Abstract
Objective
A reduction in endothelial progenitor cell (EPC) count is considered to correlate with cumulative cardiovascular risk factors including hyperglycemia. This study was conducted to elucidate the influence of glycemic variability on EPC count in patients with diabetes.
Methods
In study 1, we examined the number of EPCs in 57 patients with type 1 diabetes and 43 patients with type 2 diabetes. The number of EPCs (CD34+, CD34+CD133+, CD34+CD309+, and CD34+CD133+CD309+) was counted as the number of cells per 106 events. In study 2, we examined 37 outpatients with type 1 diabetes without macrovascular complications. We assessed associations between EPC count and seven parameters of glycemic variability (blood glucose standard deviation, mean amplitude of glycemic excursion, J index, M value, mean of daily differences, low blood glucose index, and high blood glucose index), as measured by continuous glucose monitoring. We further analyzed the correlation between EPC count and the carotid intima-media thickness (IMT) in 24 patients.
Results
In study 1, the number of circulating CD34+ and CD34+CD133+ cells was significantly decreased in patients with type 1 diabetes relative to that in patients with type 2 diabetes (p = 0.020 and 0.036, respectively). In study 2, a univariate analysis showed that the J index was negatively correlated with logCD34+ (r = −0.342, p = 0.039). LogCD34+ was significantly negatively associated with the max IMT (r = −0.486, p = 0.012) and the mean IMT (r = −0.503, p = 0.016).
Conclusions
An increase in the J index, which reflects both hyperglycemia and glycemic variability, is associated with a reduction in the EPC count, which might result in the progression of diabetic vascular complications.
Keywords: Endothelial progenitor cells, Type 1 diabetes, J index, Glycemic variability, Intima-media thickness, Glycated albumin, Continuous glucose monitoring
Introduction
Peripheral circulating endothelial progenitor cells (EPCs), which are derived from bone marrow, contribute to revascularization and endothelial homeostasis [1]. Previous studies have reported that a reduction in the EPC count may correlate with cumulative postulated cardiovascular risk factors, such as aging, hypertension, hyperlipidemia, hyperglycemia, and smoking status [2]. Low EPC counts lead to the acceleration of oxidative stress and may result in vascular endothelial dysfunction [3], which is considered one of the major changes that occur during the initial step of the atherosclerotic process [1]. However, its underlying mechanism remains unclear.
Peripheral circulating EPCs are characterized by the expression of markers such as CD34, CD133, and CD309 (VEGFR-2/KDR). CD34 is a glycoprotein expressed on both multipotent stem cells and hematopoietic precursor cells. CD133 is also a glycoprotein expressed on hematopoietic cell lines; however, its surface antigen identifies more immature progenitor cells than CD34 does alone. CD309 (VEGFR-2/KDR) is a tyrosine kinase that characterizes the vascular endothelial growth factor receptor and indicates endothelial differentiation.
Hyperglycemia is a well-known risk factor for endothelial dysfunction and atherosclerosis [4]. It has been evaluated via the mean plasma glucose level as shown in glycated hemoglobin (HbA1c). However, endothelial dysfunction was observed even in patients who have normal fasting but high postprandial plasma glucose levels in the early stages of type 2 diabetes. Prolonged and repeated exposure to postprandial hyperglycemia may accelerate the progression of cardiovascular disease [5, 6]. The outcomes of recent large-scale studies have also indicated that tight glycemic control using HbA1c alone is not sufficient to reduce the risk of macrovascular complications. Using the glucose clamp method, Cirilo et al. found that the activation of oxidative stress by acute glucose fluctuations has more deleterious effects on endothelial function than constant hyperglycemia does in patients with type 2 diabetes [7]. Torimoto et al. observed that the parameters of glycemic variability correlated with the reactive hyperemia index, which is an indicator of vascular endothelial function, measured using the peripheral arterial tonometry [8]. Severe hypoglycemia markedly increases the incidence of sudden cardiovascular death [9, 10]. These studies suggest that, in order to prevent vascular damage, it is important to not only reduce HbA1c levels but also to stabilize dynamic changes in glucose levels.
