Abstract
Our aim was to examine the effects of ipragliflozin, a selective sodium–glucose co-transporter 2 inhibitor, on blood pressure in Japanese patients with type 2 diabetes mellitus (T2DM). We conducted a pooled analysis of double-blind trials of Japanese T2DM patients, randomized to 50 mg ipragliflozin or placebo, with patient-level data for the change in systolic blood pressure (SBP) and diastolic blood pressure (DBP) from baseline to end of treatment (12–24 weeks). Data from six trials were analyzed: ipragliflozin was administered as monotherapy in two; in combination with metformin, pioglitazone, or sulfonylurea in one each; and in combination with prior therapy in patients with renal impairment in one. Overall, 628 and 368 patients were treated with ipragliflozin and placebo, respectively. The placebo-adjusted mean changes (95 % confidence interval) in SBP and DBP (mmHg) were −2.8 (−4.4, −1.3, P < 0.001) and −1.6 (−2.7, −0.6, P < 0.002), respectively, in all patients. The reductions in SBP and DBP were significantly greater in patients with baseline SBP ≥140 mmHg [−5.5 (−9.1, −1.8) and −2.9 (−5.3, −0.5), respectively] than in patients with SBP <140 mmHg [−2.1 (−3.8, −0.4) and −1.3 (−2.5, −0.1), respectively]. The reductions in SBP and DBP were also significantly greater in the ipragliflozin group than in the placebo group in patients treated with [−2.8 (−5.1, −0.4) and −2.4 (−4.0, −0.8), respectively] or without [−3.0 (−5.0, −1.0) and −1.0 (−2.4, 0.4), respectively] concomitant antihypertensive therapy. In conclusion, this pooled analysis showed that ipragliflozin was associated with significant reductions in SBP and DBP compared with placebo.
Electronic supplementary material
The online version of this article (doi:10.1007/s13340-016-0283-x) contains supplementary material, which is available to authorized users.
Keywords: Blood pressure, Efficacy, Ipragliflozin, Pooled analysis, Sodium–glucose co-transporter 2
Introduction
Hypertension and type 2 diabetes mellitus (T2DM) are major risk factors for cardiovascular events. Therefore, these diseases should be adequately controlled to reduce the risk of such events. T2DM and elevated blood pressure (BP) frequently co-exist; for example, it was reported that >70 % of Asian patients with T2DM [1] and 56 % of Japanese patients with T2DM have hypertension [2].
It is well established that lowering BP reduces the risk of cardiovascular events in patients with T2DM [3]. Consequently, the Japanese Guidelines for the Management of Hypertension recommend lowering SBP/DBP to <130/80 mmHg for diabetic patients through a combination of lifestyle modifications and the use of antihypertensive drugs [4]. BP lowering also has renoprotective effects in patients with T2DM [5]. However, BP control is more difficult in patients with T2DM than in patients without diabetes.
Several mechanisms may reduce the efficacy of conventional antihypertensive drugs in T2DM. For example, high salt sensitivity in Japanese individuals, partly as a consequence of gene polymorphisms [6], may contribute to the relatively high incidence of hypertension in Japan. Additionally, volume expansion and arterial stiffness may contribute to the pathogenesis of hypertension in patients with T2DM [7–10].
Sodium–glucose co-transporter 2 (SGLT2) is predominantly expressed in the proximal tubules in the kidney. Because of its role in the reuptake of glucose, it has been exploited as a treatment target for T2DM by the development of SGLT2 inhibitors, such as ipragliflozin [11–13]. Intriguingly, the results of clinical trials have revealed that ipragliflozin and other SGLT2 inhibitors are associated with reductions in SBP and DBP [14–16]. Currently, however, there are limited data describing the effects of ipragliflozin on BP. Therefore, the aim of this pooled analysis was to examine the effects of ipragliflozin on BP in Japanese patients with T2DM.
Methods
Inclusion criteria for selecting the clinical trials
We searched for clinical trials of ipragliflozin in Japanese patients with T2DM that had patient-level data on SBP and DBP. Only randomized, double-blind, placebo-controlled trials that used 50 mg ipragliflozin were included in this pooled analysis.
Trial design and treatments
Six trials were identified and included in this pooled analysis (trial reference no. and ClinicalTrials.gov identifier):
Phase 2 dose-finding trial (CL0103, NCT00621868) [17];
Phase 3 trial in patients with renal impairment (CL0072, NCT01316094) [18];
Phase 3 monotherapy trial (CL0105, NCT01057628) [19];
Phase 3 trial in combination with metformin (CL0106, NCT01135433) [20];
Phase 3 trial in combination with pioglitazone (CL0107, NCT01225081) [21];
Phase 3 trial in combination with a sulfonylurea (CL0109, NCT01242215) [22].
All of the trials were performed in accordance with Good Clinical Practice, International Conference on Harmonisation guidelines, applicable laws/regulations, and under approval from institutional review boards at participating institutions.
All included studies required participants to provide written informed consent before beginning the trial. The design and primary results of each trial are summarized in Supplementary Table 1.
CL0103 was a five-arm, parallel-group, phase 2 trial in which patients were randomized to 12.5, 25, 50, or 100 mg ipragliflozin or placebo once daily for 12 weeks. Only the 50-mg and placebo groups were included in this pooled analysis. CL0072 was a two-arm, parallel-group, phase 3 trial in which patients with mild to moderate renal impairment were randomized to 50 mg ipragliflozin or placebo in combination with their prior therapy (diet and exercise alone or diet/exercise in combination with an α-glucosidase inhibitor, a sulfonylurea, or pioglitazone). CL0105 was a two-arm, parallel-group, phase 3 trial in which patients were randomized to either 50 mg ipragliflozin or placebo as monotherapy for 16 weeks. CL0106, CL0107, and CL0109 were two-arm, parallel-group, phase 3 trials in which patients were randomized to either 50 mg ipragliflozin or placebo in combination with metformin (CL0106), pioglitazone (CL0107), or a sulfonylurea (CL0109).
