Abstract
The aim of this study was to clarify the incidences of and the risk factors for severe retinopathy requiring photocoagulation therapy and albuminuria in Japanese patients with childhood-onset type 1 diabetes mellitus. A total of 756 patients from a cohort study by the Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes were included in the study. Patients were registered in 1995 or 2000, and HbA1cwas measured every 4 months and analyzed in central hospital for an average of 6 years. The presence of severe retinopathy requiring laser photocoagulation and the presence of albuminuria was checked for during the period 2010–2011. During a median of 18 (range: 15–21) years, 34 out of 756 patients underwent laser photocoagulation and 57 out of 605 patients developed albuminuria. A Cox proportional hazards model showed that the risk of severe retinopathy requiring laser photocoagulation increased by 1.15 (95% confidence interval [CI] 1.03–1.29, p = 0.012) with each increase of a year in the age at onset, by 4.03 (95% CI 1.20–13.5, p = 0.024) in females, and by 2.05 (95% CI 1.69–2.49, p < 0.0001) with each increase of 1% in HbA1c. The risk of albuminuria increased significantly, by 1.09 (95% CI 1.01–1.18 p = 0.037), with each increase of a year in the age at onset and by 2.38 (95% CI 1.93–2.97 p < 0.0001) with each increase of 1% in HbA1c. In Japanese patients with childhood-onset type 1 diabetes, older age at the onset of diabetes, female rather than male gender, and higher HbA1c were found to increase the risk of requiring photocoagulation. No patients with HbA1c < 7.5% developed severe retinopathy requiring photocoagulation therapy. The risk of developing albuminuria increased with age at onset of diabetes and HbA1c. Female gender was a strong risk factor for severe retinopathy requiring photocoagulation, but not for albuminuria.
Keywords: Child-onset type 1 diabetes, Laser photocoagulation, Microalbuminuria, Macroalbuminuria, Microangiopathy
Introduction
Microvascular complications develop in many patients with type 1 diabetes mellitus, and these complications are associated with a reduction in quality of life [1]. Over the last few decades, the cumulative incidence of retinopathy and nephropathy in patients with type 1 diabetes has declined [2, 3], which has been attributed to intensified insulin treatment [4, 5]. The incidence of type 1 diabetes mellitus is much lower in Japanese than in Caucasian children [6], and the effect of blood glucose control and other clinical characteristics on retinopathy and nephropathy status has not been reported among Japanese patients with childhood-onset type 1 diabetes.
In 1991, a high mortality rate among young Japanese patients with type 1 diabetes was reported by the Diabetes Epidemiology Research International Mortality Study Group [7]. The Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT) was established in 1994 to create a registry of a large cohort of patients and facilitate prospective studies aimed at improving the quality of therapy for children with type 1 diabetes in Japan [8]. Participants in the JSGIT include pediatric diabetologists and endocrinologists who agreed to participate in the collaborative network. Thirty-one hospitals throughout Japan agreed to join the JSGIT, which started cohort studies that were multicenter, observational, non-population-based surveys of childhood-onset type 1 diabetes, including the collection of data on blood glucose control and the state of diabetic complications.
The aims of the study reported in the present paper were to clarify the risk factors for severe retinopathy requiring laser photocoagulation therapy and for albuminuria in Japanese patients with childhood-onset type 1 diabetes mellitus.
Patients and methods
Subjects
Participants were children from the Japanese 1995 and 2000 cohorts of the JSGIT study group. The subjects from the 1995 cohort were registered in 1995 and met the following criteria: (1) the ages of registered patients ranged from 6 to 18 years; (2) they were born between 1977 and 1988 and diagnosed before 1995; and (3) they were clinically diagnosed with diabetes and insulin dependency based on the World Health Organization (WHO) guidelines [9]. For the 1995 cohort, HbA1c was measured and standardized every 4 months from July 1995 to October 1999. The subjects from the 2000 cohort were registered in 2000 and met the following criteria: (1) they developed type 1 diabetes before 18 years of age; (2) they were born between 1982 and 1999 and diagnosed before 2000; and (3) they were clinically diagnosed with diabetes and insulin dependency based on the WHO guidelines [9]. For the 2000 cohort, HbA1c was measured and standardized every 4 months from July 2000 to February 2008. There were 546 patients in the 1995 cohort and 710 patients in the 2000 cohort. We used the data from the 2000 cohort for the 108 patients who were registered with both the 1995 and 2000 cohorts. All attending physicians obtained informed consent from the patients when presenting them with the questionnaire survey reported here. The participants provided their informed consent and the study was approved by the Institutional Review Board of Tokyo Women’s Medical University under the approval numbers 874 and 1723.
Methods
A questionnaire was sent to the attending physicians of the 1995 cohort in 2010 and the 2000 cohort in 2011. After obtaining informed consent from the patients, the physicians reported whether each patient had undergone laser photocoagulation or not, and whether they were positive or negative for albuminuria. If the patient had undergone photocoagulation, we also requested the date and year of the treatment. Measurement of the albumin-to-creatinine ratio (UAE) in spot urine was carried out in 2010–2011. Depending on the UAE, the renal status of each patient was classified as follows: UAE of <30 mg/g Cr was considered to indicate normoalbuminuria, UAE of 30–299 mg/g Cr indicated microalbuminuria, and UAE ≥ 300 mg/g Cr indicated macroalbuminuria.