Several lines of evidence suggest that EPC counts in patients with diabetes are remarkably decreased compared with those in healthy subjects [11–13]. It has also been found that improved glycemic control (reductions in HbA1c) increases the number of EPCs [12]. However, few studies have evaluated the impact of brittle glycemic changes on EPC counts in patients with diabetes. Therefore, we aimed to determine clinical factors associated with EPC counts in patients with diabetes, and to clarify the influence of glycemic variability (as measured by continuous glucose monitoring CGM)—on the number of EPCs in the peripheral blood of patients with type 1 and type 2 diabetes.
Materials and methods
Subjects
Study 1
We examined a total of 100 patients, 57 of whom had type 1 diabetes and 43 of whom had type 2 diabetes. All subjects were inpatients or outpatients at Osaka Medical College Hospital between 2014 and 2015. Table 1 shows clinical characteristics of the patients with two types of diabetes. The criteria applied to exclude subjects from our study were known myocardial infarction, cerebral infarction, or obstructive arteriosclerosis; concomitant malignancy or inflammatory disease; and pregnancy or lactation. We defined smoking status (+) as current smoking, hypertension (+) as a consulting room blood pressure of ≥130/80 mmHg, and dyslipidemia (+) as LDL-Chol ≥120 mg/dl and/or HDL-Chol <40 mg/dl and/or TG ≥150 mg/dl.
Table 1.
Clinical characteristics of patients with type 1 diabetes and patients with type 2 diabetes (study 1)
| Type 1 diabetes (n = 57) | Type 2 diabetes (n = 43) | p value | |
|---|---|---|---|
| Sex (male/female) | 24/33 | 20/23 | NS |
| Age (years) | 49 ± 17 | 59 ± 16 | 0.003 |
| BMI (kg/m2) | 22.4 ± 3.6 | 26.1 ± 4.3 | <0.001 |
| Duration of diabetes (years) | 9.5 ± 7.5 | 11.5 ± 9.6 | NS |
| Insulin therapy (+/−) | 57/0 | 19/24 | <0.001 |
| GA (%) | 25.0 ± 8.4 | 22.7 ± 5.2 | NS |
| HbA1c (%) | 8.4 ± 2.0 | 9.3 ± 1.8 | 0.013 |
| GA/HbA1c | 2.96 ± 0.37 | 2.45 ± 0.38 | <0.001 |
| CPR (ng/ml) | 0.30 ± 0.66 | 2.63 ± 1.42 | <0.001 |
| Microvascular complications (+/−) | 16/41 | 23/20 | 0.010 |
| Neuropathy (+/−) | 9/48 | 7/36 | NS |
| Retinopathy (+/−) | 5/52 | 10/33 | 0.045 |
| Nephropathy (+/−) | 9/48 | 17/26 | 0.007 |
| Smoking status (+/−) | 13/44 | 9/34 | NS |
| Hypertension (+/−) | 15/42 | 18/25 | NS |
| Dyslipidemia (+/−) | 22/35 | 26/17 | 0.030 |
| EPC counts/106 events | |||
| CD34+ | 366.0 (258.7, 531.5) | 499.0 (317.0, 659.0) | 0.020 |
| CD34+CD133+ | 208.0 (140.3, 319.9) | 271.7 (191.0, 433.1) | 0.036 |
| CD34+CD309+ | 8.0 (3.0, 12.0) | 5.0 (2.0, 10.0) | NS |
| CD34+CD133+CD309+ | 5.0 (2.0, 10.0) | 3.6 (1.0, 8.0) | NS |
Smoking status (+): current smoking, hypertension (+): blood pressure ≥130/80 mmHg, dyslipidemia (+): LDL-Chol ≥120 mg/dl and/or HDL-Chol <40 mg/dl and/or TG ≥150 mg/dl
Data are expressed as n (+/−), mean ± SD, or median (interquartile range)
P < 0.05 was considered statistically significant. NS denotes not significant. Differences between the two groups were calculated using Pearson’s chi-squared test, Student’s t test, or the Mann–Whitney U test, depending on the normality of the examined sample
Study 2
Thirty-seven outpatients with type 1 diabetes who had been treated at the Osaka Medical College Hospital between 2014 and 2015 were recruited for study 2. Table 2 shows their clinical characteristics. The exclusion criteria were known myocardial infarction, cerebral infarction, or obstructive arteriosclerosis, concomitant malignancy or inflammatory disease, and pregnancy or lactation. The definitions of smoking status, hypertension, and dyslipidemia were same as those in study 1. In addition, patients were excluded if they met one or more of the following criteria: a maximum carotid intima-media thickness (max IMT) ≥1.4 mm, an ST-T abnormality, and an abnormal Q wave with 12-lead electrocardiograms, and an ankle–brachial index of ≤0.90 or >1.40. We also excluded patients with unstable HbA1c levels, defined as an absolute variation in HbA1c over the past 3 months of ≥1.2%, as well as individuals with diabetic nephropathy stage 3, 4, or 5, those with diabetic proliferative retinopathy, underweight patients (BMI <17), and moderately or severely obese patients (BMI >30).