All of the studies included a 4-week screening period, followed by a 2-week placebo run-in, the specified treatment phase, and a follow-up phase of 4–6 weeks after completing treatment. CL0106 included a 6-week washout period, and CL0107 and CL0109 included a 4-week washout period in which previously used antidiabetic drugs were washed out, except for the specified combination drug, before the screening period. In CL0103 and CL0105, previously used antidiabetic drugs were washed out in the screening period. In CL0072, patients who had used an oral antidiabetic drug (α-glucosidase inhibitor, sulfonylurea, or pioglitazone) for ≥12 weeks before enrollment could continue using it throughout the treatment period; changes in the regimen or switching to an alternative drug was prohibited. As described in the original reports, the study protocols specified that the prescribed diet therapies should be continued throughout the study to avoid any confounding of the effects of the study drugs on glycemic control. Diet therapies included a reduction in salt intake. The protocols did not include any specific requirements of antihypertensive therapies.
After the 24-week treatment period in CL0072, CL0106, CL0107, and CL0109, patients were eligible for a 28-week open-label phase in which all patients received 50 or 100 mg ipragliflozin. Only data from the double-blind treatment periods were included in this pooled analysis. Established randomization procedures managed by central registration centers, blinding, and breaking of the treatment code were applied in each trial. The test drug and placebo were identical in appearance and packaging. Patients were prohibited from using antidiabetic drugs other than the relevant combination drug in all six trials. Continued use of corticosteroids, immunosuppressants, and loop diuretics, except for topical and temporary use, was prohibited in all of the studies; however, loop diuretics were permitted in CL0103.
In each trial, BP was measured in the clinic at each visit with the patient in a seated position. BP was routinely measured as a safety-related variable, not as an efficacy endpoint. The primary endpoint of each trial was the change in HbA1c from baseline to the end of treatment.
Patients
The patient eligibility criteria applied in each trial are summarized in Supplementary Table 1. Generally, patients aged ≥20 years who were diagnosed with T2DM ≥12 weeks before screening/washout, HbA1c of ≥7.4 % (57 mmol/mol), and body mass index (BMI) ≥20 kg/m2 were eligible for the trials. For CL0072, additional criteria included the presence of renal impairment classified as mild [estimated glomerular filtration rate (eGFR) ≥60 to <90 ml/min/1.73 m2] or moderate (eGFR ≥30 to <60 ml/min/1.73 m2).
Data analysis
BP data and other clinically relevant data pooled from the individual trials were compared between the ipragliflozin and placebo groups at baseline and at weeks 2, 4, 8, 12, 16, 20, and 24. For CL0103 and CL0105, data were included up to the last available visit (i.e., weeks 12 and 16, respectively).
Subgroup analyses were conducted by dividing patients into the following subgroups according to their baseline characteristics: sex (male vs. female), age (<65 vs. ≥65 years), BMI (<25 vs. ≥25 kg/m2), diabetes duration (<60 vs. ≥60 months), SBP (<140 vs. ≥140 mmHg), HbA1c [<8.0 vs. ≥8.0 % (64 mmol/mol)], fasting plasma glucose (FPG: <160 vs. ≥160 mg/dl), fasting serum insulin (FSI: <6 vs. ≥6 µU/ml), eGFR (30–<60 ml/min/1.73 m2, 60–<90 ml/min/1.73 m2, ≥90 ml/min/1.73 m2), hematocrit (<42 vs. ≥42 %), and the use of concomitant antihypertensive drugs (yes vs. no). The cutoff values for HbA1c and FPG were determined based on their clinical relevance in real-world settings. The cutoff values for other continuous variables were selected as values close to the median value for each variable. The pooled analyses were conducted using the patient-level BP data from the safety analysis set (i.e., all patients who received at least one dose of the ipragliflozin/placebo in each trial).
Descriptive statistics [mean, standard deviation (SD), n, percent] were calculated for the baseline characteristics for all patients combined. The changes in SBP and DBP from baseline to the end of treatment were assessed by analysis of covariance in which the treatment group and clinical trial were included as fixed effects and the baseline value was included as a covariate, and the results are presented as the placebo-adjusted mean difference with 95 % confidence intervals (CIs). Values of P < 0.05 were considered statistically significant.
The association between patient characteristics and the change in SBP was evaluated by stepwise regression analysis in which baseline/general characteristics with an F value of >2.0 were included in the prediction model.
Markers of fluid volume, including the changes in hematocrit and blood urea nitrogen (BUN) from baseline to the end of treatment, were also compared between the ipragliflozin and placebo groups to provide clues to potential underlying mechanisms of any change in BP.
Data processing, summarization, and analyses were performed using SAS Drug Development (ver. 3.4) and PC-SAS (ver. 9.1.3) (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of subjects
Data for a total of 628 patients treated with 50 mg ipragliflozin and 368 patients treated with placebo were available for this pooled analysis. Two patients in the ipragliflozin group and one in the placebo group lacked SBP/DBP data at the end of treatment. Table 1 summarizes the patient characteristics at baseline in the 50 mg ipragliflozin and placebo groups. Both groups were generally well matched in terms of their baseline characteristics. Although there were statistically significant differences in BMI and eGFR between the ipragliflozin and placebo groups, these differences were not clinically meaningful. The majority of patients in each group treated with antihypertensive drugs were using renin-angiotensin system (RAS) inhibitors and calcium channel blockers (Table 1).
Table 1.
