Abstract
The incidence of metabolic disorders such as obesity and diabetes is on the rise, and food quality is not alone to blame. Sleep disturbances, altered feeding time and circadian disruption are linked to metabolic disturbances in many clinical research studies and cross-sectional analyses. This review tried to summarize the role of the circadian timing system and sleep on energy and metabolic homeostasis. We also tried to explain the molecular and endocrine mechanisms behind circadian misalignment and sleep disorders that lead to metabolic disorders.
Keywords: Circadian misalignment, Diabetes, Metabolic disorder, Obesity, Sleep disturbance
Introduction
Obesity is a growing problem in the world, and it is estimated that more than 1.6 billion people are currently either overweight or obese [1]. Recent data from National Health and Nutrition Examination Survey (NHANES) indicate that around 33.9 % of the US adults (age 20 or more) are overweight [body mass index (BMI) of 25.0–29.9 kg/m2], 35.1 % are obese (BMI of 30.0–39.9 kg/m2), and 6.4 % are extremely obese (BMI of 40 kg/m2 or more) [2]. Obesity is a major health problem, which is responsible for myriad other health problems such as diabetes, hypertension, hyperlipidemia, sleep apnea, osteoarthritis, depression and cancer [3–5]. According to the Centers for Disease Control and Prevention (CDC), more than 230 million people (9.3 % of the US population) are estimated to have diagnosed or undiagnosed diabetes, whereas more than 1 of 3 (86 million US adults) have pre-diabetes [6]. Therefore, identifying and characterizing the etiological factors are very important in counteracting the epidemics of obesity and diabetes.
Obesity is mainly caused by overconsumption of food rich in calories and saturated fat. Recent findings suggest quality of food is not alone to blame as timing of food also leads to metabolic derangement, causing obesity [4, 5]. In human beings, most of the essential biological functions (physiologic, metabolic and behavioral processes) show daily variations, called the circadian rhythm, which allow individuals to anticipate and prepare for periodic changes in the environment such as the light-dark (LD) cycle and food availability [5].
The widespread use of electricity (light) and increased social demands (work, school, family) in the last few decades have significantly changed human sleeping patterns [7]. The average sleep duration has decreased from a healthy 7–9 h to ≤6 h in 30 % (40.6 million) of civilian employed US adults [8]. Sleep has been causally linked to the regulation of glucose homeostasis and appetite control [7]. Experimental and epidemiological data have linked sleep disturbances (both quantity and quality) to insulin resistance, obesity and diabetes in non-diabetic individuals as well as poor glycemic control in type 2 diabetes (T2DM) [7, 9]. Clinical research studies and cross-sectional analyses have also revealed that circadian misalignment (behavioral sleep-wake cycle not in synchrony with the biological circadian timing system) has an association with similar metabolic problems as seen with the sleep disturbances [9].
Circadian timing system
The circadian timing system, a peculiar feature of most living organisms, allows organisms to anticipate and adjust their biological functions in response to environmental changes such as the LD cycle and feeding-fasting cycle [5, 10, 11]. Numerous biological functions of mammals, such as sleep-wake cycles, feeding behavior, central and peripheral tissue metabolism, hormonal secretion and cell cycle progression, are under direct control of the circadian timing system [5, 9–11]. The circadian timing system consists of a master (central) clock and a multitude of peripheral clocks. The master clock, located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus, is synchronized (i.e., reset) to the 24-h LD cycle primarily by light signals conducted via the retinohypothalamic tract (RHT) [5, 9]. The synchronized master clock then transmits the information via neuronal [central and autonomic nervous system (ANS)] and neuroendocrine pathways to the rest of the brain and to peripheral organs such as liver, heart, adipose tissue, adrenals, muscle, skin and pancreas, orchestrating the rhythmicity of many aspects of metabolism, behavior and physiology [5, 9].
Circadian rhythms are endogenously generated in the SCN by the transcription-translation negative feedback loop involving a group of “core clock” genes [12]. The essential core clock genes are CLOCK (circadian locomotor output cycles kaput) or its analog Npas2 (neuronal PAS domain protein 2), BMAL1 (brain and muscle arnt-like protein-1), Per 1-3 (period homolog 1-3), Cry 1-2 (cryptochrome 1-2), Rev-erb α and β (reverse viral erythroblastosis oncogene products α and β) and Ror α, β, and γ (retinoic acid-related orphan receptors α, β and γ) [5, 9, 13]. Three main autoregulatory feedback loops (a positive loop, a negative loop and an interconnecting loop) are responsible for the generation and fine tuning of circadian oscillations; for a detailed review, see Ref. [11]. In the positive feedback loop, CLOCK and BMAL1 dimerize (CLOCK:BMAL1) to activate E-box mediated transcription of Per and Cry genes. The negative feedback loop includes the genes Per and Cry, where they act as negative regulators of their own transcription by interacting with CLOCK:BMAL1 complexes. The interconnecting loop consists of Rev-erb and Ror genes, where they act antagonistically on the RORE (retinoic acid-related orphan receptor response element) to activate (Rev-erb genes) or repress (Ror genes) the transcription of the BMAL1 and Npas2 clock genes [5, 10, 14].