Table 2.
Clinical characteristics, circulating levels of EPCs, and parameters charting the glycemic variability of 37 patients with type 1 diabetes (study 2)
| Sex (male/female) | 13/24 |
| Age (years) | 45 ± 13 |
| BMI (kg/m2) | 22.1 ± 2.7 |
| Duration of diabetes (years) | 11.2 ± 8.5 |
| Treatment of insulin (MDI/CSII) | 10/27 |
| Fasting glucose | 146.0 ± 62.6 |
| GA (%) | 21.7 ± 4.1 |
| HbA1c (%) | 7.5 ± 1.0 |
| GA/HbA1c | 2.89 ± 0.28 |
| CPR (ng/ml) | 0.12 ± 0.31 |
| Microvascular complications (+/−) | 6/31 |
| Neuropathy (+/−) | 3/34 |
| Retinopathy (+/−) | 2/35 |
| Nephropathy (+/−) | 3/34 |
| Smoking status (+/−) | 8/29 |
| Hypertension (+/−) | 10/27 |
| Dyslipidemia (+/−) | 13/24 |
Data are expressed as n (±) or mean ± SD
MDI multiple daily injections, CSII continuous subcutaneous insulin infusion, smoking status (+): current smoking, hypertension (+): blood pressure ≥130/80 mmHg, dyslipidemia (+): LDL-Chol ≥120 mg/dl and/or HDL-Chol <40 mg/dl and/or TG ≥150 mg/dl
The study protocol was approved by the ethics committee of Osaka Medical College on July 7, 2014 (Rin-19). Each subject was informed of the purpose of the study and their written consent was obtained.
Methods
Study 1
In all patients, venous blood samples were obtained during the morning following an overnight fast. These samples were used to assess EPCs, glycemic parameters (fasting C-peptide level, glycated albumin [GA], and HbA1c), and lipid profiles (LDL cholesterol, HDL cholesterol, and triglycerides). The clinical characteristics of all patients (age, sex, BMI, smoking status, duration of diabetes in years, systolic blood pressure, and diastolic blood pressure) were obtained on the day of sample collection. We subsequently compared EPC counts for the two types of diabetes.
Study 2
The patients with type 1 diabetes were allowed to continue with either their multiple daily injections (27 patients) or their continuous subcutaneous insulin infusion (10 patients) during the study. On the first or seventh day that they were equipped with the CGM device, we collected venous blood samples under the same conditions that had been used in study 1. From the samples, the following were assessed: EPCs, glycemic parameters (fasting plasma glucose level, fasting C-peptide level, GA, and HbA1c), lipid profiles, and estimated glomerular filtration rate (eGFR). The clinical characteristics assessed were the same as those in study 1.
The protocol for study 2 included the use of the CGM system (iPro2; Medtronic, Northridge, CA, USA) to measure fluctuations in blood glucose levels for seven consecutive days. Calibrations were performed four times daily for each subject. To avoid bias which could result from the insertion and removal of the CGM, the analysis was limited to the data obtained during the five intermediary days (120 h) of recording. We determined the correlation between EPC count and glycemic variability.