Patient characteristics
| Ipragliflozin 50 mg (n = 628) | Placebo (n = 368) | P valuea | |
|---|---|---|---|
| Sex, n (%) | 0.833 | ||
| Male | 431 (68.6) | 250 (67.9) | |
| Female | 197 (31.4) | 118 (32.1) | |
| Age (at consent), years | 0.486 | ||
| Mean (SD) | 59.0 (10.14) | 58.5 (10.06) | |
| Median (range) | 60.0 (29–82) | 60.0 (31–82) | |
| Age category, n (%) | 0.780 | ||
| <65 | 421 (67.0) | 250 (67.9) | |
| ≥65 | 207 (33.0) | 118 (32.1) | |
| Body weight, kg | 0.117 | ||
| Mean (SD) | 69.08 (12.590) | 67.78 (12.434) | |
| Median (range) | 67.85 (41.5–139.0) | 66.50 (44.4–121.6) | |
| BMI, kg/m2 | 0.007* | ||
| Mean (SD) | 25.98 (3.731) | 25.33 (3.597) | |
| Median (range) | 25.44 (19.0–42.9) | 24.63 (19.1–40.6) | |
| BMI category, n (%) | 0.009* | ||
| <25 | 281 (44.7) | 197 (53.5) | |
| ≥25 | 347 (55.3) | 171 (46.5) | |
| Diabetes duration, months | 0.403 | ||
| n | 621 | 363 | |
| Mean (SD) | 100.0 (80.84) | 95.7 (73.53) | |
| Median (range) | 79.0 (3–467) | 81.0 (3–547) | |
| eGFR (at baseline), ml/min/1.73 m2 | 0.009* | ||
| Mean (SD) | 83.01 (20.857) | 86.50 (19.470) | |
| Median (range) | 80.88 (29.0–152.4) | 85.37 (37.0–162.3) | |
| eGFR category, n (%) | 0.081 | ||
| ≥90 | 207 (33.0) | 146 (39.7) | |
| ≥60 to <90 | 348 (55.4) | 191 (51.9) | |
| ≥30 to <60 | 72 (11.5) | 31 (8.4) | |
| <30 | 1 (0.2) | 0 | |
| Concomitant antihypertensive drugs, n (%) | 302 (48.1) | 169 (45.9) | 0.512 |
| RAS inhibitorsb | 231 (36.8) | 135 (36.7) | 1.000 |
| CCB | 185 (29.5) | 100 (27.2) | 0.468 |
| Diuretics | 55 (8.8) | 21 (5.7) | 0.084 |
| Others | 58 (9.2) | 28 (7.6) | 0.415 |
SD standard deviation, BMI body mass index, eGFR estimated glomerular filtration rate, RAS renin-angiotensin system, CCB calcium channel blocker
* P < 0.05
aValues were compared between the ipragliflozin and placebo groups using Fisher’s exact test for categorical variables or independent-samples t tests for continuous variables
bIncludes angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and direct renin inhibitors
Effects of ipragliflozin vs. placebo on BP
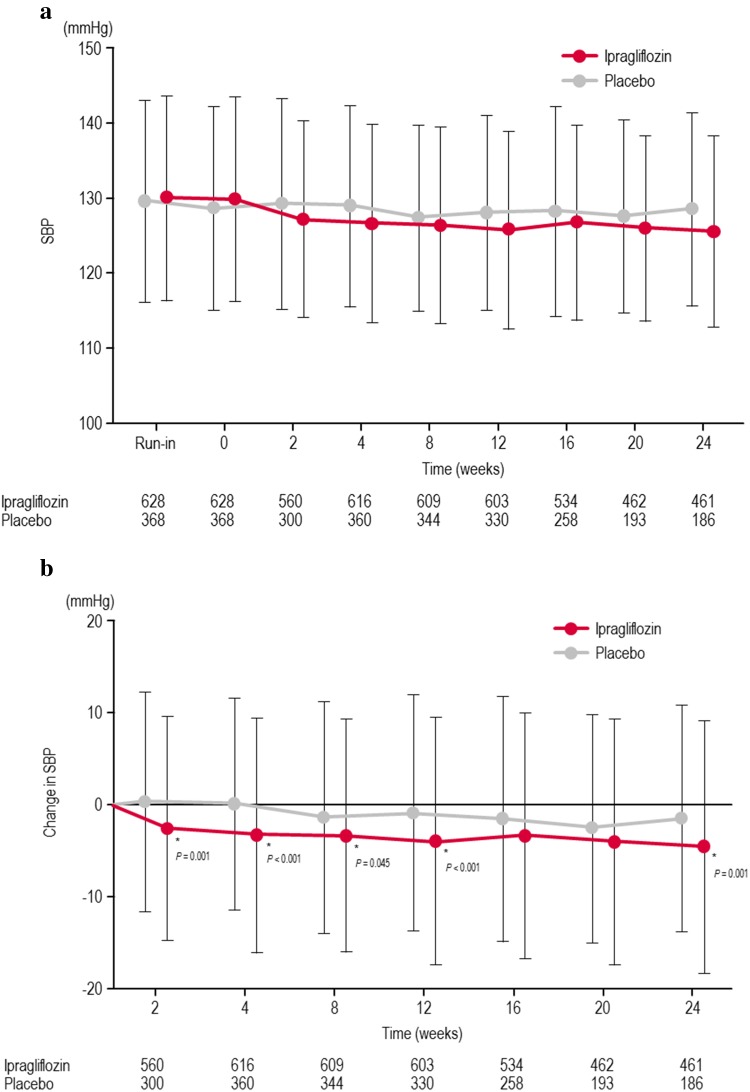
The changes in SBP and DBP from baseline until the end of treatment are shown in Fig. 1, Table 2, and Supplementary Figure 1. Although SBP and DBP decreased in both groups, the reductions in SBP and DBP were significantly greater in the ipragliflozin group than in the placebo group with an adjusted mean difference (95 % CI) of −2.8 mmHg (−4.4, −1.3 mmHg; P < 0.001) for SBP and −1.6 mmHg (−2.7, −0.6 mmHg; P = 0.002) for DBP. The change in body weight at the end of treatment showed a weak, positive correlation with the changes in SBP/DBP in the ipragliflozin group [SBP: r (partial correlation coefficient adjusted for study) = 0.121, P = 0.002; DBP: r = 0.085, P = 0.034], but not in the placebo group (SBP: r = 0.067, P = 0.203; DBP: r = 0.027, P = 0.612). The change in SBP was not correlated with the change in HbA1c in the ipragliflozin (r = −0.039, P = 0.332) or the placebo (r = 0.077, P = 0.141) groups.
