Abstract
We recently reported that vitamin D deficiency aggravates diabetic bone loss in mice. Although vitamin D affects both muscle and bone, the role of the vitamin D state in diabetic muscle loss and muscle-bone relationships remains unclear. In the present study, we examined the effects of vitamin D deficiency on muscle mass, muscle differentiation and muscle-derived humoral factors linking muscle to bone in diabetic female mice. Diabetes was induced in mice by streptozotocin (STZ) injection after feeding with a normal or vitamin D-deficient diet for 6 weeks. Quantitative computed tomography analysis showed that tibial muscle mass was significantly decreased in diabetic mice compared with control mice 4 weeks after induction of diabetes. Vitamin D deficiency accelerated muscle loss in diabetic mice. Vitamin D deficiency augmented the decreases in Pax7 mRNA levels and the increases in muscle RING-Finger Protein-1 and atrogin-1 mRNA levels induced by diabetes in the gastrocnemius muscle of mice. Moreover, vitamin D deficiency decreased the mRNA levels of insulin-like growth factor-1, fibroblast growth factor-2 and osteoglycin in muscle of diabetic mice. In conclusion, we demonstrated that vitamin D deficiency aggravates muscle loss induced by diabetes in female mice. Vitamin D may exert significant effects on the maintenance of the musculoskeletal system partly through the muscle-bone relationships in diabetic state.
Keywords: Vitamin D, Diabetes, Muscle wasting, Myoblast
Introduction
Diabetes induces numerous complications, such as peripheral vascular disorders, renal dysfunction, retinopathy and neuropathy [1]. In addition to these complications, diabetic state affects the musculoskeletal system, such as muscle and bone [2]. Several epidemiological studies revealed that fracture risk is increased in patients with type 1 and type 2 diabetes [3, 4]. Muscle wasting is one of serious complications of diabetic patients [5–8], since it might lead to an increase in falling and subsequent fracture risk, a decrease in quality life and an increase in risk of death. Muscle fiber atrophy without morphological indications of neuropathy is observed in patients with type 1 diabetes [9], suggesting that the type 1 diabetic state affects skeletal muscle through the metabolic changes prior to neuropathic complications. Russell et al. showed that a high glucose environment induces elevated protein degradation and impaired protein synthesis in myotubes in vitro [10], suggesting that hyperglycemia is a salient factor that exerts direct deleterious effects on muscle. It has been suggested that diabetic muscle wasting is caused by the impairment of muscle differentiation and the imbalance between protein synthesis and degradation induced by the metabolic changes, including hyperglycemia, decreased insulin action, increased advanced glycation end products (AGE)/oxidative stress and neuropathy [5]. However, the mechanisms of muscle wasting and osteoporosis induced by diabetes are not fully understood.
The prevalence of vitamin D insufficiency or deficiency is high in patients with diabetes [10–12]. Numerous reports have suggested that vitamin D deficiency is related to the increases in bone loss and fracture risk [13–15]. In addition, vitamin D deficiency elevates fall risk partly through muscle wasting [16, 17]. Several studies revealed that vitamin D enhances muscle differentiation and inhibits the degradation of muscle in vitro and in vivo [18, 19]. These findings suggest that vitamin D is important in the maintenance of muscle as well as bone metabolism. Our recent study demonstrated that vitamin D deficiency aggravates bone loss induced by diabetes in female mice [20]. However, the influences of vitamin D deficiency on diabetic muscle wasting and muscle/bone relationships remain unknown. In the present study, we therefore examined the effects of vitamin D deficiency on muscle mass, muscle differentiation and muscle-derived humoral factors linking muscle to bone in diabetic female mice.
Methods
Materials
Streptozotocin was purchased from Sigma-Aldrich Japan (Tokyo, Japan). Normal diet (AIN93G) and vitamin D-deficient diet (AIN93GA-2) were purchased from Oriental Yeast Co., Ltd. (Tokyo, Japan). All other chemicals used were of analytical grade.