Endothelial progenitor cells
EPCs were determined by the flow cytometry method, as reported previously [14]. Fresh peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation (Lymphoprep; Axis-Shield PoC AS, Oslo, Norway) and immediately subjected to cellular staining. The cells were stained with the following antibodies: Brilliant Violet (BV) 421 mouse anti-human CD34 (clone 581), phycoerythrin (PE) CD133/1 (clone AC133), and Alexa Fluor 647 mouse anti-human CD309 (VEGFR-2) (clone 89106). The following isotype control antibodies were used: BV 421 mouse IgG1, κ (clone X40), PE mouse IgG1, κ (clone MOPC-21), Alexa Fluor 647 (clone MOPC-21). After surface staining for 30 min at 4 °C in the dark, the cells were washed with phosphate-buffered saline solution. Surface-stained cells were then subjected to fluorescence-activated cell sorting (BD FACS Aria ™). The frequency of EPCs was defined as the number of cells per 106 events for each sample. According to the surface expression of CD34, CD133, and CD309, EPCs were determined by flow cytometry and differentiated into four subpopulations, namely CD34+, CD34+CD133+, CD34+CD309+, and CD34+CD133+CD309+ cells. These four cell subpopulations were counted as EPCs, as described previously [14].
Parameters of glycemic variability and glycemic control
The mean blood glucose level, the standard deviation (SD) of the blood glucose level, the mean amplitude of glycemic excursion (MAGE) [15], the J index [16], the M value [17], the mean of daily differences (MODD) [18], the low blood glucose index (LBGI), and the high blood glucose index (HBGI) [19] were measured from the data recorded by the CGM system as parameters of glycemic variability in study 2. The MAGE values were calculated by measuring the arithmetic mean of the differences between consecutive peaks and nadirs, provided the differences were greater than 1 SD of the mean glucose value. The J index was calculated using the following formula: J index = 0.001 × (mean glucose + SD)2. The M value is a logarithmic transformation of the glycemic deviation from an arbitrary assigned ideal glucose value. The MODD was calculated as the mean absolute value of the differences between glucose values measured at the same time for two consecutive days. The LBGI and HBGI accounted for the frequency and amplitude of hypoglycemic and hyperglycemic events, respectively, and allowed the risk for adverse glycemic events to be assessed.
The HbA1c levels were determined by high-performance liquid chromatography using an ADAMS-A1c HA-8181 instrument (Arkray Inc., Kyoto, Japan), and the serum GA level was simultaneously measured by an enzymatic method (Lucica GA-L, Asahi Kasei Pharma, Tokyo, Japan) using a Beckman Coulter (Brea, CA, USA) AU5800 autoanalyzer. The GA/HbA1c ratio was calculated by dividing the GA value by the HbA1c value.
Intima media thickness
The carotid IMT [20, 21] 5.8 ± 6.0 months (mean ± SD) after counting EPCs was measured in 24 patients randomly selected from 37. The max IMT was defined as the IMT at the site of greatest thickness on both sides. Measurements were taken on both the left side and the right side of the IMT at the site of the greatest thickness and at a point 1 cm upstream and another 1 cm downstream from the site of greatest thickness. The average of these six values were computed and used as a representative value (mean IMT) for the participant. All measurements were conducted by the same physician, who was unaware of the clinical characteristics of the subjects. A series of ultrasonographic images of the carotid artery were obtained using an echotomographic system (SSA-790A; Toshiba) with an electrical transducer (mid frequency of 7.5 MHz).
Renal function
The eGFR was calculated in 32 patients randomly selected from 37 using the following equation: eGFR = 194 × serum creatinine−1.094 × age−0.287 × 0.739 (if female), where serum creatinine is in mg/dl and age is in years [22]. eGFR(0) and ΔeGFR(3 M) were defined as the eGFR on the day of sample collection and the change in the eGFR after 3 months, respectively.
Statistical analysis
The data were expressed as the mean ± standard deviation (SD) or the median and interquartile range, depending on the sample distribution. EPC counts were log-transformed into normally distributed values. Comparisons among groups were evaluated using Pearson’s chi-square test, the unpaired Student t test, or the Mann–Whitney U test, as appropriate. Univariate regression analyses were performed to assess the statistical associations between normally distributed variables using Pearson’s correlation coefficient. p < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using the JMP® 11 software program (SAS Institute Inc., Cary, NC, USA).