Fig. 1.
Time course of changes in systolic blood pressure (a) and the changes in systolic blood pressure from baseline to each time point (b). *Statistical difference between the ipragliflozin and placebo groups. Values and vertical lines show mean and SD, respectively. The numbers in the table below the graph represent numbers of patients in each group included at each time point. SBP systolic blood pressure, SD standard deviation
Table 2.
Blood pressure at baseline and end of treatment according to the treatment received
| Ipragliflozin 50 mg (n = 626a) | Placebo (n = 367a) | |
|---|---|---|
| SBP (mmHg) | ||
| Baseline (SD) | 130.0 (13.7) | 128.7 (13.6) |
| End of treatment (SD) | 125.8 (13.7) | 128.1 (13.3) |
| Change from baseline (SD) | −4.1 (13.8) | −0.7 (12.8) |
| AMD (95 % CI) | −2.8 (−4.4, −1.3) | |
| P value | <0.001 | |
| DBP (mmHg) | ||
| Baseline (SD) | 77.6 (9.7) | 76.3 (9.9) |
| End of treatment (SD) | 75.0 (9.9) | 75.9 (9.9) |
| Change from baseline (SD) | −2.6 (9.0) | −0.4 (8.9) |
| AMD (95 % CI) | −1.6 (−2.7, −0.6) | |
| P value | 0.002 | |
SBP systolic blood pressure, SD standard deviation, AMD adjusted mean difference, CI confidence interval, DBP diastolic blood pressure
aFor two patients in the ipragliflozin group and one patient in the placebo group, there were no SBP/DBP data at end of treatment
BP reduction according to patient characteristics at baseline
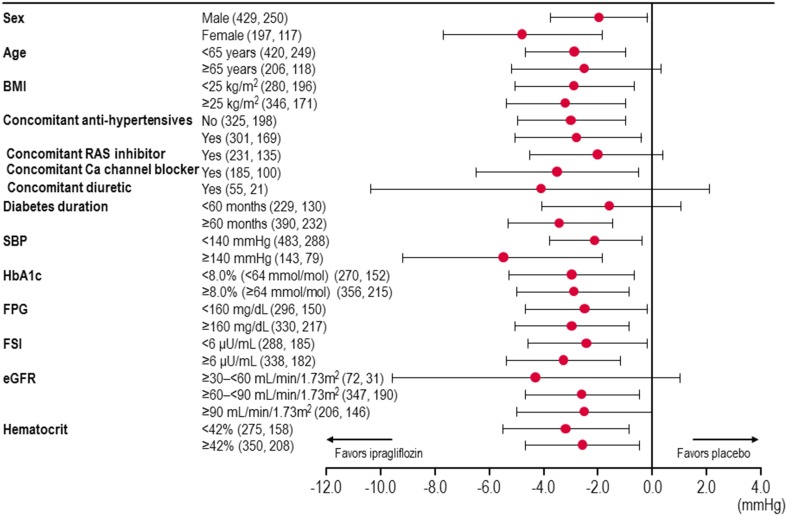
The changes in SBP were also compared between the ipragliflozin and placebo groups after dividing the patients by sex, age, BMI, diabetes duration, SBP, HbA1c, FPG, FSI, eGFR, hematocrit, and the use of antihypertensive drugs. The adjusted mean difference between ipragliflozin and placebo is presented for each subgroup of patients in Fig. 2. As shown in this figure, the reduction in SBP from baseline to the end of treatment was in favor of ipragliflozin in all subgroups of patients, except for patients aged ≥65 years, patients using a concomitant RAS inhibitor or diuretic, patients with a diabetes duration of <60 months, and patients with an eGFR of ≥30–<60 ml/min/1.73 m2 or ≥90 ml/min/1.73 m2.
Fig. 2.
Change in systolic blood pressure in patients divided by baseline/general characteristics. The number of patients is given in parentheses (ipragliflozin, placebo). Values and horizontal lines show mean and 95 % CI, respectively. BMI body mass index, eGFR estimated glomerular filtration rate, FPG fasting plasma glucose, FSI fasting serum insulin, RAS renin-angiotensin system, SBP systolic blood pressure
The reductions in SBP and DBP were independent of the use of antihypertensive drugs and were significantly greater in the ipragliflozin group than in the placebo group in subgroups of patients treated with or without concomitant antihypertensive drugs, except for the reductions in DBP in the subgroup of patients who were not using antihypertensive drugs (Table 3). In this analysis, the adjusted mean difference (95 % CI) in SBP was −3.0 mmHg (−5.0, −1.0 mmHg, P = 0.004) in patients treated without an antihypertensive drug and −2.8 mmHg (−5.1, −0.4 mmHg, P = 0.020) in patients treated with an antihypertensive drug. According to the use of individual drugs, the reduction in SBP from baseline to the end of treatment was significantly greater in the ipragliflozin group among patients treated with a calcium channel blocker (P = 0.022) and tended to be greater in the ipragliflozin group, albeit not significantly, among patients treated with an RAS inhibitor or a diuretic (Fig. 2).
Table 3.