SCN passes the time-keeping signals to the oscillators in peripheral tissues, which also contain core clock genes [15, 16]. Endogenous rhythms generated by SCN are synchronized to the outside world mostly by light [perceived by retinal photoreceptors (melanopsin, rods and cones)] via RHT [4, 15]. The master clock has specific sensitivity to the intensity of ambient light, which is a key element in the circadian timing system. Light exposure during the night can phase-shift the clock, while light exposure during the day has minimal or no phase-resetting effects [17]. Although peripheral clocks are synchronized to the LD cycle indirectly via the master clock, they can be markedly influenced by the timing cues related to the ingestion of food [15]. Not only the peripheral clocks, but also many central clocks (except for the master clock) in the brain are also easily phase-shifted by restricted feeding [15]. Food-entrained brain and peripheral clocks (e.g., liver, pancreas) become uncoupled from the SCN control and lead to a state of desynchronization [18]. Apart from ANS control by SCN and timing of food intake, peripheral clocks are also responsive to neuroendocrine hormonal signals and alterations in body temperature [19].
Circadian rhythm and energy homeostasis
A functional circadian system and resultant synchronous inter-relationships between peripheral and central oscillators, in response to changing sleep-wake and feeding-fasting cycles, are essential for maintaining appropriate homeostatic function [10, 20, 21]. Regulation of glucose homeostasis is an excellent example of coordination between central and peripheral oscillators, where plasma glucose levels are tightly controlled throughout the 24-h LD cycle in healthy individuals by the circadian regulation of β-cell insulin production, secretion and growth [10]. Many metabolically relevant hormones [cortisol, insulin, leptin, ghrelin, growth hormone (GH), adiponectin] show circadian oscillation with different daily patterns [4, 21].
Cortisol secretion reaches its zenith in the biological morning (i.e., a time according to the circadian rhythm associated with the start of behavioral activity) and has its nadir during the early biological night (i.e., a time according to the original circadian rhythm associated with the start of behavioral inactivity), around 3 a.m. [4, 21]. GH typically peaks at sleep onset during slow-wave sleep (SWS), and the insulin level displays a modest diurnal variation with a nadir between midnight and 6 a.m. and a zenith between noon and 6 p.m. [4]. Several appetite-regulating hormones, such as leptin and ghrelin, display circadian rhythms. Leptin, an appetite-suppressing hormone secreted mainly by white adipose tissue (WAT), increases after meals and during the night, and it stays low during the day [4, 22]. Leptin also increases energy expenditure (EE) [4]. Ghrelin, produced mainly in the stomach, stimulates appetite, stimulates the release of GH, decreases EE and promotes gastric emptying [23]. The ghrelin level rises with fasting and during the early part of the sleep and decreases in the morning before awakening [21, 23]. Adiponectin, secreted by adipocytes in an inverse fashion compared to fat mass, displays some diurnal variability with lower levels during the night [4]. Adiponectin influences both EE (increases) and glucose metabolism (prevents insulin resistance) [4].
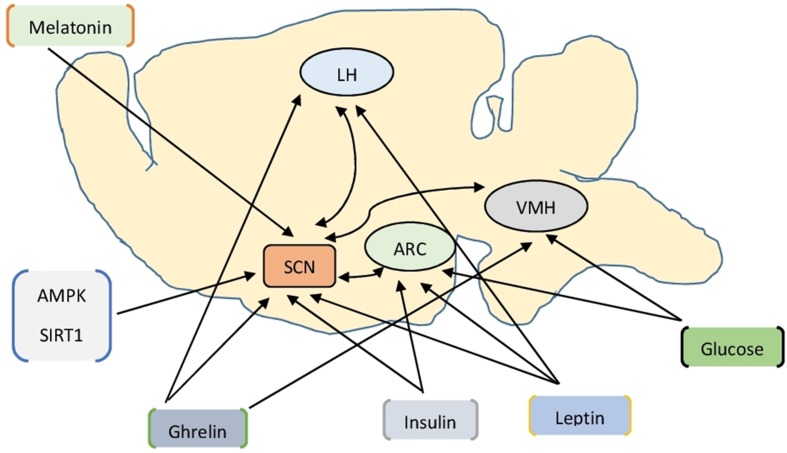
Several direct and indirect pathways, which can influence the function of the SCN, connect the endocrine system with the SCN (Fig. 1) [3, 24]. Leptin, ghrelin and insulin can affect the SCN directly via the receptors present in the SCN [3]. Ghrelin exerts its effect in the lateral hypothalamus (LH) and arcuate nucleus (ARC) via the orexigenic neuropeptide Y/agouti-related protein (NPY/AgRP) neuron [3–5, 25, 26]. Similarly, leptin exerts its effect in the ARC via the anorexigenic proopiomelanocortin/cocaine and amphetamine-regulated transcript (POMC/CART) neuron [3–5, 25]. The POMC neurons secrete α-melanocyte-stimulating hormone (α-MSH), which activates the central melanocortin signaling to reduce food intake and stimulate EE [27]. Although insulin, released from β-cells of the pancreas, lowers blood glucose peripherally and stimulates appetite, it inhibits appetite centrally in a leptin-like manner [5, 25]. The glucose-sensing neurons in the ventromedial hypothalamus (VMH), LH and ARC can communicate glucose-related information to the SCN [3, 5]. Similarly, AMP/ATP ratio-sensing enzyme, the AMP protein kinase (AMPK) and NAD+ sensing SIRT1 (sirtuin type 1) convey the body’s metabolic state to the SCN [3, 28]. A recent study showed that the circadian clock plays a critical role in the long-term homeostasis of the leptin endocrine feedback loop, ensuring a coupled circadian rhythm in food intake, physical activity, plasma leptin level, leptin receptor-B (LEBR-B)-mediated POMC signaling in ARC and EE [27].