Animal experiments
Female C57BL/6J mice were fed with a normal diet or vitamin D-deficient diet from 4 weeks of age, as previously described [20]. Diabetes was induced in those mice at age of 10 weeks by daily intraperitoneal injections of STZ (50 mg/kg body weight in saline), a pancreatic β-cell cytotoxin, for 4 days, as previously described [20]. Controls were injected with saline alone. Four days after the last injection (day 4), the nonfasting blood glucose level was measured with a glucometer (Glutest Ace, Sanwa Kagaku Kenkyusyo, Nagoya, Japan) by using blood obtained from the tail vein. Mice with blood glucose levels greater than 300 mg/dl were considered diabetic. Blood glucose levels were confirmed at least twice. Animals were maintained in metabolic cages on a 12-h light, 12-h dark cycle, and they received food and water ad libitum. At 4 weeks after induction of diabetes, quantitative computed tomography (qCT) analysis was performed to measure tibial muscle mass. All animal experiments were performed according to the guidelines of the National Institute of Health (NIH) and the institutional rules for the use and care of laboratory animals of Kindai University.
In vivo quantitative computed tomography (qCT) analysis
Mice were scanned using a LaTheta (LCT-200) experimental animal CT system (Hitachi-Aloka Medical, Tokyo, Japan), as previously described [20]. Briefly, regions of interest (ROIs) were defined as 9600-μm segments (100 slices) from the distal end of the proximal growth plate of the tibia for assessment of the muscle mass around the tibia. Parameters used for the CT scans were as follows: tube voltage, 50 kVp; tube current, 500 mA; integration time, 3.6 ms; axial field of view, 48 mm; voxel size of 48 × 96 μm with a slice thickness of 96 μm. Muscle mass was analyzed using the LaTheta software (version 3.40).
Preparation of AGEs
Advanced glycation end products 3 (AGE3) and nonglycated bovine serum albumin (BSA) were prepared as previously described [21].
Cell culture
Mouse myoblastic C2C12 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Wako Pure Chemical Industry, Ltd., Osaka, Japan) with 10 % fetal bovine serum (FBS) and 1 % penicillin streptomycin. Twenty four hours after incubation with 200 μg/ml AGE3 or BSA, cells were treated with 1,25-dihydroxyvitamin D3 [1,25 (OH)2D3] (Sigma Aldrich Japan, Tokyo, Japan) for 24 h.
Real-time polymerase chain reaction (PCR) analysis
Total RNA was extracted from a homogenized sample of gastrocnemius muscle tissue and the cultured cells using RNeasy mini kit fibrous tissue (Life Technologies Japan, Tokyo, Japan). Real-time PCR was performed using StepOne Plus and the Fast SYBR Green PCR Master mix (Life Technologies Japan, Tokyo, Japan) as previously described [20]. The primer sets are shown as follows: Pax7; forward: 5′-CCCTCAGTGAGTTCGATTAGCC-3′, reverse: 5′-GGTCGGGTTCTGATTCCACA-3′, MyoD; forward: 5′-AGCACTACAGTGGCGACTCAG-3′, reverse: 5′-AGGCGGTGTCGTAGCCATTC-3′, Myf6; forward: 5′-ATGGTACCCTATCCCCTTGC-3′, reverse: 5′-TAGCTGCTTTCCGACGATCT-3′, myogenin; forward: 5′-GCTGCCTAAAGTGGAGATCCT-3′, reverse: 5′-GCGCTGTGGGAGTTGCAT-3′, atrogin-1; forward: 5′-GCAGAGAGTCGGCAAGTC-3′, reverse: 5′-CAGGTCGGTGATCGTGAG-3′, muscle RING-Finger Protein-1 (MuRF-1); forward: 5′-TGTGCAAGGAACACGAAG-3′, reverse, 5′-TGAGAGATGATCGTCTGC-3′, insulin-like growth factor-1 (IGF-1); forward: 5′-CAAGCCCACAGGCTATGGC-3′, reverse: 5′-TCTGAGTCTTGGGCATGTCAG-3′, fibroblat growth factor-2 (FGF-2); forward: 5′-GCGACCCACACGTCAAACTA-3′, reverse: 5′-CCGTCCATCTTCCTTCATAGC-3′, transforming growth factor-β (TGF-β); forward: 5′-CCTCTGTCACCTGCTCAACA-3′, reverse: 5′-GATGAATTGGCGTGGAATCT-3′, irisin; forward: 5′-TCATTGTTGTGGTCCTCTTC-3′, reverse: 5′-GCTCGTTGTCCTTGATGATA-3′, osteoglycin; forward: 5′-TGCTTTGTGGTCACATGGAT-3′, reverse: 5′-GAAGCTGCACACAGCACAAT-3′, myostatin; forward: 5′-CTGTAACCTTCCCAGGACCA-3′, reverse: 5′-TCTTTTGGGTGCGATAATCC-3′, β-actin; forward: 5′-TACCACAGGCATTGTGATGG-3′, reverse: 5′-TTTGATGTCACGCACGATTT-3′. The mRNA levels in the tissues of mice and in the cultured cells were normalized relative to the amount of β-actin and GAPDH mRNA, respectively.
