Abstract
Type 2 diabetes is a typical multifactorial disease, but the causes can largely be divided into genetic and environmental factors. In recent years, focus has shifted to the interaction between these factors (i.e., gene–environment interactions). It has become widely known that changes in the intrauterine environment such as intrauterine growth restriction result in gene expression changes in various tissues, which ultimately lead to the onset of diabetes. Epigenetic modification is considered to be a particularly important mechanism in these effects, as it is easily affected by environmental changes that occur during the fetal and neonatal periods. Moreover, recent reports have revealed that epigenetic modifications are passed down through generations. Although genome-wide association studies have identified many type 2 diabetes susceptibility genes, these genes do not pose a significantly high risk when isolated as single factors. In particular, it has been suggested that the interaction of the FTO or KCNQ1 genes with environmental factors increases the incidence of diabetes. These findings suggest that detailed analyses of individual gene–environment interactions hold promise for gaining new insight into the mechanisms and risk factors contributing to type 2 diabetes, with application to personalized diagnoses and treatments. We look forward to future developments in this regard.
Keywords: Development origins of health and disease, Intrauterine growth restriction, Epigenetics, Genome-wide association study, Gene–environment interaction
Introduction
The number of type 2 diabetic patients has increased rapidly worldwide in recent decades. As of 2015, there were more than 400 million affected people, and this figure is estimated to reach 600 million by 2040 [1]. In particular, the western Pacific region, which includes East Asia, has seen an explosive increase in diabetic patients. This pattern is generally attributed to dietary changes and decreases in physical activity after World War II. It has been suggested that East Asians have an inherent weakness in insulin secretion when compared to Western populations, and are therefore considered to be genetically prone to pancreatic β-cell dysfunction.
To date, studies using pancreatic β-cell-specific genetically modified mice have revealed various insights into the importance of insulin signaling in pancreatic β-cells. Our group has demonstrated that when phosphoinositide-dependent kinase-1 (PDK1), a component of insulin signaling, is deficient in the pancreatic β-cells of mice, the pancreatic β-cell mass declines markedly, resulting in significant hyperglycemia [2]. In addition, we have reported that mice deficient in tuberous sclerosis complex 2 (TSC2) protein, which acts further downstream in the insulin signaling pathway, exhibit hyperinsulinemia in adolescence due to an increase in pancreatic β-cell mass, whereas degradation of insulin signaling occurs in older age due to autophagy obstruction or negative feedback, leading to hyperglycemia accompanied by the reduction in pancreatic β-cell mass [3–5].
In most laboratories around the world, including our own, diabetes research is focused on studies using genetically modified mice. These studies have uncovered the numerous important roles that different genes play in the onset and progression of type 2 diabetes; nevertheless, it is well known that type 2 diabetes is a multifactorial disorder. It is extremely rare that the disease develops from a single gene abnormality, such as in the case of maturity onset diabetes of the young; instead, it is generally believed that a combination of genetic and environmental factors contributes to the onset of the disease. The rapid rise of type 2 diabetes patients in East Asia is undoubtedly caused by the dual presence of inherent genetic factors and recent environmental changes.