Fig. 1.
Hormones and metabolic signals influence suprachiasmatic nucleus function (refer to text for details). AMPK AMP protein kinase, ARC arcuate nucleus, LH lateral hypothalamus, SCN suprachiasmatic nucleus, SIRT1 sirtuin type 1, VMH ventromedial hypothalamus
Melatonin, a hormone secreted by the pineal gland under the influence of SCN, is exclusively secreted during the night as light inhibits its production [4]. Melatonin, through its G protein-coupled membrane receptors MT1 and MT2, exerts its effects on SCN and peripheral tissues (especially pancreas α and β cells) [29]. Proinflammatory factors, such as interleukin 6 (IL-6), interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α) and C-reactive protein (CRP), also display diurnal oscillations [4]. IL-2 displays double peaks, one peak at around 2 a.m. and the other peak at around 5 a.m. The IL-1β level reaches its nadir at 8 a.m., and the TNF-α level has its zenith at around 6 a.m. and nadir at around 3 p.m.; the CRP level increases in the morning [4]. These proinflammatory mediators (especially IL-6 and TNF-α) stimulate cortisol secretion [4]. Orexin neuropeptides (A and B), released from LH under the influence of SCN, leptin, ghrelin and glucose, play an important role in modulating both the metabolic physiology and sleep-wake behavior [3, 4, 30].
Sleep
Human sleep is divided into four distinct stages based on the patterns of eye movement, brain electric activity and skeletal muscle tone [7]: Stage 1, 2 and 3 [collectively called non-rapid-eye-movement (NREM)] sleep and rapid-eye-movement (REM) sleep. Stage 3 of the NREM sleep is also known as SWS [4, 9]. During a normal night of sleep, there are about four to six sleep cycles [9]. NREM and REM stages oscillate every 90–120 min, with short episodes of REM initially, but they increase progressively as the night advances [4, 7, 9]. Humans sleep approximately one-third of their lives, with NREM sleep taking 75–80 % of the share [4, 9]. The sleep-wake cycle is regulated in part by the internal circadian clock, which sets the body’s internal sleep-wake timing, and in part by the homeostatic mechanism, which monitors the previous waking duration [3, 9, 21]. The circadian phase at which sleep occurs affects the distribution of sleep stages: the preferential distribution of SWS sleep toward the beginning of the sleep episode is linked to homeostatic mechanism, and the preferential distribution of REM sleep toward the latter part of the night is mediated by circadian oscillation [21].
Energy metabolism during sleep
Sleep and wakefulness are contrasting physiological processes, and their differences are mirrored in the regulation of energy metabolism (substrate utilization and EE) [7, 21]. Metabolic needs for processes such as gut motility, breathing, muscular activity and heart rate are decreased during sleep, thereby reducing the energy requirement [4]. The whole body EE drops by 15–30 % during physiologic sleep, with the lowest EE during SWS [31]. Several observational studies indicate that REM sleep, which has the highest sleep-metabolic rate (SMR), may have a role in energy metabolism and obesity [21, 32, 33].
Circadian sleep-wake oscillations have been described in glucose and lipid metabolism, which are independent of metabolic changes induced by food intake [7]. Plasma glucose levels show strong circadian oscillations with progressively increasing levels during sleep with the highest level in the early morning [34]. Increased hepatic output (SCN mediated), decreased skeletal muscle glucose uptake (SCN mediated reduction of skeletal muscle blood flow) and decreased glucose utilization by the brain (decreased neuronal activity during SWS) during sleep progressively increase the plasma glucose level [7]. Similarly, increased activities of lipoprotein lipase (LPL) and fatty acid synthase (FAS) in the adipose tissue during sleep progressively decrease the plasma lipid level [7, 35].