Statistical analysis
Data were expressed as mean ± SEM. Statistical significance was assessed using one-way ANOVA followed by Tukey-Kramer post hoc tests. Statistical values at p < 0.05 were considered significant. All statistical analysis were performed using StatView version 5.0 software (SAS Institute; Cary, NC, USA).
Results
Influences of diabetic state and vitamin D deficiency on muscle mass
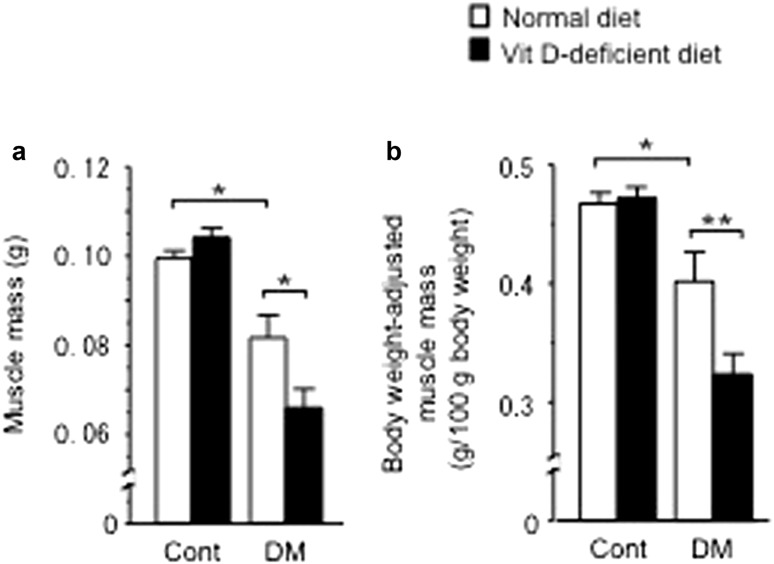
STZ treatment decreased the body weight as previously described [20] (body weight of 4 weeks after diabetes induction: control with normal diet, 21.1 ± 0.3 g; control with vitamin D-deficient diet, 22.1 ± 0.2 g; STZ with normal diet 17.0 ± 0.8 g*, STZ with vitamin D-deficient diet, 17.0 ± 0.6 g**; *p < 0.01 vs. control mice fed with normal diet. **p < 0.01 vs. control mice fed with vitamin D-deficient diet). STZ treatment equally increased the levels of blood glucose in female mice fed with a normal and vitamin D-deficient diet (blood glucose levels: control with normal diet, 145.5 ± 8.6 mg/dl; control with vitamin D-deficient diet, 140.2 ± 9.8 mg/dl; STZ with normal diet 363.8 ± 17.6 mg/dl*, STZ with vitamin D-deficient diet, 366.1 ± 30.2 mg/dl**; *p < 0.01 vs. control mice fed with normal diet; **p < 0.01 vs. control mice fed with vitamin D-deficient diet), although vitamin D-deficient diet feeding markedly reduced the levels of serum 25-hydroxyvitamin D (25OHD) in both control and diabetic mice as previously described [20]. In the present study, STZ treatment significantly decreased muscle mass as well as body weight-adjusted muscle mass in female mice compared with control (Fig. 1a, b). Vitamin D deficiency aggravated muscle mass decreased by STZ treatment, whereas vitamin D deficiency itself did not affect muscle mass in control mice (Fig. 1a, b).