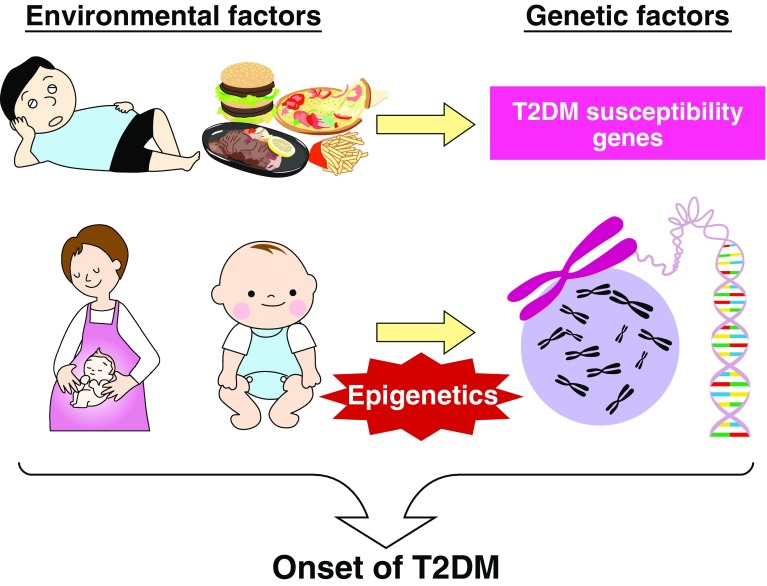
Moreover, it has been recently suggested that not only the additive effects but also synergistic effects of genetic and environmental factors play important roles in disease onset (Fig. 1). In other words, type 2 diabetes is suggested to be more prone to develop through the influence of certain environmental factors on genetic factors than based on the presence of genetic risk factors alone. We expect that further detailed exploration of these gene–environment interactions will lead to a better understanding of the underlying mechanisms contributing to the disease, allowing improvements in the individualization of diet and exercise therapy. Thus, in this review, we present an overview of the gene–environment interactions in type 2 diabetes as revealed from prior studies and data from our own experiments.
Fig. 1.
Model of gene–environment interaction. Environmental factors such as food and exercise have a notable effect on gene expression. The onset of type 2 diabetes occurs when insulin resistance and pancreatic β-cell failure are triggered. Among the environmental factors, excessive or deficient nutrient intake, particularly in the prenatal period and during infancy, have notable effects on the offspring. Epigenetics is considered to be significantly involved in this mechanism. With regard to genetic factors, the type 2 diabetes susceptibility genes identified by genome-wide association studies increase the risk of diabetes through synergistic effects with environmental factors
Phenotypic variations among mouse strains and human ethnicities
As the first example to help illustrate the effects of gene–environment interactions in type 2 diabetes, we will focus on the influence of diet treatments in mouse strains with different genetic backgrounds. Currently, the C57BL/6J mouse strain is the most common model used in experiments on type 2 diabetes and obesity. This model is particularly useful given that C57BL/6J mice are prone to expressing specific phenotypes through induction of a high-fat diet, including obesity. Specifically, C57BL/6J mice do not become obese when raised on a normal chow diet, but exhibit hyperglycemia, hyperinsulinemia, and hyperleptinemia when raised on a high-fat diet [6–8]. Furthermore, the 129/Sv and A/J mice strains are less likely to become obese or diabetic on a high-fat diet [9]. When both C57BL/6J and 129/Sv mice were simultaneously fed a high-fat diet, the 129/Sv mice showed an accelerated rate of thermogenesis and difficulty in displaying insulin resistance. When further comparing the genetic differences between the two strains through a genome-wide scan, a significant difference was revealed in the locus located on chromosome 14, which is associated with insulin resistance [10]. Therefore, we consider that such genetic differences yielded phenotypic differences only under a high-fat diet.
A similar phenomenon has also been observed in humans. Various differences exist between East Asians and Caucasians, such as in the tendency to become obese, energy consumption levels, insulin resistance, and insulin secretion [11–13]. More recently, it has been shown that the regulation of pancreatic β-cells varies among different ethnic groups. Saisho et al. [14] compared the pancreatic β-cell mass between lean and obese groups in healthy Caucasian subjects and found that the obese group had a significant increase in pancreatic β-cells. This effect was considered to be due to a compensatory mechanism for insulin resistance. However, when conducting the same analysis in healthy Japanese subjects, they did not find a similar increase in pancreatic β-cells in the obese group, which actually showed a slight decreased tendency [15]. These results indicate that the compensatory mechanisms of pancreatic β-cells under conditions of insulin resistance such as obesity differ between Japanese and Caucasian subjects. Therefore, it appears that phenotypes can vary according to intrinsic genetic factors despite similar eating habits.