Several hormones, responsible for the modulation of EE, glucose metabolism and appetite, show diurnal variability. Thyroid hormone, adiponectin and sympathovagal balance (a ratio between sympathetic and parasympathetic nervous system activity) influence EE; cortisol, GH and insulin modulate glucose metabolism; leptin and ghrelin regulate appetite [4]. The adiponectin and sympathovagal balance also has an influence on glucose control, and insulin shows an influence on central appetite regulation as well [4]. The thyroid-stimulating hormone (TSH) level rises at night [4]; the adiponectin level decreases during the night [4]. The sympathovagal balance reaches a lower level during the night because of increased vagal tone [4], and other hormones (cortisol, GH, insulin, leptin and ghrelin) show circadian variations as described earlier in this review.
Sleep disturbance and altered energy balance
Sleep disturbances, whether from insufficient sleep duration, abnormal sleep timing or poor quality sleep, are the results of human behavior overriding the normal physiologic sleep control mechanism [9]. Sleep disturbances have been implicated not only in the metabolic disorders (T2DM, obesity, insulin resistance) (Fig. 2), but also in the impairment of cognitive performance as well as in the increased risk of morbidity and mortality [7, 9].
Fig. 2.
A simplified schematic representation of the pathways linking sleep disturbances and circadian misalignment to altered glucose homeostasis and type 2 diabetes (refer to text for details). EE energy expenditure, GH growth hormone, OSA obstructive sleep apnea, REM rapid eye movement, SWS slow-wave sleep, TSH thyroid stimulating hormone, T2DM type 2 diabetes mellitus
Sleep deprivation (partial or short sleep) is associated with increased appetite (increase ghrelin, decrease leptin levels) and increased cravings for foods rich in calories, leading to a positive energy balance (increased energy intake but reduced EE) [3, 4, 7, 21, 36]. Sleep deprivation reduces the amount of REM sleep, thereby decreasing the SMR, causing central obesity (especially in women) and increased BMI in children and adolescents [4, 21, 32]. It has been shown that REM sleep may affect the normal functioning of the HPA axis [21]. Insulin resistance and β-cell dysfunction develop in sleep deprivation, causing fasting hyperglycemia and postprandial hyperglycemia [37, 38]. It is believed that there is a causal role of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic activation in the onset of β-cell dysfunction and insulin resistance [7, 9, 21]. The increased evening levels of cortisol in sleep deprived individuals are thought to show an impairment in negative feedback control of the HPA axis causing obesity [4, 9, 21].
Similarly, there is an increased respiratory quotient (RQ), indicating a shift from fat toward carbohydrate oxidation, leading to increased fat deposition and causing weight gain and obesity [4, 39]. Several other hormones, which have roles in energy metabolism, also show deviation from the normal circadian oscillations after sleep deprivation. The TSH level decreases, and GH (insulin counter-regulatory hormone) shows a biphasic pattern (which may decrease muscle glucose uptake), causing a positive energy balance as well as insulin resistance [4, 9]. There is an increased sympathovagal balance (increased sympathetic activity and decreased parasympathetic activity) after acute sleep deprivation, decreasing insulin secretion [4, 9]. Increased sympathetic activity also stimulates lipolysis and increases free fatty acids (FFAs), leading to insulin resistance [40]. Short sleep stimulates proinflammatory mediators (IL-6, IL-1β, CRP and TNF-α), which, in turn, increase the risk of cardiovascular disease (CVD) and T2DM [9, 41].
The sleep disorder obstructive sleep apnea (OSA) is worth mentioning; it has not only been recognized as an independent risk factor for CVD, but also been associated with a number of metabolic alterations such as insulin resistance, dyslipidemia, glucose intolerance and T2DM [7, 42, 43]. OSA is characterized by repetitive obstructions of the upper airways during sleep, causing intermittent hypoxia (IH) and fragmented sleep (total sleep duration is preserved but continuous sleep and its architecture are interrupted) [7, 9]. The prevalence of OSA in the general population is around 3–7 %, but its prevalence is on the rise because of the increasing prevalence of obesity (a major risk factor for OSA) [7, 44]. Several cross-sectional studies implicated OSA, as measured by homeostatic model assessment of insulin resistance (HOMA-IR), in impaired glucose tolerance and/or insulin sensitivity even after adjustment of various confounding factors including BMI [45, 46].