Fig. 1.
Influences of diabetic state and vitamin D deficiency on muscle mass (a) and body weight-adjusted muscle mass (b) in female mice. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, n = 5 in each group. Cont control, DM diabetes, VitD vitamin D
Influences of diabetic state and vitamin D deficiency on muscle differentiation and degradation
We examined the levels of the genes related to muscle differentiation and degradation in the gastrocnemius muscle of female mice. STZ treatment seemed to decrease, but not significantly, the mRNA levels of Pax7, a marker for muscle satellite cells, and MyoD, a master transcription factor for muscle differentiation, in muscle of mice fed with normal diet, whereas STZ treatment did not affect the mRNA levels of Myf6 and myogenin, myogenic genes, in muscle (Fig. 2a–d). Vitamin D deficiency aggravated the levels of Pax7 and MyoD mRNA decreased by STZ treatment, although its effects on MyoD mRNA were not statistically significant (Fig. 2a–d). We next examined the mRNA levels of muscle-specific E3 ubiquitin ligase, such as MuRF-1 and atrogin-1, in the gastrocnemius muscle of mice to clarify the influences of vitamin D deficiency on muscle protein degradation in diabetic mice. STZ treatment seemed to increase, but not significantly, the levels of MuRF-1 and atrogin-1 mRNA in muscle compared with control mice (Fig. 2e, f). Vitamin D deficiency augmented the mRNA levels of MuRF-1 and atrogin-1 enhanced by STZ treatment in muscle, whereas vitamin D deficiency did not affect the levels of these genes in non-diabetic mice (Fig. 2e, f).
Fig. 2.
Influences of diabetic state and vitamin D deficiency on the mRNA levels of myogenic genes, such as Pax7 (a), MyoD (b), myogenin (c) and Myf6 (d) and atrogenes such as Atrogin-1 (e) and MuRF-1 (f) in the gastrocnemius muscle of female mice. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, n = 5 in each group. Cont control, DM diabetes, VitD vitamin D
Influence of AGEs and vitamin D on muscle differentiation and degradation in myoblasts
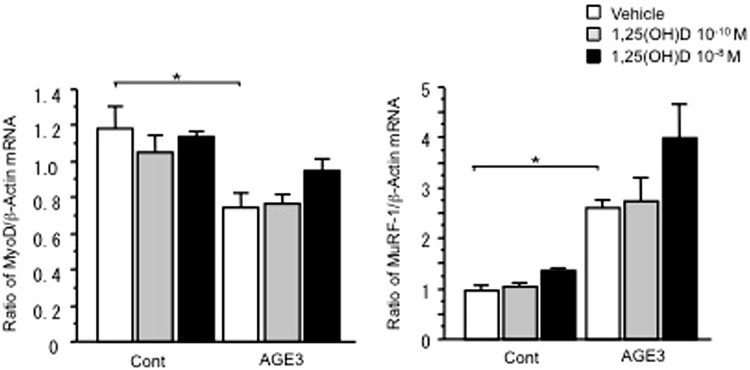
AGE3 treatment significantly decreased the levels of MyoD mRNA in C2C12 myoblasts, whereas 1,25 (OH)2D3 seemed to blunt the levels of MyoD mRNA decreased by AGE3 in C2C12 myoblasts (Fig. 3a). On the other hand, AGE3 treatment significantly increased the levels of MuRF-1 mRNA in C2C12 myoblasts, although 1,25 (OH)2D3 did not affect the levels of MuRF-1 mRNA in C2C12 myoblasts (Fig. 3b).
Fig. 3.