Development origins of health and disease
In 1986, Barker et al. [16] proposed the theory that a low nutritional status during the fetal period is a risk factor for the subsequent development of ischemic heart disease and diabetes in adulthood (Barker hypothesis). This hypothesis is also referred to as the development origins of health and disease, and implies that the environmental conditions during the fetal and neonatal periods have a substantial influence on the future onset of lifestyle-related diseases. It has been reported that children born to mothers who experienced the Dutch famine (“Hunger winter”) during the latter part of World War II exhibited high blood pressure, obesity, and impaired glucose tolerance based on an epidemiological analysis conducted 50 years later [17].
In addition, the mechanisms contributing to this phenomenon are becoming clearer through the use of model animals. Yura et al. [18] established intrauterine growth retardation (IUGR) mice with ligated uterine arteries as an animal model to replicate a low-nutrition condition during the fetal period. Although the IUGR mice were born with a lower body weight compared to control mice, they subsequently underwent a rapid weight increase, thereby eliminating the difference from control mice (catch-up growth). Nevertheless, when a high-fat diet was given to both groups after maturity, the IUGR mice exhibited significant obesity and an impaired glucose tolerance [18]. This is believed to be caused by a “neonatal leptin surge,” when the blood leptin concentration increases in IUGR mice during the neonatal period. This neonatal leptin surge is considered to have contributed to the development of subsequent high-fat diet-induced obesity by causing leptin resistance.
Other reports have confirmed this association between catch-up growth and future obesity in both mice and humans [19, 20], which is now considered to be a very important phenomenon in the development of lifestyle-related diseases. In our lab, we have revealed that during the catch-up growth period, pancreatic β-cells also undergo a catch-up growth phenomenon, similar to the rest of the body, and we suggested the importance of insulin signaling as the primary mechanism [21]. Another study showed that vitamin B12 deficiency in the mother during pregnancy results in the production of offspring with a greater tendency for insulin resistance in adulthood [22]. In addition, providing a low-protein diet to pregnant rats also resulted in insulin resistance in the offspring due to the decline in protein kinase C-zeta expression in the muscles [23, 24].
Recently, excessive lipid intake has been identified as a public health problem in Japan. It was recently reported that offspring of monkeys in which the mothers were fed a high-fat diet showed an increase in the expression of gluconeogenic enzymes and transcription factors in the liver, and consequently exhibited non-alcoholic fatty liver disease [25]. More interestingly, experiments using rats revealed that the female offspring of fathers that were fed a high-fat diet demonstrated pancreatic β-cell dysfunction and impaired glucose tolerance [26]. Moreover, the female offspring from high-fat diet-fed fathers showed changes in the expression of 642 genes in the pancreatic islets when compared to the control group. These changes are considered to have caused pancreatic β-cell dysfunction.
Collectively, the results of the experiments describe above clearly demonstrate an influence of the environmental conditions during the fetal and neonatal periods on genetic factors that lead to type 2 diabetes and obesity. The most important and notable molecular mechanism driving these effects appears to be epigenetics.
Epigenetics
Epigenetics has been recognized as a key molecular mechanism contributing to a variety of diseases, including metabolic diseases, and researchers worldwide are actively engaged in understanding these mechanisms. Epigenetics is defined as a mechanism by which gene expression is influenced through DNA and histone modification without a change in the nucleotide sequence. Since changes in the environment affect gene expression, no discussion on gene–environment interactions can be complete without considering epigenetics. In particular, it is believed that intrauterine environmental changes are more likely to influence epigenetic modification. Indeed, the nutritional status of the fetal period has been found to be recorded in the tissue (i.e., metabolic memory), which can ultimately influence the future onset of disease.