Studies have shown that IH induces acute insulin resistance in rodents (e.g., mice) through various mechanisms, such as activation of hepatic lipid synthesis (causes hepatic insulin resistance), activation of the sympathetic nervous system and HPA axis (impairs insulin secretion), alterations in adipokines (e.g., adiponectin) and increased oxidative stress (causes β-cell proliferation and death) [47–49]. Healthy human volunteers, exposed to acute IH, showed a decrease in insulin sensitivity and glucose effectiveness (the ability of glucose itself in stimulating glucose uptake and suppressing glucose production) [50]. Recently, OSA has been associated with non-alcoholic fatty liver disease (NAFLD), and it was suggested that IH promotes hepatic injury (inflammation and fibrosis) and increase in key lipid biosynthesis [51]. Experimental studies have shown that sleep fragmentation causes decreased insulin sensitivity and impaired insulin secretion, suggesting an important role in glucose homeostasis [52]. Elevated night and morning cortisol levels, sympathetic activation and reduction in SWS duration have been implicated in the altered metabolic control in sleep fragmentation [53].
Interestingly, some recent meta-analyses suggested a U-shaped relationship between sleep duration and the risk of type 2 diabetes [54, 55]. A recent study found that the long sleep duration can also adversely affect the metabolic outcomes, causing weight gain and increasing the risk of diabetes [56]. It has been suggested that long sleep duration also influences the energy intake and expenditure, albeit in a different way than for the short sleepers [56]. It has been proposed that reduced time for physical activity and age-related modification of the association between sleep and metabolic outcomes might have some role in the onset of metabolic abnormality in the long sleepers [56, 57]. Ongoing studies might shed some light in the mechanism of metabolic derangement in the long sleepers.
To summarize, sleep disturbances, whether sleep deprivation, sleep fragmentation or OSA, cause disturbances in the normal circadian rhythm of several factors responsible for appetite regulation, glucose metabolism and EE, leading to a positive energy balance [3, 4, 21]. The mechanism for metabolic derangement in the long sleepers is still under investigation.
Circadian disturbance and metabolic disorders
Circadian timing system can be disrupted mainly in two ways: an altered endogenous clockwork and altered rhythmic environment (change in the timing of LD cycles and change in behavioral activities such as sleeping, feeding and physical activity) [5, 9]. As mentioned earlier, clock genes are involved in numerous biological processes such as lipid and glucose metabolism [9, 11]. Alteration in the clock genes can have a detrimental effect on energy metabolism [5, 9].
It has been shown that CLOCK mutant mice shift their activity and feeding behavior to their normal rest period (daytime) and have reduced EE at normally active period (nighttime), leading to obesity and severe metabolic alterations (hyperlipidemia, hepatic steatosis and hyperglycemia) [58]. BMAL1 clock gene deletion is found to increase sensitivity to insulin and suppress gluconeogenesis in mice, causing hypoglycemia during the resting period [5, 59]. Per clock gene mutation is found to have variable effects in mice: Per1 mutation caused increased glucose tolerance [60], Per2 mutation caused attenuation of cortisone rhythm [5], and Per3 mutation caused obesity [61]. Similarly, Rorα and Rev-erbα clock genes have been shown to take part in lipid homeostasis [62, 63]. It has been found that mice deficient in BMAL1, Per or Cry genes display distinct phenotypes of energy homeostasis under both entrained and jet-lagged conditions [27]. The transgenic mice lacking Per or Cry genes show opposite phenotypes of EE, body weight and body composition because of a distinct disruption of the leptin-mediated central melanocortin signaling [27]. An experimental study on leptin lacking (ob/ob) mice showed that peripheral circadian clock impairment precedes the metabolic abnormalities, suggesting that leptin deficiency may cause peripheral clock impairment, leading to metabolic abnormalities such as obesity, hyperglycemia, hyperinsulinemia and hypercholesterolemia [64]. Studies have shown that rhythmic expression of clock genes is attenuated in the peripheral organs such as liver and adipose tissue of experimental models of diabetes and obesity, such as KK (mild form of obesity and diabetes) and KK-Ay (severe form of obesity and diabetes) mice, suggesting the role of peripheral clocks in the energy homeostasis [65]. It is to be noted that 10 % of genes in the liver exhibit circadian regulation, and the analysis of gene expression based on a single observation may overlook the effect of obesity and diabetes on the hepatic mRNA levels of several clock genes [66]. Similarly, peripheral tissue such as leucocytes in patients with T2DM show a dampened rhythmic mRNA expression, which appears to be closely associated with the pathophysiology of T2DM [67].