Results
Study 1
Table 1 presents a comparison of the baseline characteristics for patients with type 1 diabetes and patients with type 2 diabetes. Patients with type 1 diabetes had a significantly lower mean age (49 ± 17 vs. 59 ± 16 years; p = 0.003) and HbA1c level (8.4 ± 2.0 vs. 9.3 ± 1.8%; p = 0.013) than patients with type 2 diabetes. The prevalence of microvascular complications (28.1 vs. 53.5%; p = 0.010) and dyslipidemia (38.6 vs. 60.5%; p = 0.030) was also significantly lower among patients with type 1 diabetes than among patients with type 2 diabetes. In contrast, the GA/HbA1c ratio was significantly higher in patients with type 1 diabetes than in patients with type 2 diabetes (2.96 ± 0.37 vs. 2.45 ± 0.38%; p < 0.001). The number of CD34+ cells (expressed as [median (interquartile range)]) was significantly lower for patients with type 1 diabetes [366.0 (258.7, 531.5)] than for patients with type 2 diabetes [499.0 (317.0, 659.0)] (p = 0.020). The number of CD34+CD133+ cells was also significantly lower for patients with type 1 diabetes [208.0 (140.3, 319.9)] than for patients with type 2 diabetes [271.7 (191.0, 433.1)] (p = 0.036). No significant between-group difference was found for CD34+CD309+ cells [8.0 (3.0, 12.0) vs. 5.0 (2.0, 10.0)] or CD34+CD133+CD309+ cells [5.0 (2.0, 10.0) vs. 3.6 (1.0, 8.0)].
Study 2
The number of circulating CD34+ cells (expressed as [median (interquartile range)]) was 435.5 (263.2, 597.0), and that of CD34+CD133+ cells was 263.8 (169.0, 355.7). The results of the measurements of the four parameters of glycemic variability by CGM were as follows: SD: 60.6 ± 16.5 (mg/dl), M value: 36.4 ± 17.7 (mg/dl), J index: 46.4 ± 21.1, MODD: 65.5 ± 18.2 (mg/dl), MAGE: 140.4 ± 40.6 (mg/dl), LBGI: 6.1 ± 3.8, and HBGI: 10.0 ± 5.3 (mean ± SD).
Table 3 shows correlation coefficients of the natural-logarithm-scaled CD34+ cell count (logCD34+) and the natural-logarithm-scaled CD34+CD133+ cell count (logCD34+CD133+) with markers of glycemic variability. LogCD34+ exhibited a significantly negative association with the J index (r = −0.342, p = 0.039) among the seven parameters of glycemic variability, but logCD34+CD133+ did not show any correlation with any of the parameters of glycemic variability.