Park et al. [27] established IUGR rats through uterine artery ligation and reported that these rats exhibited pancreatic β-cell dysfunction. Analysis of potential epigenetic modifications of the pancreatic islets of these IUGR rats revealed an increase in DNA methylation and changes in histone modifications in the promoter region of the pancreatic and duodenal homeobox 1 (Pdx1), and the expression levels of Pdx1 decreased. In addition, another study showed that rat offspring born to mothers that were fed a low-protein diet during pregnancy developed type 2 diabetes in adulthood, and showed a decline in the expression of hepatocyte nuclear factor 4-alpha (HNF4α) in the pancreatic islets, which was due to inhibition of the promoter–enhancer interaction at the Hnf4α gene [28]. Moreover, in humans, analysis of the cord blood stem cells from neonates with IUGR demonstrated changes in DNA methylation in a number of genes, including HNF4α [29]. The authors predicted that, regardless of the species and tissue, IUGR significantly affects the expression levels of these transcription factors.
Studies have also been carried out on the effects of IUGR on other insulin target organs. For example, in the skeletal muscle of IUGR rats, the histone modification of the glucose transporter type 4 (Glut4) gene promoter was found to be changed in the direction toward transcriptional repression, contributing to the decreased expression of Glut4 [30]. In addition, Ehara et al. [31] demonstrated that the expression level of the fatty acid synthase GPAT1 in the mouse liver is controlled by DNA methylation in the promoter of the gene. More interestingly, the influence of IUGR on the expression of lipogenic genes in the liver of male offspring (F1 mice) could be passed on to the next generation (F2 mice), with observed changes in the expression level of the liver X receptor alpha (Lxra) gene and DNA methylation in the genetic region [32]. The fact that IUGR can affect not only the first generation but also the second generation suggests a large impact of environmental influence, which implies that the effects of WWII might linger in contemporary populations.
Gene–environment interactions of the fat mass and obesity-associated gene (FTO), a type 2 diabetes susceptibility gene
In recent years, genome-wide association studies (GWAS) have identified many types of type 2 diabetes susceptibility genes, including FTO, which was reported in 2007 [33]. Although the majority of the genes identified through this GWAS were those involved in insulin secretion, the FTO gene was identified as a molecule that is strongly related to obesity [33]. Subsequently, in 2008, it was discovered that harboring the risk allele in the FTO gene could impact the eating habits of children. Specifically, subjects with a single nucleotide polymorphism in the FTO gene tended to consume more greasy, high-calorie meals compared to those with the control group [34]. In addition, recent reports have shown that the relationship between the FTO variant and body mass index becomes stronger with increasing sodium intake [35]. The specific molecular mechanism by which the FTO variant causes obesity had remained unclear. Recent studies revealed that the FTO variant induces a shift from brown fat cells to white fat cells through the increased expression of Iroquois homeobox protein 3 (IRX3), causing fat accumulation [36, 37]. However, the mechanism by which the FTO variant influences food preference is not yet elucidated. These emerging patterns of the influence of genetic factors on environmental factors are very interesting, and we await future research to decipher the specific underlying mechanisms.