Circadian misalignment, both a phase advance and phase delay, results in the disruption of the normal phase relationship between REM and SWS sleep (Fig. 2) [21]. REM sleep becomes relatively phase advanced to SWS with reduced REM sleep latency and increased REM sleep duration [21]. This results in a shorter REM sleep duration during the second part of the night, disrupting the normal HPA axis rhythm [21]. The overall effect of this disruption is higher cortisol levels, higher fasting glucose levels and higher HOMA-IR index, leading to insulin resistance and obesity [21, 68]. Circadian misalignment (e.g., night shift work, jet lag) in humans impairs glucose tolerance and reduces sensitivity to insulin [34]. The degree of circadian misalignment is dependent on the chronotype (an individual’s preference for being a particular time person) of an individual, and late chronotypes have a greater degree of circadian misalignment [9]. Late chronotypes were associated with increased risk of diabetes and metabolic syndrome [9].
Mice exposed to constant bright light or dim light during their usual biological night were shown to shift their food intake into the inactive phase [9, 69]. This was associated with reduced glucose tolerance and increased body mass [69]. Aligning meals in a circadian way may have beneficial metabolic effects on insulin sensitivity, diet-induced thermogenesis and fasting lipid profiles [21]. Regular eating was associated with greater postprandial thermogenesis, lower energy intake, and lower fasting total and low-density lipoprotein (LDL) cholesterol [21]. When the temporal pattern of food intake is altered, the body’s metabolic state is conveyed to SCN via AMPK, SIRT1 and glucose-sensing neurons in VMH, LH and ARC, and then the function of SCN will be influenced [3]. Food intake at the wrong time (biological inactive phase) has been linked to obesity [3]. Also, low nocturnal melatonin levels, decreased as a result of increased duration of light exposure, have been associated with diabetes and metabolic disturbances [9, 70]. Genetic studies have also recently linked MTNR1B (gene encoding MT2) to abnormal glucose metabolism and diabetes risks [71, 72]. It is plausible that melatonin may act as an internal signal synchronizing SCN and peripheral clocks, especially pancreas α and β cells, and help in maintaining glucose homeostasis [9].
As mentioned earlier, the circadian clock plays a critical role in controlling the leptin endocrine feedback loop to maintain the homeostasis of energy balance. The effects of acute and chronic circadian disruption/dysfunction are different. With acute disruption, there will be reduced expression of leptin in the adipose tissue, leading to a shift in energy balance and weight gain [27]. However, in the chronic circadian disruption, such as chronic jet lag, there will be progressive gain in body fat and weight and an increase in the serum leptin, but the POMC signaling in the ARC neurons will be dampened, resulting in leptin resistance [27]. It is postulated that chronic circadian disruption/dysfunction may contribute to obesity in humans [27].
Some recent studies on nocturnal and diurnal organisms demonstrated multiple physiologic benefits of time-restricted feeding (TRF) such as reduced obesity, elevated lean body mass, longer sleep duration and gut homeostasis, without altering the quality and quantity of the diet [73, 74]. It has been demonstrated in a recent study that most humans eat frequently and erratically during the wakeful hours, and changes in the eating timing during weekdays and weekends, due to change in the sleeping pattern, can lead to so-called “metabolic jetlag” [75]. The same study also pointed out that reducing the temporal eating period can restore the feeding/fasting diurnal rhythm and reduce the “metabolic jetlag,” leading to decreased body weight and improved sleep [75].
Conclusion
Metabolic disorders, such as obesity, insulin resistance and T2DM, are the undesirable gifts of the modern lifestyle. Changing patterns of sleep, feeding and activity are all to blame for the circadian misalignment and sleep disorders. We reviewed the molecular and endocrine mechanisms behind the circadian misalignment and sleep disorders that lead to altered energy and metabolic homeostasis. On the basis of this review, it is very tempting to recommend sleep hygiene, a regular feeding pattern and healthy food choices to curtail and/or prevent metabolic disorders in the general public. However, whether a ‘one size fits all’ approach will work in this situation is hard to answer.
The current researches have not specifically addressed the effects of age, sex, ethnicity, BMI, socioeconomic status and health status on the circadian and sleep disturbances. Most of the available data are either from rodent studies or from acute sleep restrictions in human studies, whose results cannot be translated into real-life situations as such. Further research is needed before we can make a firm recommendation. Recent findings of clock gene mutations and their effect on EE and metabolic homeostasis point to yet another exciting area to be explored. Future research on drugs targeting the molecular and endocrine mechanisms involved in circadian disruption and sleep disorders could shed some light on the treatment of metabolic disorders.
Acknowledgments
The authors alone are responsible for the content and writing of the article.
Conflict of interest
The authors do not have any conflict of interest to declare.
Ethics policy
No human or animal subjects were used in this study by any of the authors.
Contributor Information
Navin Adhikary, Email: Adhikary_Navin@outlook.com.
Santosh Lal Shrestha, Email: shrestha03@hotmail.com.