Influences of AGE3 and vitamin D on the mRNA levels of MyoD (a) and MuRF-1 (b) in C2C12 myoblasts. Subconfluent C2C12 cells pretreated with 200 μg/ml BSA (Cont) or AGE3 were cultured with or without the indicated concentrations of 1,25(OH)2D3, then total RNA was extracted. Real-time PCR of MyoD, MuRF-1 and GAPDH mRNA was performed. Data are expressed as mean ± SEM. *p < 0.05, n = 3 in each group
Influences of diabetic state and vitamin D deficiency on the mRNA levels of muscle-derived humoral factors in muscle
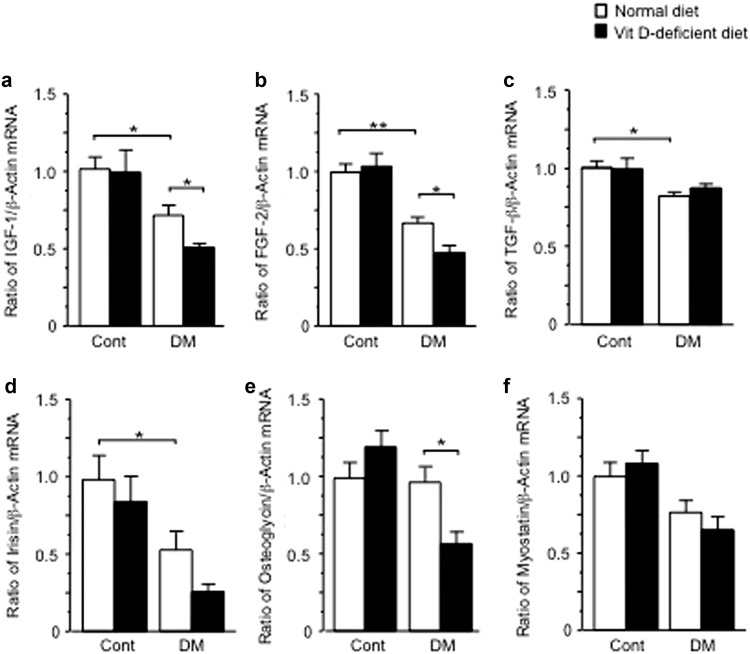
IGF-1, FGF-2, TGF-β, irisin, osteoglycin and myostatin are included in putative muscle-derived humoral factors linking muscle to bone [22]. We examined the mRNA levels of these factors in the gastrocnemius muscle of female mice. STZ treatment decreased the mRNA levels of IGF-1, FGF-2, TGF-β and irisin in muscle compared with control (Fig. 4a–d). Vitamin D deficiency aggravated the levels of IGF-1 and FGF-2, but not TGF-β, decreased by STZ treatment in muscle (Fig. 4a–d). Vitamin D deficiency significantly decreased the levels of osteoglycin mRNA in muscle of diabetic mice, although STZ treatment did not affect them (Fig. 4e). Neither STZ treatment nor vitamin D deficiency affected the mRNA levels of myostatin, a muscle-mass suppressor in muscle (Fig. 4f).
Fig. 4.
Influences of diabetic state and vitamin D deficiency on the mRNA levels of IGF-1 (a), FGF-2 (b), TGF-β (c), irisin (d), osteoglycin (e) and myostatin (f) in the gastrocnemius muscle of female mice. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, n = 5 in each group. Cont control, DM diabetes, VitD vitamin D
Discussion
In the present study, vitamin D deficiency aggravated muscle loss induced by diabetic state without affecting the levels of blood glucose in mice. This finding suggests that vitamin D deficiency affects skeletal muscle independently of glucose metabolism abnormality in diabetic state. The impaired muscle differentiation is associated with muscle wasting induced by several pathological conditions, such as diabetes and aging [23]. Muscle differentiation is initiated from the activation of muscle stem cells, called satellite cells; they differentiate into myoblasts and consequently form myotubes [24]. A previous study suggests that diabetic state impairs satellite cell function and muscle differentiation [25]. It has been also reported that vitamin D deficiency impairs muscle differentiation in rats in vivo [19]. Moreover, active vitamin D enhances the differentiation and tube formation via vitamin D receptor in C2C12 myoblasts in vitro [26, 27]. In the present study, we revealed that vitamin D deficiency aggravates the decrease in Pax7 levels induced by diabetic state in the gastrocnemius muscle of mice, whereas neither diabetic state nor vitamin D deficiency affected the mRNA levels of myogenin and Myf6 (myogenic markers at more differentiated stage). These data suggest that vitamin D deficiency aggravates muscle differentiation decreased by diabetic state by acting at an early differentiation stage in mice.