Interrelationship between type 2 diabetes susceptibility gene mutations and high-fat diets
Finally, we would like to introduce the insight gained in our recent analyses of the gene–environment interactions of type 2 diabetes. In 2008, the potassium voltage-gated channel subfamily Q member 1 (KCNQ1) gene was identified as a type 2 diabetes susceptibility gene through a large-scale single nucleotide polymorphism analysis targeting Japanese type 2 diabetes patients [38, 39]. Subsequent reports from facilities all over the world confirmed the association between the mutation in the KCNQ1 gene and a decrease in insulin secretion [40–42], but there is insufficient information to link this mechanism to the onset of diabetes. We recently reported that, in mice, the decreased expression of the non-coding RNA Kcnq1ot1, expressed within the Kcnq1 genetic region, triggers the enhanced expression of cyclin-dependent kinase inhibitor 1C (Cdkn1c) through epigenetic modification, and causes the decrease in pancreatic β-cell mass [43]. The Kcnq1 gene is an imprinted gene, and Kcnq1ot1 expression was found to be reduced in the pancreatic β-cells of mice only when the Kcnq1 genetic mutation is inherited from the father. As a result, when Cdkn1c expression is enhanced, the number of pancreatic β-cells decreases. These results may partially clarify the onset mechanism of type 2 diabetes caused by the KCNQ1 genetic mutation. However, the odds ratio of the onset risk due to the KCNQ1 genetic mutation is only about 1.4, indicating that type 2 diabetes does not develop solely from the genetic mutation. Therefore, we focused on the fact that there is a CCAAT sequence in the Cdkn1c promoter region. This CCAAT sequence serves as a binding site for the C/EBP family of transcription factors, and therefore we hypothesized that the binding of C/EBP may further enhance the expression of Cdkn1c. Previously, we found that the expression of C/EBPβ was enhanced in the pancreatic islets of db/db mice, a diabetic mouse model, and that pancreatic β-cells became vulnerable to endoplasmic reticulum stress due to the accumulated C/EBPβ, contributing to pancreatic β-cell dysfunction [44]. In addition, recent studies have confirmed that C/EBPβ also accumulates in the pancreatic islets of mice loaded with a high-fat diet. The accumulation of C/EBPβ from a high-fat diet increases the possibility of pancreatic β-cell dysfunction induced by the enhanced expression of Cdkn1c in addition to endoplasmic reticulum stress, and is considered to be a factor that greatly influences type 2 diabetes onset risk.
Thus, we examined the influences of the accumulation of C/EBPβ in pancreatic β-cells under the state of reduced Kcnq1ot1 expression. A significant increase in fed blood glucose levels and a decrease in serum insulin levels were observed in the mice with decreased Kcnq1ot1 expression and overexpression of C/EBPβ in the pancreatic β-cells in comparison to the control group, which was accompanied by a significant decrease in pancreatic β-cell mass. Moreover, Cdkn1c expression levels in the pancreatic islets were significantly elevated, confirming enhancement of Cdkn1c expression in pancreatic β-cells as the underlying mechanism for these effects. This is in turn presumed to be due to the decrease in Kcnq1ot1 expression, which causes changes in epigenetic modifications such as loosening of the chromatin structure, enabling C/EBPβ to more easily bind to the Cdkn1c promoter (Fig. 2). Further, even when mice with decreased Kcnq1ot1 expression were loaded with a high-fat diet, significant enhancement in the expression levels of C/EBPβ and Cdkn1c was observed in the pancreatic islets, as well as the suppression of the increase in pancreatic β-cells. These findings verified that the synergistic effect of genetic factors (Kcnq1 genetic mutation) and environmental factors (C/EBPβ accumulation) can induce notable pancreatic β-cell dysfunction.
Fig. 2a–b.
Regulation of the expression of the Kcnq1 gene region and the onset mechanism of pancreatic β-cell failure. a Upper: control of imprinting in the Kcnq1 gene region. The non-coding RNA Kcnq1ot1 expressed on the paternal allele controls the expression of neighboring gene clusters. However, since Kcnq1ot1 is not expressed on the maternal allele, expression of the neighboring gene clusters can be seen. Lower: if a decrease in Kcnq1ot1 expression results in the formation of an open chromatin structure, binding with the transcription factor C/EBPβ occurs, subsequently enhancing the expression of Cdkn1c. b. Mechanism of pancreatic β-cell failure following administration of a high-fat diet to mice with decreased Kcnq1ot1 expression
Conclusion
The explosive worldwide increase in the number of type 2 diabetes patients in recent years can largely be attributed to changes in the environment given that there have been no significant or consistent changes in genetic factors. However, changes in environmental factors are likely to have an impact on genetic factors. In the present review, we presented evidence for the relationship between IUGR and type 2 diabetes susceptibility genes. However, it is believed that such gene–environment interactions apply universally to the modern-day pathology of type 2 diabetes patients. Therefore, further detailed analyses of this gene–environment interaction should provide clues for individualized medicine. We look forward to further progress in this field in the future.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research from MEXT (22590981 to YK); grants from Astellas Foundation for Research on Metabolic Disorders and from Novo Nordisk Insulin Awards (to YK); grants from the Japan Diabetes Foundation (to YK).
