. Repeated measures ANOVA with post hoc Bonferroni test was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001 versus the value at switching from sitagliptin to linagliptin. # P < 0.05, † P < 0.01, †† P < 0.001 versus the value at the initiation of sitagliptin
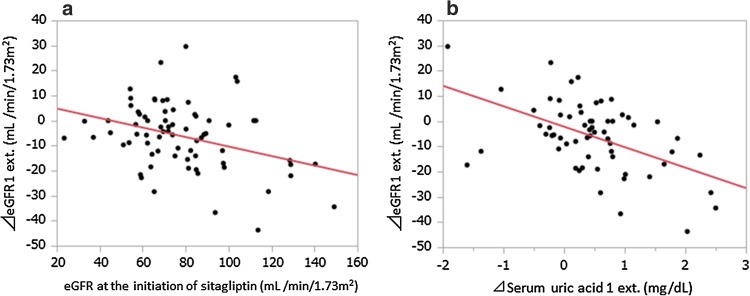
The change in eGFR for 1 year before switching from S to L (ΔeGFR1) was negatively correlated with the value of eGFR at 1 year before switching and Δserum uric acid 1 (Table 3). In multiple regression analysis of ΔeGFR1 as an outcome variable, those two variables were again selected as significant predictive variables (standardized βs were −0.363 and −0.279, P < 0.01 and 0.05, respectively). The change in eGFR from the initiation of S to the switching to L (ΔeGFR1 ext.) showed a stronger correlation with the value of eGFR at the initiation of S (r = −0.351, P < 0.01) and Δserum uric acid 1 ext. (r = −0.508, P < 0.0001) (Fig. 2) and was again supported by multivariate analysis (standardized βs were −0.311 and −0.468, P < 0.01 and 0.0001, respectively). Fifty-four patients who had been completely followed for 2 years under S treatment were selected and stratified into three groups according to their eGFR levels at the initiation of S. A significant decline in eGFR for 2 years was only observed in patients whose initial eGFR had been over 90 ml/min/1.73 m2 (106.6 ± 12.6 → 94.1 ± 19.7, P < 0.05, n = 13) with no significant changes in the remaining two groups (eGFR 60–90; 74.7 ± 8.6 → 71.3 ± 9.6, n = 31, eGFR 30–60; 53.5 ± 6.2 → 53.7 ± 11.6, n = 10). After switching from S to L, ΔeGFR2 was not correlated with any variable at all (data not shown).
Table 3.
Relationship between ΔeGFR1 and each variable
| Predictive variable | r value | P value |
|---|---|---|
| Age (years old) | 0.161 | NS |
| BMI | 0.07 | NS |
| HbA1c (%) | 0.026 | NS |
| Systolic blood pressure (mmHg) | −0.201 | NS |
| Diastolic blood pressure (mmHg) | −0.140 | NS |
| ΔSystolic blood pressure 1 (mmHg) | 0.061 | NS |
| ΔDiastolic blood pressure 1 (mmHg) | −0.074 | NS |
| eGFR (ml/min/1.73 m2) | −0.346 | <0.01 |
| Serum creatinine concentration (mg/dl) | 0.139 | NS |
| Serum uric acid concentration (mg/dl) | 0.154 | NS |
| ΔSerum uric acid concentration 1 (mg/dl) | −0.307 | <0.05 |
| Serum Na concentration (mEq/l) | 0.145 | NS |
| Serum K concentration (mEq/l) | −0.158 | NS |
| ΔSerum Na concentration 1 (mEq/l) | −0.046 | NS |
| ΔSerum K concentration 1 (mEq/l) | −0.079 | NS |
Abbreviations are shown in the text. Pearson’s correlation coefficient. Each value is that at 1 year before switching. Δvalue 1 = (a value at switching) − (a value at 1 year before switching)
NS Not significant
Fig. 2.
Correlations of the change in eGFR (ΔeGFR ext.) with eGFR at the initiation of S (A) and with the change in serum uric acid concentration (Δserum uric acid 1 ext. B). Pearson’s correlation coefficient was used for statistical analysis
Discussion
Here we demonstrated a quite different effect of two types of DPP-4 inhibitors with different excretion routes on systemic and renal hemodynamics. The decrease in eGFR during S treatment is in line with its diuretic effect based on the increase in both serum creatinine and uric acid levels with a concomitant decrease in systolic and diastolic blood pressure. Some previous reports demonstrated similar results regarding renal functions and blood pressure [2, 3]. It is plausible that the decrease in eGFR caused by S administration may be attributable to a tubule-glomerular feedback mechanism leading to a renal afferent arteriole constriction and a drop in GFR [8]. In this study, a decrease in eGFR was mainly observed in patients with higher eGFR levels, suggesting an amelioration of glomerular hyperfiltration [9].