Jia Zhong Sun, Email: sjz300@163.com.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and extreme obesity among adults: United States, 1960–1962 through 2011–2012. Atlanta: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2014. http://www.cdc.gov/nchs/data/hestat/obesity_adult_11_12/obesity_adult_11_12.pdf
- 3.Coomans CP, Lucassen EA, Kooijman S, et al. Plasticity of circadian clocks and consequences for metabolism. Diabetes Obes Metab. 2015;17(suppl 1):65–75. doi: 10.1111/dom.12513. [DOI] [PubMed] [Google Scholar]
- 4.Lucassen EA, Rother KI, Cizza G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity. Ann NY Acad Sci. 2012;1264:110–134. doi: 10.1111/j.1749-6632.2012.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delezie J, Challet E. Interactions between metabolism and circadian clocks: reciprocal disturbances. Ann NY Acad Sci. 2011;1243:30–46. doi: 10.1111/j.1749-6632.2011.06246.x. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Diabetes report card 2014. Atlanta: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2015. http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf
- 7.Briançon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr. 2015;7:25. doi: 10.1186/s13098-015-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Short sleep duration among workers-United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(16):281–285. [PubMed] [Google Scholar]
- 9.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann NY Acad Sci. 2014;1311:151–173. doi: 10.1111/nyas.12355. [DOI] [PubMed] [Google Scholar]
- 10.Rakshit K, Qian J, Colwell CS, Matveyenko AV. The islet circadian clock: entrainment mechanisms, function and role in glucose homeostasis. Diabetes Obes Metab. 2015;17:115–122. doi: 10.1111/dom.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13(2):125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 14.Crumbley C, Wang Y, Kojetin DJ, Burris TP. Characterization of the core mammalian clock component, NPAS2, as a REV- ERBalpha/RORalpha target gene. J Biol Chem. 2010;285(46):35386–35392. doi: 10.1074/jbc.M110.129288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Challet E. Keeping circadian time with hormones. Diabetes Obes Metab. 2015;17:76–83. doi: 10.1111/dom.12516. [DOI] [PubMed] [Google Scholar]
- 16.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90(3):1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 18.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saini C, Suter DM, Liani A, Gos P, Schibler U. The mammalian circadian timing system: synchronization of peripheral clocks. Cold Spring Harb Symp Quant Biol. 2011;76:39–47. doi: 10.1101/sqb.2011.76.010918. [DOI] [PubMed] [Google Scholar]
- 20.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonnissen HKJ, Hulshof T, Westerterp-Plantenga MS. Chronobiology, endocrinology, and energy- and food-reward homeostasis. Obes Rev. 2013;14(5):405–416. doi: 10.1111/obr.12019. [DOI] [PubMed] [Google Scholar]
- 22.Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metab. 2011;96(9):E1512–E1516. doi: 10.1210/jc.2011-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegel K, Tasali E, Leproult R, Scherberg N, Van Cauter E. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011;96(2):486–493. doi: 10.1210/jc.2010-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsang AH, Barclay JL, Oster H. Interactions between endocrine and circadian systems. J Mol Endocrinol. 2014;52(1):R1–R16. doi: 10.1530/JME-13-0118. [DOI] [PubMed] [Google Scholar]
- 25.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91(2):389–411. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogueiras R, Tschöp MH, Zigman JM. Central nervous system regulation of energy metabolism: ghrelin versus leptin. Ann NY Acad Sci. 2008;1126:14–19. doi: 10.1196/annals.1433.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kettner NM, Mayo SA, Hua J, Lee C, Moore DD, Fu L. Circadian dysfunction induces leptin resistance in mice. Cell Metab. 2015;22(3):448–459. doi: 10.1016/j.cmet.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orozco-Solis R, Ramadori G, Coppari R, Sassone-Corsi P. SIRT1 relays nutritional inputs to the circadian clock through the Sf1 neurons of the ventromedial hypothalamus. Endocrinology. 2015;156(6):2174–2184. doi: 10.1210/en.2014-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peschke E, Mühlbauer E. New evidence for a role of melatonin in glucose regulation. Best Pract Res Clin Endocrinol Metab. 2010;24(5):829–841. doi: 10.1016/j.beem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 31.Katayose Y, Tasaki M, Ogata H, Nakata Y, Tokuyama K, Satoh M. Metabolic rate and fuel utilization during sleep assessed by whole-body indirect calorimetry. Metabolism. 2009;58(7):920–926. doi: 10.1016/j.metabol.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 32.Theorell-Haglow J, Berne C, Janson C, Sahlin C, Lindberg E. Associations between short sleep duration and central obesity in women. Sleep. 2010;33(5):593–598. [PMC free article] [PubMed] [Google Scholar]