Baseline systolic blood pressure levels and blood pressure reductions at end of treatment from baseline in patients with/without antihypertensive drugs
| Patients treated without antihypertensive drugs | Patients treated with antihypertensive drugs | SBP <140 mmHg at baseline | SBP ≥140 mmHg at baseline | |||||
|---|---|---|---|---|---|---|---|---|
| Ipragliflozin (n = 325) | Placebo (n = 198) | Ipragliflozin (n = 301) | Placebo (n = 169) | Ipragliflozin (n = 483) | Placebo (n = 288) | Ipragliflozin (n = 143) | Placebo (n = 79) | |
| SBP (mmHg) | ||||||||
| Baseline (SD) | 126.4 (13.1) | 124.5 (12.6) | 133.8 (13.3) | 133.7 (13.0) | 124.5 (9.8) | 123.7 (9.9) | 148.3 (7.4) | 147.3 (7.9) |
| End of treatment (SD) | 122.8 (12.9) | 124.8 (12.9) | 129.1 (13.9) | 131.8 (12.9) | 123.6 (12.7) | 125.2 (12.5) | 133.3 (14.6) | 138.5 (10.8) |
| Change from baseline (SD) | −3.6 (13.0) | 0.3 (12.2) | −4.7 (14.6) | −1.9 (13.5) | −0.9 (11.9) | 1.5 (12.1) | −15.0 (14.2) | −8.7 (12.2) |
| AMD (95 % CI) | −3.0 (−5.0, −1.0) | −2.8 (−5.1, −0.4) | −2.1 (−3.8, −0.4) | −5.5 (−9.1, −1.8) | ||||
| P value | 0.004 | 0.020 | 0.013 | 0.003 | ||||
| DBP (mmHg) | ||||||||
| Baseline (SD) | 76.7 (9.7) | 74.7 (8.8) | 78.5 (9.3) | 78.1 (10.7) | 75.7 (9.3) | 74.7 (8.7) | 83.7 (8.5) | 81.9 (11.7) |
| End of treatment (SD) | 74.4 (10.0) | 74.2 (8.9) | 75.6 (9.9) | 77.8 (10.6) | 74.1 (10.0) | 74.8 (9.2) | 77.9 (9.2) | 79.9 (11.2) |
| Change from baseline (SD) | −2.3 (8.5) | −0.6 (8.2) | −2.9 (9.4) | −0.3 (9.7) | −1.6 (8.8) | 0.0 (8.4) | −5.8 (8.9) | −2.0 (10.4) |
| AMD (95 % CI) | −1.0 (−2.4, 0.4) | −2.4 (−4.0, −0.8) | −1.3 (−2.5, −0.1) | −2.9 (−5.3, −0.5) | ||||
| P value | 0.149 | 0.004 | 0.030 | 0.017 | ||||
SBP systolic blood pressure, SD standard deviation, AMD adjusted mean difference, CI confidence interval, DBP diastolic blood pressure
The adjusted mean difference in the change in SBP was numerically greater in patients with a baseline SBP of ≥140 mmHg than in the other subgroups of patients. By way of comparison, the adjusted mean difference (95 % CI) was −5.5 mmHg (−9.1, −1.8 mmHg, P = 0.003) in patients with a baseline SBP of ≥140 mmHg compared with −2.1 mmHg (−3.8, −0.4 mmHg, P = 0.013) in patients with a baseline SBP of <140 mmHg (Table 3). The change in DBP from baseline to the end of treatment was also numerically greater in patients with a baseline SBP of ≥140 mmHg (−2.9 mmHg; 95 % CI: −5.3, −0.5 mmHg; P = 0.017) than in patients with a baseline SBP of <140 mmHg (−1.3 mmHg; 95 % CI: −2.5, −0.1 mmHg; P = 0.030). Consistent with these data, SBP at baseline showed a strong association with the reduction in SBP from baseline to the end of treatment in the ipragliflozin group (r = −0.496) and in the placebo group (r = −0.486). However, the placebo-subtracted reductions in SBP and DBP were not significantly greater in patients with a baseline SBP ≥140 mmHg than in patients with a baseline SBP <140 mmHg (P = 0.0517 and P = 0.1390 for SBP and DBP, respectively), according to an interaction test using ANOVA with the treatment group, study, baseline SBP category, and treatment group × baseline SBP category as fixed effects.
Prediction model for the change in SBP from baseline
Stepwise linear regression was used to develop a prediction model for the change in SBP from baseline to the end of treatment using the following baseline variables: treatment group, SBP, study, age, sex, diabetes duration, BMI, HbA1c, FPG, FSI, eGFR, and hematocrit. The final model comprised the following four variables: baseline SBP [regression coefficient (β) = −0.510, P < 0.0001], treatment group (ipragliflozin—placebo; β = −2.993, P = 0.0001), age (β = 0.112, P = 0.0044), and BMI (β = 0.192, P = 0.0708). In this model, baseline SBP, treatment group, and age were significantly associated with the change in SBP (Table 4).
Table 4.
Stepwise linear regression for the change in systolic blood pressure from baseline
| Explanatory variables | Estimate | SE | F value | P value |
|---|---|---|---|---|
| Baseline SBP (mmHg) | −0.510 | 0.028 | 333.64 | <0.0001 |
| Treatment group (ipragliflozin––placebo) | −2.993 | 0.769 | 15.16 | 0.0001 |
| Age (years) | 0.112 | 0.039 | 8.13 | 0.0044 |
| BMI (kg/m2) | 0.192 | 0.106 | 3.27 | 0.0708 |
Stepwise linear regression was used to develop a prediction model for the change in SBP from baseline to the end of treatment using the following baseline variables: treatment group, SBP, study, age, sex, diabetes duration, BMI, HbA1c, fasting plasma glucose, fasting serum insulin, estimated glomerular filtration rate, and hematocrit. The association between patient characteristics and the change in SBP was evaluated by stepwise regression analysis in which baseline/general characteristics with an F value of >2.0 were included in the prediction model. The final model comprised the following four variables: baseline SBP, treatment group, age, and BMI
SE standard error, SBP systolic blood pressure, BMI body mass index
Markers of fluid volume
As markers of fluid volume and as potential contributors to the change in SBP, we also compared the changes in hematocrit and BUN from baseline to the end of treatment between the two groups. The change (±SD) in hematocrit was +1.6 ± 2.4 % in the ipragliflozin group (baseline: 42.5 ± 4.2 %) compared with −0.3 ± 2.5 % in the placebo group (baseline 42.7 ± 3.9 %). The change in BUN was +1.7 ± 3.5 mg/dl in the ipragliflozin group (baseline: 15.0 ± 4.4 mg/dl) compared with −0.3 ± 3.3 mg/dl in the placebo group (baseline: 14.9 ± 4.1 mg/dl). The differences in hematocrit and BUN between the ipragliflozin and placebo groups were statistically significant (both: P < 0.001). The change in hematocrit was not significantly correlated with the change in SBP at week 2 in either the ipragliflozin group (r = 0.016, P = 0.703) or the placebo group (r = −0.015, P = 0.802). In addition, the change in hematocrit was not significantly correlated with the change in SBP in either the ipragliflozin (r = 0.032, P = 0.423) or the placebo (r = 0.052, P = 0.322) groups at the end of treatment. However, the change in hematocrit was negatively correlated with the change in body weight at week 2 in both the ipragliflozin group (r = −0.239, P < 0.001) and the placebo group (r = −0.257, P < 0.001), indicating that hematocrit increased in patients with a decrease in body weight.