Enhancement of protein degradation is considered to be one of the underlying mechanisms of diabetic muscle wasting [28]. Although protein degradation includes three pathways—the ubiquitin proteasome pathway, the lysosomal pathway and the cytosolic calcium-activated pathway—it has been suggested that the ubiquitin-proteasome pathway is the principal mechanism of protein degradation in skeletal muscle [29, 30]. The process of protein ubiquitination for proteasomal degradation comprises three steps as follows: (1) activation of ubiquitin at its C-terminus by an E1 ubiquitin-activating enzyme, (2) conjugation of ubiquitin to an E2 ubiquitin-conjugating enzyme and (3) transfer of ubiquitin to the substrate protein by an E3 ubiquitin ligase [31]. Atrogin-1 and MuRF-1 are muscle-specific E3 ubiquitin ligases, which are crucial in the development of muscle wasting [32, 33]. Lambertucci et al. reported that STZ treatment enhances the expression of MuRF-1 and atrogin-1 as well as protein degradation in muscle of rats [34]. Moreover, a recent animal study suggests that vitamin D deficiency accelerates protein degradation through the ubiquitin-proteasome system in muscle of rats [19]. In the present study, we revealed that vitamin D deficiency augments the increases in mRNA levels of atrogin-1 and MuRF-1 induced by diabetic state in the gastrocnemius muscle of mice. These data suggest that vitamin D deficiency accelerates protein degradation through the activation of the ubiquitin-proteasome pathway in muscle of diabetic mice. Taken together, vitamin D may protect against muscle wasting through the inhibition of muscle degradation as well as the enhancement of early stage muscle differentiation in diabetic mice.
In the present study, STZ treatment significantly decreased muscle mass at the tibial region in mice, although STZ treatment did not affect the levels of the genes related to muscle differentiation or degradation significantly in the gastrocnemius muscle of these mice. We speculated that the additive effects of STZ treatment on early muscle differentiation and muscle degradation might induce a decrease in muscle mass, since STZ treatment seemed to decrease and increase the levels of Pax7/MyoD mRNA and the levels of MuRF-1/atrogin-1, respectively, without statistical significance. Alternatively, this discrepancy might be due to the differences of muscle sites of interest between qCT and mRNA analysis.
Chiu et al. recently reported that AGEs are associated with diabetic muscle wasting through the enhancement of muscle degradation and impairment of muscle differentiation in vivo and in vitro [35]. In the present study, we showed that AGE3 significantly decreased the levels of MyoD mRNA and increased the levels of MuRF-1 mRNA in myoblasts. Moreover, we found that active vitamin D seemed to increase the levels of MyoD mRNA decreased by AGE3, whereas it did not affect the levels of MuRF-1 mRNA increased by AGE3 in myoblasts. In addition, we previously showed that active vitamin D blunts MyoD protein level decreased by AGEs in myoblasts [21]. These findings suggest that vitamin D might improve diabetic muscle wasting partly through the amelioration of myogenesis impaired by AGEs. On the other hand, vitamin D deficiency-enhanced levels of muscle degradation-related genes in diabetes state in vivo might be due to factors such as oxidative stress and inflammatory cytokines, but not AGEs [5].
It is well known that vitamin D affects both muscle and bone [36]. Our previous study showed that vitamin D deficiency aggravates bone loss induced by diabetic state in mice [20]. Recent evidence suggested that muscle/bone relationships are related to the pathological disorders of muscle and bone [22, 37]. We previously revealed that osteoglycin and FAM5c might be included in muscle-derived humoral factors linking muscle to bone [38, 39]. Moreover, muscle tissues secrete several positive regulators of osteogenesis, such as IGF-1, FGF-2, TGF-β and irisin [22]. These findings suggest that those muscle-derived humoral factors might be related to the influences of vitamin D and diabetic state on muscle and bone. In the present study, we showed that vitamin D deficiency aggravates the mRNA levels of IGF-1 and FGF-2 decreased by diabetic state in the gastrocnemius muscle of mice. These data are consistent with our evidence about the changes in bone mass induced by diabetic state and vitamin D deficiency in mice [20]. Moreover, vitamin D deficiency significantly decreased the levels of osteoglycin mRNA in muscle tissues in diabetic mice, which were compatible with our previous results that active vitamin D and its analog treatment increased the expression of osteoglycin in myoblasts in vitro [21]. Taken together, vitamin D deficiency might enhance diabetic bone loss through the impaired secretion of muscle-derived humoral factors linking muscle to bone. Further studies will be necessary to clarify the influences of vitamin D and diabetic state on muscle-bone relationships in detail.