- 33.Horne J. REM sleep, energy balance and optimal foraging. Neurosci Biobehav Rev. 2009;33(3):466–474. doi: 10.1016/j.neubiorev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bray MS, Young ME. Regulation of fatty acid metabolism by cell autonomous circadian clocks: time to fatten up on information? J Biol Chem. 2011;286(14):11883–11889. doi: 10.1074/jbc.R110.214643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1(5):266–273. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohkuma T, Fujii H, Iwase M, et al. Impact of sleep duration on obesity and the glycemic level in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Care. 2013;36(3):611–617. doi: 10.2337/dc12-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93(6):1229–1236. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 39.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153(7):435–441. doi: 10.7326/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hucking K, Hamilton-Wessler M, Ellmerer M, Bergman RN. Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest. 2003;111(2):257–264. doi: 10.1172/JCI14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54:S114–S124. doi: 10.2337/diabetes.54.suppl_2.S114. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Jimenez F, Sert Kuniyoshi FH, Gami A, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. Chest. 2008;133(3):793–804. doi: 10.1378/chest.07-0800. [DOI] [PubMed] [Google Scholar]
- 43.Priou P, Le Vaillant M, Meslier N, et al. Independent association between obstructive sleep apnea severity and glycated hemoglobin in adults without diabetes. Diabetes Care. 2012;35(9):1902–1906. doi: 10.2337/dc11-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 45.Seicean S, Kirchner HL, Gottlieb DJ, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the sleep heart health study. Diabetes Care. 2008;31(5):1001–1006. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 46.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 47.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 2010;24(5):843–851. doi: 10.1016/j.beem.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Long YS, Gozal D, Epstein PN. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med. 2009;46(6):783–790. doi: 10.1016/j.freeradbiomed.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 49.Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity. 2011;19(11):2167–2174. doi: 10.1038/oby.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;1065:1538–1544. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polotsky VY, Patil SP, Savransky V, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179(3):228–234. doi: 10.1164/rccm.200804-608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105(3):1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38:529–537. doi: 10.2337/dc14-2073. [DOI] [PubMed] [Google Scholar]
- 56.Cespedes EM, Bhupathiraju SN, Li Y, Rosner B, Redline S, Hu FB. Long-term changes in sleep duration, energy balance and risk of type 2 diabetes. Diabetologia. 2016;59(1):101–109. doi: 10.1007/s00125-015-3775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Lin L, Lv L, et al. U-shaped relationships between sleep duration and metabolic syndrome and metabolic syndrome components in males: a prospective cohort study. Sleep Med. 2015;16(8):949–954. doi: 10.1016/j.sleep.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 58.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudic RD, McNamara P, Curtis AM, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2(11):e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dallmann R, Touma C, Palme R, Albrecht U, Steinlechner S. Impaired daily glucocorticoid rhythm in Per1 (Brd) mice. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192(7):769–775. doi: 10.1007/s00359-006-0114-9. [DOI] [PubMed] [Google Scholar]
- 61.Dallmann R, Weaver DR. Altered body mass regulation in male mPeriod mutant mice on high-fat diet. Chronobiol Int. 2010;27(6):1317–1328. doi: 10.3109/07420528.2010.489166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau P, Nixon SJ, Parton RG, Muscat GE. RORalpha regulates the expression of genes involved in lipid homeostasis in skeletal muscle cells: caveolin-3 and CPT-1 are direct targets of ROR. J Biol Chem. 2004;279(35):36828–36840. doi: 10.1074/jbc.M404927200. [DOI] [PubMed] [Google Scholar]
- 63.Le Martelot G, Claudel T, Gatfield D, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7(9):e1000181. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ando H, Kumazaki M, Motosugi Y, et al. Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology. 2011;152(4):1347–1354. doi: 10.1210/en.2010-1068. [DOI] [PubMed] [Google Scholar]
- 65.Ando H, Yanagihara H, Hayashi Y, et al. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146(12):5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 66.Ando H, Oshima Y, Yanagihara H, et al. Profile of rhythmic gene expression in the livers of obese diabetic KK-A(y) mice. Biochem Biophys Res Commun. 2006;346(4):1297–1302. doi: 10.1016/j.bbrc.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 67.Ando H, Takamura T, Matsuzawa-Nagata N, et al. Clock gene expression in peripheral leucocytes of patients with type 2 diabetes. Diabetologia. 2009;52(2):329–335. doi: 10.1007/s00125-008-1194-6. [DOI] [PubMed] [Google Scholar]
- 68.Wu H, Stone WS, Hsi X, et al. Effects of different sleep restriction protocols on sleep architecture and daytime vigilance in healthy men. Physiol Res. 2010;59(5):821–829. doi: 10.33549/physiolres.931895. [DOI] [PubMed] [Google Scholar]
- 69.Fonken LK, Workman JL, Walton JC, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107(43):18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cizza G, Requena M, Galli G, De Jonge L. Chronic sleep deprivation and seasonality: implications for the obesity epidemic. J Endocrinol Investig. 2011;34(10):793–800. doi: 10.3275/7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prokopenko L, Langenberg C, Florez JC, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41(1):77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lyssenko V, Nagorny CL, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347:1265–1269. doi: 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]