Safety
The overall incidence of treatment-emergent adverse events was 67.9 % in the placebo group and 72.9 % in the ipragliflozin group. Adverse events potentially associated with BP included hypertension (placebo vs. ipragliflozin: 1.6 vs. 0.3 %), blood pressure increased (0.5 vs. 0.2 %, respectively), blood pressure decreased (0 vs. 0.2 %, respectively), orthostatic hypotension (0 vs. 0.3 %), and renal disorders (0 vs. 0.3 %, respectively). No major difference was observed in the occurrence of these treatment-emergent adverse events between the ipragliflozin and placebo groups.
Serum magnesium, serum phosphorus, urine calcium, urine magnesium, and urine phosphorus concentrations increased in the ipragliflozin group from week 2 onwards compared with the baseline concentrations. The percentage changes from baseline to the end of treatment were 5, 4, 14, 19, and 20 % for serum magnesium, serum phosphorus, urine calcium, urine magnesium, and urine phosphorus, respectively.
Discussion
Hypertension is a common complication in patients with T2DM. Accordingly, antihypertensive medicines are prescribed to many patients with T2DM; however, BP control often remains suboptimal. Interventions aimed at lowering BP are essential to reduce cardiovascular risk in patients with T2DM, and there is increasing awareness that antidiabetic medicines may influence BP. Therefore, evaluating the potential effects of antidiabetic medicines on BP is an important topic for clinical research. Accordingly, we performed a pooled analysis to examine the effects of ipragliflozin on BP using data of six clinical trials concerning the recently approved SGLT2 inhibitor for the treatment of T2DM.
Of note, we observed significant reductions in SBP and DBP in all patients combined [adjusted mean difference (95 % CI): −2.8 mmHg (−4.4, −1.3 mmHg) for SBP and −1.6 mmHg (−2.7, −0.6 mmHg) for DBP]. The magnitudes of the reductions in SBP and DBP were greater in patients with SBP ≥140 mmHg at baseline [adjusted mean difference (95 % CI): −5.5 mmHg (−9.1, −1.8 mmHg) for SBP and −2.9 mmHg (−5.3, −0.5 mmHg) for DBP]. The reduction in SBP was also strongly correlated with the patient’s baseline SBP. The reductions in SBP were consistently greater in the ipragliflozin group than in the placebo group in patients divided into subgroups by sex, age, BMI, diabetes duration, baseline HbA1c, FPG, FSI, eGFR, hematocrit, and the use or nonuse of antihypertensive drugs.
Several meta-analyses/systematic reviews have examined the effects of SGLT2 inhibitors on BP. In particular, in one systematic review of 27 trials of the SGLT2 inhibitors canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and remogliflozin, it was reported that this class of drugs reduced SBP and DBP relative to placebo, with a weighted mean difference (WMD) (95 % CI) of −4.0 mmHg (−4.4, −3.5 mmHg) for SBP and −1.6 mmHg (−1.9, −1.3 mmHg) for DBP [23]. Likewise, Imprialos et al. described a mild class effect of SGLT2 inhibitors on reducing BP in clinical trials [24].
Analyses of individual drugs have revealed that 100 mg canagliflozin was associated with a significant reduction in SBP (placebo-subtracted change: −3.9 mmHg) [25], 25 mg empagliflozin was associated with significant reductions in SBP and DBP [WMD (95 % CI) to placebo: −4.19 mmHg (−5.17, −3.20 mmHg) for SBP and −1.88 mmHg (−2.73, −1.04 mmHg) for DBP] [26], and dapagliflozin was associated with significant reductions in SBP and DBP [WMD (95 % CI) to placebo: −3.57 mmHg (−4.38, −2.77 mmHg) for SBP and −1.49 mmHg (−1.98, −0.99 mmHg) for DBP] [27].
It has been proposed that SGLT2 inhibitors may reduce cardiovascular risk [28] through a variety of mechanisms, including reductions in BP, body weight, visceral adiposity, hyperinsulinemia, arterial stiffness, albuminuria, circulating uric acid levels, and oxidative stress. In this pooled analysis, we focused on the effect of ipragliflozin on one of these factors, namely BP, and observed significant reductions in SBP and DBP, consistent with the effects of other SGLT2 inhibitors as shown in pooled analysis, systematic review, and meta-analysis [23, 25–27].