Conflict of interest
The author has no conflict of interest to declare.
Animal
Animal studies were approved by the animal ethics committee of Kobe University Graduate School of Medicine.
References
- 1.International Diabetes Federation . Diabetes atlas. 7. Brussels: International Diabetes Federation; 2015. [PubMed] [Google Scholar]
- 2.Hashimoto N, Kido Y, Uchida T, Asahara S, Shigeyama Y, Matsuda T, Takeda A, Tsuchihashi D, Nishizawa A, Ogawa W, Fujimoto Y, Okamura H, Arden KC, Herrera PL, Noda T, Kasuga M. Ablation of PDK1 in pancreatic β cells induces diabetes as a result of loss of β-cell mass. Nat Genet. 2006;38:589–593. doi: 10.1038/ng1774. [DOI] [PubMed] [Google Scholar]
- 3.Shigeyama Y, Kobayashi T, Kido Y, Hashimoto N, Asahara S, Matsuda T, Takeda A, Inoue T, Shibutani Y, Koyanagi M, Uchida T, Inoue M, Hino O, Kasuga M, Noda T. Biphasic response of pancreatic β cell mass to ablation of TSC2 in mice. Mol Cell Biol. 2008;28:2971–2979. doi: 10.1128/MCB.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyanagi M, Asahara S, Matsuda T, Hashimoto N, Shigeyama Y, Shibutani Y, Kanno A, Fuchita M, Mikami T, Hosooka T, Inoue H, Matsumoto M, Koike M, Uchiyama Y, Noda T, Seino S, Kasuga M, Kido Y. Ablation of TSC2 enhances insulin secretion by increasing the number of mitochondria through activation of mTORC1. PLoS One. 2011;6:e23238. doi: 10.1371/journal.pone.0023238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartolomé A, Kimura-Koyanagi M, Asahara S, Guillén C, Inoue H, Teruyama K, Shimizu S, Kanno A, García-Aguilar A, Koike M, Uchiyama Y, Benito M, Noda T, Kido Y. Pancreatic β cell failure mediated by mTORC1 hyperactivity and autophagic impairment. Diabetes. 2014;63:2996–3008. doi: 10.2337/db13-0970. [DOI] [PubMed] [Google Scholar]
- 6.Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG. Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6 J mice. Diabetes. 1999;48:1662–1666. doi: 10.2337/diabetes.48.8.1662. [DOI] [PubMed] [Google Scholar]
- 7.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6 J mice. Diabetes. 1998;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 8.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 9.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffé-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6 J and A/J mice. Metabolism. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-X. [DOI] [PubMed] [Google Scholar]
- 10.Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53:3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- 11.Chiu KC, Cohan P, Lee NP, Chuang LM. Insulin sensitivity differs among ethnic groups with a compensatory response in beta-cell function. Diabetes Care. 2000;23:1352–1358. doi: 10.2337/diacare.23.9.1353. [DOI] [PubMed] [Google Scholar]
- 12.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE, American Diabetes Association GENNID Study Group. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the US. Diabetes. 2002;51:2170–2178. doi: 10.2337/diabetes.51.7.2170. [DOI] [PubMed] [Google Scholar]
- 13.Gerstein HC, Anand S, Yi QL, Vuksan V, Lonn E, Teo K, Malmberg K, McQueen M, Yusuf S, SHARE Investigators The relationship between dysglycemia and atherosclerosis in South Asian, Chinese, and European individuals in Canada: a randomly sampled cross-sectional study. Diabetes Care. 2003;26:144–149. doi: 10.2337/diacare.26.1.144. [DOI] [PubMed] [Google Scholar]
- 14.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-Cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36:111–7. [DOI] [PMC free article] [PubMed]