Table 3.
Association between logCD34+ and the parameters of glycemic variability (study 2)
| Parameter | r | p value | |
|---|---|---|---|
| logCD34+ | SD | −0.068 | NS |
| M value | −0.292 | NS | |
| J index | −0.342 | 0.039 | |
| MAGE | −0.380 | NS | |
| MODD | −0.054 | NS | |
| LBGI | 0.256 | NS | |
| HBGI | −0.246 | NS | |
| logCD34+CD133+ | SD | 0.058 | NS |
| M value | −0.137 | NS | |
| J index | −0.158 | NS | |
| MAGE | 0.085 | NS | |
| MODD | 0.284 | NS | |
| LBGI | −0.098 | NS | |
| HBGI | 0.140 | NS |
The relationship was calculated using Pearson’s correlation coefficient
NS denotes not significant
p < 0.05 was considered to indicate statistical significance
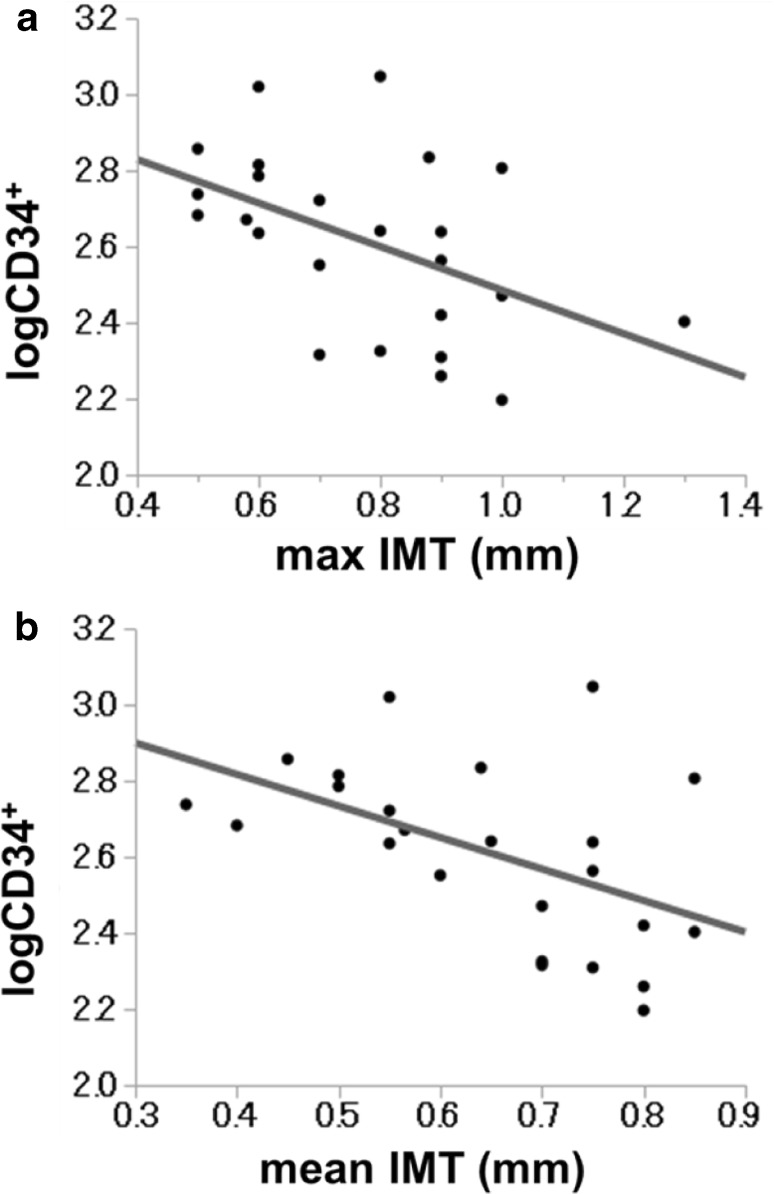
In 24 patients, the average (±SD) values of the max IMT and the mean IMT were 0.78 ± 0.09 (mm) and 0.65 ± 0.07 (mm), respectively. LogCD34+ was significantly negatively associated with the max IMT (r = −0.486, p = 0.012) and the mean IMT (r = −0.503, p = 0.016) (Table 4A; Fig. 1). On the other hand, logCD34+CD133+ showed no correlation with the max or mean IMT.
Table 4.
Association between EPCs and markers of macrovascular complications (study 2)
| Parameter | r | p value | |
|---|---|---|---|
| (A) Association between EPCs and intima-media thickness (IMT) | |||
| logCD34+ | max IMT | −0.486 | 0.012 |
| mean IMT | −0.503 | 0.016 | |
| logCD34+CD133+ | max IMT | −0.309 | NS |
| mean IMT | −0.386 | NS (0.062) | |
| (B) Association between EPCs and estimated glomerular filtration (eGFR) | |||
| CD34+ | eGFR(0) | −0.011 | NS |
| ΔeGFR(3 M) | 0.354 | 0.047 | |
| CD34+CD133+ | eGFR(0) | 0.099 | NS |
| ΔeGFR(3 M) | 0.152 | NS | |
For definitions of max and mean IMT, see the main text
p < 0.05 was considered to indicate statistical significance. NS denotes not significant
In (A), the relationship was calculated using Pearson’s correlation coefficient
In (B), the relationship was calculated using Spearman’s correlation coefficient
Fig. 1.