Conversely, the decrease in eGFR caused by S was related to the increase in uric acid, suggesting a hypovolemic state through its diuretic nature. Up to September 2007, the FDA’s Adverse Event Reporting System revealed 96 renal injury events (3.2%) caused by this drug [10]. In Japan, the S package insert was revised in September 2010 including the possibility of acute renal failure as a severe adverse effect. It is notable that the correlation between the decline of eGFR and the increase in uric acid was stronger from the initiation of S to the switching to L than that for the restricted term of 1 year before the switching, suggesting a possibility that longer exposure to S might produce the worse renal outcomes. Although the mechanism of renal damage by S is largely unknown, caution must be exercised in case of the use of S for patients with pre-existing renal insufficiency.
In contrast, the preservation of eGFR during L treatment reflects its outstanding safety regarding renal function, as previously reported, with no change in eGFR up to 52 weeks requiring no dose adjustment in patients with chronic kidney disease [7]. These renal safety results deserve recognition, taking the annual decline in eGFR in type 2 diabetic patients into account [11]. The efficacy and safety of L have been confirmed in patients older than 70 years in whom a risk of renal insufficiency is much higher than in younger patients [12]. The complete restorations of the serum uric acid level and blood pressure by switching from S to L are in line with its non-renal excretion nature without any effect on renal hemodynamics. There might be a possibility that DPP-4 and the related NHE3 activity in renal tubules are not affected by L, leading to an abolishment of facilitated natriuresis by S beforehand.
The limitations of this study are as follows. Results obtained from a limited number of participants in limited institutions and from only Japanese patients are not generally applied to other ethnicities. Because our study is not a head-to-head comparison or placebo-controlled study, and data during S were retrieved retrospectively, there might be some room left for including potential bias in this study design. The strength of this study is its crossover design, which possibly makes comparisons within the same individuals much easier with lower cost, although no randomization of the turn of either S or L as the first treatment alternative is a weak point of this study.
In conclusion, we first demonstrated quite different effects of two DPP-4 inhibitors with different excretion routes on systemic and renal hemodynamics for a long period of administration. It is important to use these two agents properly, taking the renal function of patients into account.
Acknowledgements
YT is a guarantor and takes full responsibility for the fidelity of this work. The work received no funding support.
Conflict of interest
MT and YT declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
References
- 1.Kirino Y, Sato Y, Kamimoto T, et al. Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes, dyslipidaemia and nephropathy: a streptozotocin-induced model using wild-type and DPP4-deficient rats. J Endocrinol. 2009;200:53–61. doi: 10.1677/JOE-08-0424. [DOI] [PubMed] [Google Scholar]
- 2.Kubota A, Maeda H, Kanamori A, et al. Pleiotropic effects of sitagliptin in the treatment of type 2 diabetes mellitus patients. J Clin Med Res. 2012;4:309–313. doi: 10.4021/jocmr1061w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takihata M, Nakamura A, Tajima K, et al. Comparative study of sitagliptin with pioglitazone in Japanese type 2 diabetic patients: the COMPASS randomized controlled trial. Diabetes Obes Metab. 2013;15:455–462. doi: 10.1111/dom.12055. [DOI] [PubMed] [Google Scholar]
- 4.Maeda H, Kubota A, Tanaka Y, et al. The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes. Diabetes Res Clin Pract. 2012;95:e20–e22. doi: 10.1016/j.diabres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Girardi AC, Fukuda LE, Rossoni LV, et al. Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol. 2008;294:F414–F422. doi: 10.1152/ajprenal.00174.2007. [DOI] [PubMed] [Google Scholar]
- 6.Girardi AC, Degray BC, Nagy T, et al. Association of Na(+)-H(+) exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J Biol Chem. 2001;276:46671–46677. doi: 10.1074/jbc.M106897200. [DOI] [PubMed] [Google Scholar]
- 7.Scott D. Treatment of type 2 diabetes in chronic kidney disease: a case for linagliptin in the treatment of diabetes in severe renal impairment. Diabetes Metab Syndr Obes. 2013;6:359–363. doi: 10.2147/DMSO.S51795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallon V. Tubuloglomerular feedback and the control of glomerular filtration rate. News Physiol Sci. 2003;18:169–174. doi: 10.1152/nips.01442.2003. [DOI] [PubMed] [Google Scholar]
- 9.Vora JP, Dolben J, Dean JD, et al. Renal hemodynamics in newly presenting non-insulin dependent diabetes mellitus. Kidney Int. 1992;41:829–835. doi: 10.1038/ki.1992.127. [DOI] [PubMed] [Google Scholar]
- 10.Center for Drug Evaluation and Research. Adverse event reporting system. Center for Drug Evaluation and Research Website. http://www.fda.gov/cder/aers. Accessed 25 Oct 2007.
- 11.Zoppini G, Targher G, Chonchol M, et al. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol. 2012;7:401–408. doi: 10.2215/CJN.07650711. [DOI] [PubMed] [Google Scholar]
- 12.Barnett AH, Huisman H, Jones R, et al. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1413–1423. doi: 10.1016/S0140-6736(13)61500-7. [DOI] [PubMed] [Google Scholar]