- 15.Kou K, Saisho Y, Satoh S, Yamada T, Itoh H. Change in β-cell mass in Japanese nondiabetic obese individuals. J Clin Endocrinol Metab. 2013;98:3724–3730. doi: 10.1210/jc.2013-1373. [DOI] [PubMed] [Google Scholar]
- 16.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;10:1077–1081. doi: 10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 17.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185:93–98. doi: 10.1016/S0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 18.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1:371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- 20.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194–199. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida Y, Fuchita M, Kimura-Koyanagi M, Kanno A, Matsuda T, Asahara S, Hashimoto N, Isagawa T, Ogawa W, Aburatani H, Noda T, Seino S, Kasuga M, Kido Y. Contribution of insulin signaling to the regulation of pancreatic beta-cell mass during the catch-up growth period in a low birth weight mouse model. Diabetol Int. 2014;5:43–52. doi: 10.1007/s13340-013-0127-x. [DOI] [Google Scholar]
- 22.Stewart CP, Christian P, Schulze KJ, Arguello M, LeClerq SC, Khatry SK, West KP., Jr Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J Nutr. 2011;141:1912–1917. doi: 10.3945/jn.111.144717. [DOI] [PubMed] [Google Scholar]
- 23.Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, Hartil K, Petry CJ, Buckley AJ, Mosthaf-Seedorf L. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J Endocrinol. 2003;177:235–241. doi: 10.1677/joe.0.1770235. [DOI] [PubMed] [Google Scholar]
- 24.Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia. 2005;48:547–552. doi: 10.1007/s00125-005-1669-7. [DOI] [PubMed] [Google Scholar]
- 25.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008;118:2316–2324. doi: 10.1172/JCI32011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandovici I, Smith NH, Nitert MD, Ackers-Johnson M, Uribe-Lewis S, Ito Y, Jones RH, Marquez VE, Cairns W, Tadayyon M, O’Neill LP, Murrell A, Ling C, Constância M, Ozanne SE. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci USA. 2011;108:5449–5454. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Einstein F, Thompson RF, Bhagat TD, Fazzari MJ, Verma A, Barzilai N, Greally JM. Cytosine methylation dysregulation in neonates following intrauterine growth restriction. PLoS One. 2010;5:e8887. doi: 10.1371/journal.pone.0008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J Biol Chem. 2008;283:13611–13626. doi: 10.1074/jbc.M800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehara T, Kamei Y, Takahashi M, Yuan X, Kanai S, Tamura E, Tanaka M, Yamazaki T, Miura S, Ezaki O, Suganami T, Okano M, Ogawa Y. Role of DNA methylation in the regulation of lipogenic glycerol-3-phosphate acyltransferase 1 gene expression in the mouse neonatal liver. Diabetes. 2012;61:2442–2450. doi: 10.2337/db11-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez D, Pentinat T, Ribó S, Daviaud C, Bloks VW, Cebrià J, Villalmanzo N, Kalko SG, Ramón-Krauel M, Díaz R, Plösch T, Tost J, Jiménez-Chillarón JC. In utero undernutrition in male mice programs liver lipid metabolism in the second-generation offspring involving altered Lxra DNA methylation. Cell Metab. 2014;19:941–951. doi: 10.1016/j.cmet.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 33.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–2566. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 35.Young AI, Wauthier F, Donnelly P. Multiple novel gene-by-environment interactions modify the effect of FTO variants on body mass index. Nat Commun. 