Several mechanisms have been proposed by which SGLT2 inhibitors may reduce BP [29]. First, inhibition of SGLT2 promotes urinary glucose excretion by preventing its reuptake in an insulin-independent manner. Because SGLT2 co-transports glucose and sodium in the same directions, blockade of SGLT2 also inhibits sodium reuptake. The increases in the urine glucose and sodium concentrations together increase urine volume, termed osmotic diuresis and natriuresis. These effects of SGLT2 inhibitors may therefore reduce the body fluid volume. This possibility is also supported by the slight but significant increases in hematocrit and BUN, which indicate that ipragliflozin had a weak hemo-concentrating effect and likely caused a slight reduction in fluid volume relative to placebo. If osmotic diuresis is the main factor responsible for the BP-lowering effects of SGLT2 inhibitors, then the magnitude of the change in BP should decrease with worsening kidney function [30]. Contrary to this hypothesis, we observed a poor correlation between the change in SBP and baseline eGFR. Additionally, the change in SBP was similar between the subgroups of patients divided on the basis of baseline eGFR. Furthermore, the change in SBP was not correlated with the change in the hematocrit at 2 weeks, which indicates that the change in SBP was less likely to be related to changes in fluid volume.
Although SGLT2 inhibitors have mild natriuretic effects, hyponatremia was not reported in patients treated with SGLT2 inhibitors, and excess sodium released into the proximal nephron is reabsorbed in the distal nephron to minimize sodium loss [30]. Sodium loss does not usually cause hyponatremia if patients are able to regulate their free water intake. In this context, serum sodium more closely reflects the water balance than the sodium balance. Accordingly, sodium loss is unlikely to have a major impact on the BP-lowering effects of these drugs.
Changes in body weight may also contribute to the change in BP. Indeed, we observed a weak, but statistically significant correlation between the change in body weight and the changes in SBP and DBP in the ipragliflozin group, but not in the placebo group. In an earlier trial, it was reported that 300 mg canagliflozin was associated with reductions in body weight and BP over 12 weeks of treatment [31]. The authors also noted that canagliflozin was associated with a reduction in plasma volume, primarily in the first week of treatment, but this reduction was attenuated by week 12. Likewise, there was a modest increase in urine volume at week 1, but the increase was attenuated by week 12. Their results suggest that a change in body fluid within a few days of starting treatment may contribute to the rapid change in BP observed at 1 week, but that other factors are likely to explain the sustained reduction in BP at week 12.
It is feasible that sustained reductions in body weight (e.g., via changes in body composition [32]) may contribute to the reduction in BP. However, in the trial by Sha et al. [31], although a reduction in body weight was observed at week 1, the reduction was much greater at week 12. By contrast, the reduction in BP was similar at both times, which suggests that changes in body weight are unlikely to explain the sustained reduction in BP, especially in the early stage of treatment. The reductions in BP are unlikely to be related to changes in lifestyle interventions (i.e., diet, including reduction in salt intake) because the patients were instructed by their physician to maintain these interventions unchanged for the duration of the trial.
It has also been hypothesized that SGLT2 inhibitors promote renal remodeling [33] and reduce arterial stiffness [34]. However, these possibilities could not be examined in the present pooled analysis. The current data also suggest that the BP-lowering effects of ipragliflozin in Japanese patients with T2DM are greater than those observed in Western patients [35]. It has been reported that Japanese have a higher daily salt intake and may be more susceptible to salt-sensitive hypertension than Caucasians [6]. Therefore, Japanese patients with T2DM may be more sensitive to diuretics, and the reduction in BP is expected to be greater in Japanese patients than in Caucasian patients. However, this pooled analysis was unable to directly confirm whether the reduction in BP is associated with or is independent of osmotic diuresis or natriuresis.
It has been demonstrated that dipeptidyl peptidase-4 inhibitors lowered BP by increasing urine sodium excretion and normalizing dips in BP in a model of salt-dependent hypertension [36]. Similar studies are necessary to verify the mechanisms of action of SGLT2 inhibitors on BP.
The results of this pooled analysis must be evaluated after considering the limitations, including the different treatments used in each study and the different durations of treatment in the monotherapy studies compared with the combination therapy studies. Although we attempted to overcome this possible bias by including trial as a fixed effect in the analyses, this approach may not be sufficient to eliminate this source of bias. However, as illustrated in Fig. 1, the change in SBP at week 12 was similar to that at the end of treatment, which suggests that the duration of treatment is unlikely to influence the change in SBP. Owing to the slight differences in baseline characteristics among the six trials, it is not possible to examine the impact of co-administration of other oral antidiabetic drugs with ipragliflozin on the reduction in SBP. Finally, antihypertensive drugs used in both the ipragliflozin group and the placebo group during the study were changed at the discretion of the attending physician and were not recorded. Therefore, we could not rule out the possibility that unreported changes in the types of antihypertensive drugs prescribed might have contributed to the changes in BP. Nevertheless, reductions in SBP and DBP were observed in the ipragliflozin group compared with the placebo group in patients treated with or without concomitant antihypertensive drugs, which indicates that the greater reductions in BP in the ipragliflozin group were due to ipragliflozin itself, not the use of antihypertensive drugs.
Despite these limitations, pooled analyses of patient-level data may be more informative than meta-analyses, which incorporate the mean changes in clinical variables and may not fully address the relationship between the baseline value and the change from baseline. Large-scale trials are necessary to confirm the magnitudes of the reductions in BP and whether the reduction in BP is related to specific patient characteristics.
Conclusions
This pooled analysis of six randomized, placebo-controlled trials revealed that ipragliflozin was associated with mild, but clinically relevant reductions in SBP and DBP compared with placebo. The reduction in SBP was correlated with the baseline SBP and was greater in patients with baseline SBP ≥140 mmHg than in patients with a baseline SBP <140 mmHg and other subgroups of patients. The reductions in SBP and DBP were also greater in the ipragliflozin group than in the placebo group in patients treated with or without antihypertensive drugs. Ipragliflozin and other SGLT2 inhibitors may improve BP control through multiple pathways, including osmotic diuresis, weight loss, sodium excretion, and volume contraction; however, more trials are needed to clarify the relative contributions of these factors. Future trials should also address the impact of the reduction in BP on cardiovascular risk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors wish to thank all of the investigators involved in each trial. This study was sponsored by Astellas Pharma Inc., Japan. Medical writing and editorial support was funded by Astellas and provided by Dr. Nicholas D. Smith (Edanz Group Ltd.) and Elsevier/ELMCOM™.