Association between logCD34+ and the carotid IMT among 24 patients with type 1 diabetes. a Association between logCD34+ and the max IMT. b Association between logCD34+ and the mean IMT
In 32 patients, the average (±SD) levels of the eGFR(0) and the ΔeGFR(3 M) were 84.4 ± 15.5 (ml/min/1.73 m2) and −3.0 ± 5.8 (ml/min/1.73 m2), respectively. The CD34+ cell count showed a significant positive association with ΔeGFR(3M) (r = 0.354, p = 0.047), but was not associated with eGFR(0) (r = −0.011, NS) (Table 4B). The number of CD34+CD133+ cells was associated with neither eGFR(0) nor ΔeGFR(3 M).
Discussion
This is the first study to demonstrate (1) lower circulating EPC levels in patients with type 1 diabetes than in patients with type 2 diabetes, (2) an inverse association between EPC count and the J index among parameters of glycemic variability in patients with type 1 diabetes, and (3) an inverse association between EPC count and IMT or ΔeGFR(3 M) in patients with type 1 diabetes.
First, study 1 revealed that the number of EPCs (CD34+ cells and CD34+CD133+ cells) was significantly decreased in patients with type 1 diabetes compared to the number of EPCs in patients with type 2 diabetes. It is well known that plasma glucose levels are usually more unstable in patients with type 1 diabetes than in those with type 2 diabetes. Indeed, the GA/HbA1c ratio, a marker of glucose fluctuation [23], was significantly higher in patients with type 1 diabetes than in patients with type 2 diabetes. Therefore, we hypothesized that increased glycemic excursions, in addition to other risk factors for arteriosclerosis, may reduce the number of EPCs. In study 2, we aimed to elucidate the influence of glycemic variability, as indicated by the CGM, on the number of EPCs in the peripheral blood of patients with diabetes.
Second, in the cross-sectional study 2, we found that the J index, a parameter of glycemic variability calculated from the CGM recordings, was significantly and inversely correlated with logCD34+. The EPC count based on CD34+ was also found to be lower in subjects with higher levels of all other parameters except the J index than in subjects with lower levels of those parameters, although the differences were not significant.
The J index was proposed by Wojcicki in 1995 to be a parameter that charts glycemic variability; it is known to be related to the period during which the glucose levels change within the targeted blood glucose range (80–180 mg/dl). The J index is characterized by high sensitivity to both the mean glucose level and the glycemic variability [16]. Moreover, among these parameters of glycemic variability, the J index may be the most specific parameter that reflects both chronic hyperglycemia and increased glycemic excursion, and may be the most suitable for evaluating the glucose variation in subjects with relatively stable glucose levels, such as patients with type 1 diabetes. The J index presented a significant negative association with EPC count in this study, suggesting that the degree of glycemic excursion in the hyperglycemic state may be related to the reduced number of circulating EPCs in patients with type 1 diabetes.
Third, when evaluating the cause–effect relationship between low EPC count and chronic vascular complications, we found a positive correlation between EPC count and IMT in a carotid echogram or eGFR. A previous study reported that a lower eGFR was independently associated with cardiovascular events [24]. Few have reported that chronic hyperglycemia and increased glucose variation induce accelerated atherosclerosis synergistically. However, it is well known that each of these factors (chronic hyperglycemia and increased glucose variation) results in progression of arteriosclerosis [4]. Some reports have shown that glucose fluctuations during the postprandial period have a more specific triggering effect on oxidative stress than sustained chronic hyperglycemia [25]. The significant association of the number of EPCs with IMT and ΔeGFR(3 M) led us to speculate that increased glycemic excursions could result in the progression of diabetic vascular complications by reducting the EPC count. However, the mechanisms by which increased glycemic excursions can lead to a reduction in the EPC count have not yet been clarified.
The mechanism underlying the association between EPCs and endothelial repair remains unknown. Previous articles have reported that the cytokine SDF-1 [26] and the growth factors VEGF [27] and G-CSF [28] are involved in the production of EPCs or in their mobilization to the local part of the blood vessel. A decrease in EPCs may cause an increase in IMT thickness and a decrease in eGFR through the action of these cytokines and growth factors.
One limitation of our study was its cross-sectional design, which did not allow a longitudinal follow-up of the patients. This limitation could be addressed through subsequent longitudinal studies.