2016;7:12724. doi: 10.1038/ncomms12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gómez-Marín C, Aneas I, Credidio FL, Sobreira DR, Wasserman NF, Lee JH, Puviindran V, Tam D, Shen M, Son JE, Vakili NA, Sung HK, Naranjo S, Acemel RD, Manzanares M, Nagy A, Cox NJ, Hui CC, Gomez-Skarmeta JL, Nóbrega MA. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507:371–375. doi: 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, Glunk V, Sousa IS, Beaudry JL, Puviindran V, Abdennur NA, Liu J, Svensson PA, Hsu YH, Drucker DJ, Mellgren G, Hui CC, Hauner H, Kellis M. FTO obesity variant circuitry and adipocyte browning in humans. N Engl J Med. 2015;373:895–907. doi: 10.1056/NEJMoa1502214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, Hirota Y, Mori H, Jonsson A, Sato Y, Yamagata K, Hinokio Y, Wang HY, Tanahashi T, Nakamura N, Oka Y, Iwasaki N, Iwamoto Y, Yamada Y, Seino Y, Maegawa H, Kashiwagi A, Takeda J, Maeda E, Shin HD, Cho YM, Park KS, Lee HK, Ng MC, Ma RC, So WY, Chan JC, Lyssenko V, Tuomi T, Nilsson P, Groop L, Kamatani N, Sekine A, Nakamura Y, Yamamoto K, Yoshida T, Tokunaga K, Itakura M, Makino H, Nanjo K, Kadowaki T, Kasuga M. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet. 2008;40:1092–1097. doi: 10.1038/ng.207. [DOI] [PubMed] [Google Scholar]
- 39.Unoki H, Takahashi A, Kawaguchi T, Hara K, Horikoshi M, Andersen G, Ng DP, Holmkvist J, Borch-Johnsen K, Jørgensen T, Sandbaek A, Lauritzen T, Hansen T, Nurbaya S, Tsunoda T, Kubo M, Babazono T, Hirose H, Hayashi M, Iwamoto Y, Kashiwagi A, Kaku K, Kawamori R, Tai ES, Pedersen O, Kamatani N, Kadowaki T, Kikkawa R, Nakamura Y, Maeda S. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet. 2008;40:1098–1102. doi: 10.1038/ng.208. [DOI] [PubMed] [Google Scholar]
- 40.Hu C, Wang C, Zhang R, Ma X, Wang J, Lu J, Qin W, Bao Y, Xiang K, Jia W. Variations in KCNQ1 are associated with type 2 diabetes and beta cell function in a Chinese population. Diabetologia. 2009;52:1322–1325. doi: 10.1007/s00125-009-1335-6. [DOI] [PubMed] [Google Scholar]
- 41.Jonsson A, Isomaa B, Tuomi T, Taneera J, Salehi A, Nilsson P, Groop L, Lyssenko V. A variant in the KCNQ1 gene predicts future type 2 diabetes and mediates impaired insulin secretion. Diabetes. 2009;58:2409–2413. doi: 10.2337/db09-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan JT, Nurbaya S, Gardner D, Ye S, Tai ES, Ng DP. Genetic variation in KCNQ1 associates with fasting glucose and beta-cell function: a study of 3734 subjects comprising three ethnicities living in Singapore. Diabetes. 2009;58:1445–1449. doi: 10.2337/db08-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asahara S, Etoh H, Inoue H, Teruyama K, Shibutani Y, Ihara Y, Kawada Y, Bartolome A, Hashimoto N, Matsuda T, Koyanagi-Kimura M, Kanno A, Hirota Y, Hosooka T, Nagashima K, Nishimura W, Inoue H, Matsumoto M, Higgins MJ, Yasuda K, Inagaki N, Seino S, Kasuga M, Kido Y. Paternal allelic mutation at the Kcnq1 locus reduces pancreatic β-cell mass by epigenetic modification of Cdkn1c. Proc Natl Acad Sci USA. 2015;112:8332–8337. doi: 10.1073/pnas.1422104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuda T, Kido Y, Asahara S, Kaisho T, Tanaka T, Hashimoto N, Shigeyama Y, Takeda A, Inoue T, Shibutani Y, Koyanagi M, Hosooka T, Matsumoto M, Inoue H, Uchida T, Koike M, Uchiyama Y, Akira S, Kasuga M. Ablation of C/EBPbeta alleviates ER stress and pancreatic beta cell failure through the GRP78 chaperone in mice. J Clin Invest. 2010;120:115–126. doi: 10.1172/JCI39721. [DOI] [PMC free article] [PubMed] [Google Scholar]