Conflict of interest
AK has acted as a consultant for Astellas Pharma Inc. and has received consulting fees/honoraria from Astellas Pharma Inc. TO has acted as a consultant for Kotobuki Pharmaceutical Co., Ltd., and has received consulting fees/honoraria from Kotobuki Pharmaceutical Co., Ltd. YK is an employee of Kotobuki Pharmaceutical Co., Ltd. The other authors are employees of Astellas Pharma Inc., Japan.
Human rights statement and informed consent
All included studies in this analysis were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients for being included in the studies.
References
- 1.Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes. 2013;6:327–338. doi: 10.2147/DMSO.S51325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministry of Health, Labour, and Welfare, Japan. Patient Survey 2011 (Disease and Injury). Available at: http://www.mhlw.go.jp/toukei/saikin/hw/kanja/10syoubyo/dl/h23syobyo.pdf. Last accessed, April 2015. [In Japanese].
- 3.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 4.Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014) Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2013.80. [DOI] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–13. doi: 10.1136/bmj.317.7160.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521–525. doi: 10.1291/hypres.26.521. [DOI] [PubMed] [Google Scholar]
- 7.Agabiti-Rosei E. From macro- to microcirculation: benefits in hypertension and diabetes. J Hypertens Suppl. 2008;26:S15–S19. doi: 10.1097/01.hjh.0000334602.71005.52. [DOI] [PubMed] [Google Scholar]
- 8.Brillante DG, O’Sullivan AJ, Howes LG. Arterial stiffness in insulin resistance: the role of nitric oxide and angiotensin II receptors. Vasc Health Risk Manag. 2009;5:73–78. [PMC free article] [PubMed] [Google Scholar]
- 9.Kozakova M, Morizzo C, Bianchi C, Di Filippi M, Miccoli R, Paterni M, et al. Glucose-related arterial stiffness and carotid artery remodeling: a study in normal subjects and type 2 diabetes patients. J Clin Endocrinol Metab. 2014;99:E2362–E2366. doi: 10.1210/jc.2014-2028. [DOI] [PubMed] [Google Scholar]
- 10.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Relation between insulin and aortic stiffness: a population-based study. J Hum Hypertens. 2004;18:1–7. doi: 10.1038/sj.jhh.1001620. [DOI] [PubMed] [Google Scholar]
- 11.Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–1277. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- 12.Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, Pongratz RL, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic β-cell function. Diabetes. 2011;60:890–898. doi: 10.2337/db10-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Investig. 1994;93:397–404. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens. 2014;8:330–339. doi: 10.1016/j.jash.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5:382–391. doi: 10.1111/jdi.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashiwagi A, Takahashi H, Ishikawa H, Yoshida S, Kazuta K, Utsuno A, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–160. doi: 10.1111/dom.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashiwagi A, Kazuta K, Takinami Y, Yoshida S, Utsuno A, Nagase I. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int. 2014;6:8–18. doi: 10.1007/s13340-014-0164-0. [DOI] [Google Scholar]
- 20.Kashiwagi A, Kazuta K, Goto K, Yoshida S, Ueyama E, Utsuno A. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2014;17:304–308. doi: 10.1111/dom.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashiwagi A, Shiga T, Akiyama N, Kazuta K, Utsuno A, Yoshida S, et al. Efficacy and safety of ipragliflozin as an add-on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study (the SPOTLIGHT study) Diabetol Int. 2014;6:104–116. doi: 10.1007/s13340-014-0182-y. [DOI] [Google Scholar]
- 22.Kashiwagi A, Akiyama N, Shiga T, Kazuta K, Utsuno A, Yoshida S, et al. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetol Int. 2014;6:125–138. doi: 10.1007/s13340-014-0184-9. [DOI] [Google Scholar]
- 23.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(262–75):e9. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Imprialos KP, Sarafidis PA, Karagiannis AI. Sodium-glucose cotransporter-2 inhibitors and blood pressure decrease: a valuable effect of a novel antidiabetic class? J Hypertens. 2015;33:2185–2197. doi: 10.1097/HJH.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair A, Bode B, Harris S, Vijapurkar U, Mayer C, Fung A, et al. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. BMC Endocr Disord. 2014;14:37. doi: 10.1186/1472-6823-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liakos A, Karagiannis T, Athanasiadou E, Sarigianni M, Mainou M, Papatheodorou K, et al. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:984–993. doi: 10.1111/dom.12307. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Zhang L, Wu B, Song H, An Z, Li S. Dapagliflozin treatment for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2014;30:204–221. doi: 10.1002/dmrr.2479. [DOI] [PubMed] [Google Scholar]
- 28.Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diabetes Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens. 2015;9:48–53. doi: 10.1016/j.jash.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Majewski C, Bakris GL. Blood pressure reduction: an added benefit of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes. Diabetes Care. 2015;38:429–430. doi: 10.2337/dc14-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, et al. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16:1087–1095. doi: 10.1111/dom.12322. [DOI] [PubMed] [Google Scholar]
- 32.Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 33.Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, et al. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. doi: 10.1186/s12933-014-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. doi: 10.1186/1475-2840-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilding JP, Ferrannini E, Fonseca VA, Wilpshaar W, Dhanjal P, Houzer A. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes Obes Metab. 2013;15:403–409. doi: 10.1111/dom.12038. [DOI] [PubMed] [Google Scholar]
- 36.Sufiun A, Rafiq K, Fujisawa Y, Rahman A, Mori H, Nakano D, et al. Effect of dipeptidyl peptidase-4 inhibition on circadian blood pressure during the development of salt-dependent hypertension in rats. Hypertens Res. 2015;38:237–243. doi: 10.1038/hr.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.