In conclusion, this study demonstrated a negative correlation between the number of EPCs and the J index, which reflects both hyperglycemia and glycemic variability, in patients with type 1 diabetes. This indicates that increased glycemic variability, as well as hyperglycemia, may have a strong reducing effect on the number of EPCs. Our study may indicate that it is important to minimize hyperglycemic spikes in order to prevent endothelial damage, and suggests that vascular burden may be reduced in individuals with diabetes by controlling fluctuations in glucose levels.
Acknowledgements
We are grateful to Ms. Akiko Irie for her support and excellent technical assistance, Professor Masaaki Hoshiga for his valuable suggestion, and to Professor Yasuichiro Nishimura for his advice regarding our statistical analysis.
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent or a substitute for it was obtained from all patients before they were included in the study.
References
- 1.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care. 2011;34(Suppl 2):S285–S290. doi: 10.2337/dc11-s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. [DOI] [PubMed]
- 4.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lamerie NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Parmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito K, Ciotola M, Carleo D, Schisano B, Sardelli L, Di Tommaso D, Misso L, Saccomanno F, Ceriello A, Giugliano D. Post-meal glucose peaks at home associate with carotid intima-media thickness in type 2 diabetes. J Clin Endocrinol Metab. 2008;93(4):1345–1350. doi: 10.1210/jc.2007-2000. [DOI] [PubMed] [Google Scholar]
- 7.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 8.Torimoto K, Okada Y, Mori H, Tanaka Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:1. doi: 10.1186/1475-2840-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999;34:146–154. doi: 10.1016/S0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 10.Action to Control Cardiovascular Risk in Diabetes Study Group, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. [DOI] [PMC free article] [PubMed]
- 11.Avogaro A, Fadini GP, Gallo A, Pagnin E, de Kreutzenberg S. Endothelial dysfunction in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2006;16(Suppl 1):S39–S45. doi: 10.1016/j.numecd.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Hortenhuber T, Rami-Mehar B, Satler M, Nagl K, Hobaus C, Hollerl F, Koppensteiner R, Schernthaner G, Schober E, Schernthaner GH. Endothelial progenitor cells are related to glycemic control in children with type 1 diabetes over time. Diabetes Care. 2013;36:1647–1653. doi: 10.2337/dc12-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 14.Fadini GP, Sartore S, Agostini C, Avogaro A. Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care. 2007;30:1305–1313. doi: 10.2337/dc06-2305. [DOI] [PubMed] [Google Scholar]
- 15.FJ Service, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 16.Wojcicki JM. “J”-index. A new proposition of the assessment of current glucose control in diabetic patients. Horm Metab Res. 1995;27:41–42. doi: 10.1055/s-2007-979906. [DOI] [PubMed] [Google Scholar]
- 17.Schlichtkrull J, Munck O, Jersild M. The M-valve, an index of blood-sugar control in diabetics. Acta Med Scand. 1965;177:95–102. doi: 10.1111/j.0954-6820.1965.tb01810.x. [DOI] [PubMed] [Google Scholar]
- 18.Molnar GD, Taylor WF, Ho MM. Day-to-day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia. 1972;8:342–348. doi: 10.1007/BF01218495. [DOI] [PubMed] [Google Scholar]
- 19.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21:1870–1875. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 20.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.CIR.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 21.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine. A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. [DOI] [PubMed]
- 23.Yoshiuchi K, Matsuhisa M, Katakami N, Nakatani Y, Sakamoto K, Matsuoka T, Umayahara Y, Kosugi K, Kaneto H, Yamasaki Y, Hori M. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J. 2008;55:503–507. doi: 10.1507/endocrj.K07E-089. [DOI] [PubMed] [Google Scholar]
- 24.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 25.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–7. [DOI] [PubMed]
- 26.Yamaguchi J, Fukushima K, Masuo O, Kawamoto A, Marcy S, Murasawa S, Marta BM, Matsuda H, Douglas WL, Jeffrey MI, Ashihara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitement for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.CIR.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 27.Asahara T, Takahashi T, Masuda H, Christoph K, Donghui C, Iwaguro H, Inai Y, Marcy S, Jeffery MS. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18(14):3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiffany MP, Jonathan DP, Jonathan MH, Micheael T, Moshe B, Maria R, Philip JM, Elizabeth JR, Hanh MK, Susan FL, Toren F, Cannon RO., III Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25(2):296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]