Our present and previous [20] studies showed that vitamin D deficiency affects muscle and bone in diabetic state but not in normal condition in mice. These findings suggest that vitamin D may exert significant effects on the maintenance of the musculoskeletal system in pathological conditions such as diabetes rather than normal conditions. Vitamin D supplementation induced reductions in the fall risk and fracture risk as well as increases in muscle mass and function in elderly patients with a vitamin D-deficient state [18]. Ito et al. recently reported that active vitamin D analog treatment increases muscle mass in elderly patients with severe osteoporosis [40]. Therefore, vitamin D treatment may be useful for the prevention and treatment of muscle wasting as well as osteoporosis in diabetic patients.
In conclusion, the present study demonstrated that vitamin D deficiency aggravates diabetic muscle wasting partly through the decreases in Pax7 mRNA levels and the increases in MuRF1 and atrogin-1 in female mice. Vitamin D deficiency and diabetic state might affect muscle and bone through the modulation of muscle-derived humoral factors linking muscle to bone. The precise mechanisms of diabetes- and/or vitamin D deficiency-induced muscle wasting still remain unknown in the present study. This is the major limitation of this study, and further studies are necessary to clarify these mechanisms.
Acknowledgments
This study was supported by Grants-in-aid 26860152 and 15K08220 from the Ministry of Science, Education and Culture of Japan (to Y.T. and to H.K., respectively) and a grant from The Nakatomi Foundation.
Conflict of interest
None.
Ethical standards
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Ahlqvist E, van Zuydam NR, Groop LC, McCarthy MI. The genetics of diabetic complications. Nat Rev Nephrol. 2015;11:277–287. doi: 10.1038/nrneph.2015.37. [DOI] [PubMed] [Google Scholar]
- 2.Saller A, Maggi S, Romanato G, Tonin P, Crepaldi G. Diabetes and osteoporosis. Aging Clin Exp Res. 2008;20:280–289. doi: 10.1007/BF03324857. [DOI] [PubMed] [Google Scholar]
- 3.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 4.Merlotti D, Gennari L, Dotta F, Lauro D, Nuti R. Mechanisms of impaired bone strength in type 1 and 2 diabetes. Nutr Metab Cardiovasc Dis. 2010;20:683–690. doi: 10.1016/j.numecd.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Krause MP, Riddell MC, Hawke TJ. Effects of type 1 diabetes mellitus on skeletal muscle: clinical observations and physiological mechanisms. Pediatr Diabetes. 2011;12:345–364. doi: 10.1111/j.1399-5448.2010.00699.x. [DOI] [PubMed] [Google Scholar]
- 6.Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 8.Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813–1818. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 9.Reske-Nielsen E, Harmsen A, Vorre P. Ultrastructure of muscle biopsies in recent, short-term and long-term juvenile diabetes. Acta Neurol Scand. 1977;55:345–362. doi: 10.1111/j.1600-0404.1977.tb05654.x. [DOI] [PubMed] [Google Scholar]
- 10.Russell ST, Rajani S, Dhadda RS, Tisdale MJ. Mechanism of induction of muscle protein loss by hyperglycaemia. Exp Cell Res. 2009;315:16–25. doi: 10.1016/j.yexcr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 12.Svoren BM, Volkening LK, Wood JR, Laffel LM. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J Pediatr. 2009;154:132–134. doi: 10.1016/j.jpeds.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff AE, Jones AN, Hansen KE. Vitamin D and musculoskeletal health. Nat Clin Pract Rheumatol. 2008;4:580–588. doi: 10.1038/ncprheum0921. [DOI] [PubMed] [Google Scholar]
- 14.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 15.Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25:585–591. doi: 10.1016/j.beem.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Dawson-Hughes B. Serum 25-hydroxyvitamin D and muscle atrophy in the elderly. Proc Nutr Soc. 2012;71:46–49. doi: 10.1017/S0029665111003260. [DOI] [PubMed] [Google Scholar]
- 17.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99:4336–4345. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 18.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34:33–83. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 19.Bhat M, Kalam R, Qadri SS, Madabushi S, Ismail A. Vitamin D deficiency-induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats. Endocrinology. 2013;154:4018–4029. doi: 10.1210/en.2013-1369. [DOI] [PubMed] [Google Scholar]
- 20.Mao L, Tamura Y, Kawao N, Okada K, Yano M, Okumoto K, et al. Influence of diabetic state and vitamin D deficiency on bone repair in female mice. Bone. 2014;61:102–108. doi: 10.1016/j.bone.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, Kanazawa I, Yamaguchi T, Yano S, Kaji H, Sugimoto T. Active vitamin D possesses beneficial effects on the interaction between muscle and bone. Biochem Biophys Res Commun. 2014;450:482–487. doi: 10.1016/j.bbrc.2014.05.145. [DOI] [PubMed] [Google Scholar]
- 22.Kawao N, Kaji H. Interactions between muscle tissues and bone metabolism. J Cell Biochem. 2015;116:687–695. doi: 10.1002/jcb.25040. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 24.Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 25.D’Souza DM, Al-Sajee D, Hawke TJ. Diabetic myopathy: impact of diabetes mellitus on skeletal muscle progenitor cells. Front Physiol. 2013;4:379. doi: 10.3389/fphys.2013.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology. 2011;152:2976–2986. doi: 10.1210/en.2011-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girgis CM, Clifton-Bligh RJ, Mokbel N, Cheng K, Gunton JE. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology. 2014;155:347–357. doi: 10.1210/en.2013-1205. [DOI] [PubMed] [Google Scholar]
- 28.Workeneh B, Bajaj M. The regulation of muscle protein turnover in diabetes. Int J Biochem Cell Biol. 2013;45:2239–2244. doi: 10.1016/j.biocel.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–165. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- 30.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- 31.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 32.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 33.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambertucci AC, Lambertucci RH, Hirabara SM, Curi R, Moriscot AS, Alba-Loureiro TC, et al. Glutamine supplementation stimulates protein-synthetic and inhibits protein-degradative signaling pathways in skeletal muscle of diabetic rats. PLoS ONE. 2012;7:e50390. doi: 10.1371/journal.pone.0050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu CY, Yang RS, Sheu ML, Chan DC, Yang TH, Tsai KS, et al. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J Pathol. 2016;238:470–482. doi: 10.1002/path.4674. [DOI] [PubMed] [Google Scholar]
- 36.Sanders KM, Scott D, Ebeling PR. Vitamin D deficiency and its role in muscle-bone interactions in the elderly. Curr Osteoporos Rep. 2014;12:74–81. doi: 10.1007/s11914-014-0193-4. [DOI] [PubMed] [Google Scholar]
- 37.Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272–277. doi: 10.1097/MCO.0b013e32835fe6a5. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K, Matsumoto E, Higashimaki Y, Katagiri T, Sugimoto T, Seino S, et al. Role of osteoglycin in the linkage between muscle and bone. J Biol Chem. 2012;287:11616–11628. doi: 10.1074/jbc.M111.292193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka K, Matsumoto E, Higashimaki Y, Sugimoto T, Seino S, Kaji H. FAM5C is a soluble osteoblast differentiation factor linking muscle to bone. Biochem Biophys Res Commun. 2012;418:134–139. doi: 10.1016/j.bbrc.2011.12.147. [DOI] [PubMed] [Google Scholar]
- 40.Ito S, Harada A, Kasai T, Sakai Y, Takemura M, Matsui Y, et al. Use of alfacalcidol in osteoporotic patients with low muscle mass might increase muscle mass: an investigation using a patient database. Geriatr Gerontol Int. 2014;14(Suppl 1):122–128. doi: 10.1111/ggi.12222. [DOI] [PubMed] [Google Scholar]