In the present study, the HbA1c determination was initially performed according to the standard of the Japanese Diabetes Society (JDS) [10]. The HbA1c (NGSP) value was calculated using the formula HbA1c (%) = HbA1c (JDS) (%) + 0.4% [11]. The median HbA1c was calculated based on the HbA1c values obtained from July 1995 to October 1999 for patients in the 1995 cohort, and from July 2000 to February 2008 for patients in the 2000 cohort. The recent HbA1c value was determined by calculating the median HbA1c value at the time of the questionnaire.
Statistical analysis
The SPSS ver.21 (IBM Japan, Tokyo, Japan) statistical software package was used for data evaluation and statistical analysis. The relative contribution of covariates to the risk for photocoagulation was analyzed using a Cox proportional hazards model and the stepwise selection of parameters. We could not evaluate the date of appearance of albuminuria, so the risk of albuminuria was analyzed by logistic regression analysis. The cumulative incidence of laser photocoagulation was analyzed by the Kaplan–Meier method, and the log-rank test was used to compare incidence curves. A p value of <0.05 was considered to indicate statistical significance.
Results
Patient background
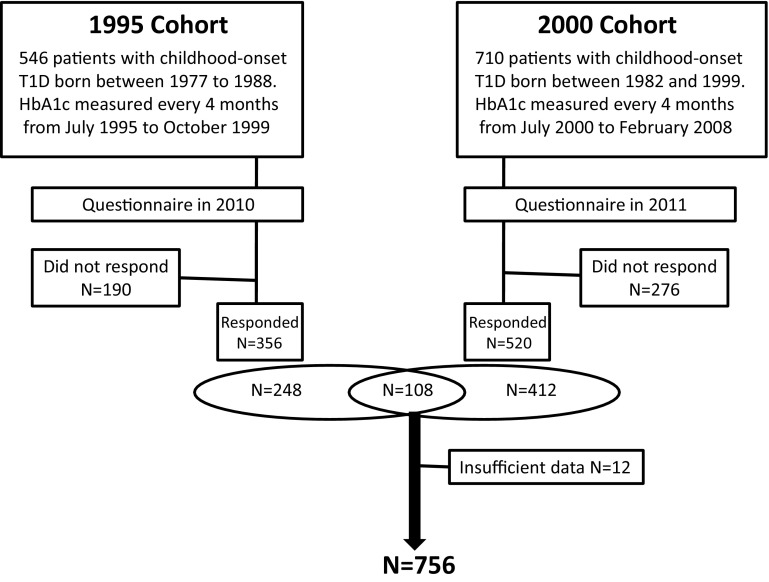
Questionnaires were sent to the attending physicians of all 546 patients registered in the 1995 cohort in 2010 and to the attending physicians of all 710 patients registered in the 2000 cohort in 2011. Twelve patients were excluded due to missing information. We collected 356 responses from the 1995 cohort and 520 responses from the 2000 cohort. One hundred eight of the patients were included in both the 1995 cohort and the 2000 cohort. Finally, at the time of analysis, the number of study subjects was 756 (Fig. 1).
Fig. 1.
Flow diagram of the patients who participated in this study
There was no difference in age and gender between those who responded to our questionnaire and those who did not (Table 1). For the 756 patients included in the final analysis, the median age of diagnosis was 7 (range: 4–10) years, and 38.4% were males. At cohort registration in 1995 or 2000, the median age was 13 (9–16) years, and we measured HbA1c every 4 months for 6 (4–7) years. Median HbA1c during the observational period was 8.2 (7.6–9.0) %. At the time of the questionnaire survey in 2010 or 2011, the median age and diabetes duration were 27 (23–29) years and 18 (15–21) years, respectively. Median HbA1c in 2010 or 2011 was 7.5 (6.7–8.4) %. Among these patients, 34 had undergone laser photocoagulation, 3 had developed blindness, and 57 had developed albuminuria.
Table 1.
Characteristics of the study participants and nonparticipants
| Participants | Nonparticipants | p | |
|---|---|---|---|
| N = 756 | N = 359 | ||
| Gender (male/female) | 290/466 | 156/203 | ns.* |
| Age at onset of diabetes (years) | 7 (4–10) | 7 (4–11) | ns.** |
| Cohort registration in 1995 or 2000 | |||
| Age (years) | 13 (9–16) | 13 (10–16) | ns.** |
| Median observational period in years | 6.1 (4.0–7.3) | ||
| Median HbA1c during observational period (%) | 8.2 (7.6–9.0) | ||
| Questionnaire survey in 2010 or 2011 | |||
| Age in years | 27 (23–29) | ||
| Diabetes duration in years | 18 (15–21) | ||
| Median HbA1c in 2010 or 2011, in % | 7.5 (6.7–8.4) | ||
Median (interquartile range) values are generally shown in the table
* Comparison with participants using the chi-squared test
** Comparison with participants using the Mann–Whitney test
Influence of HbA1c on the cumulative incidence of laser photocoagulation
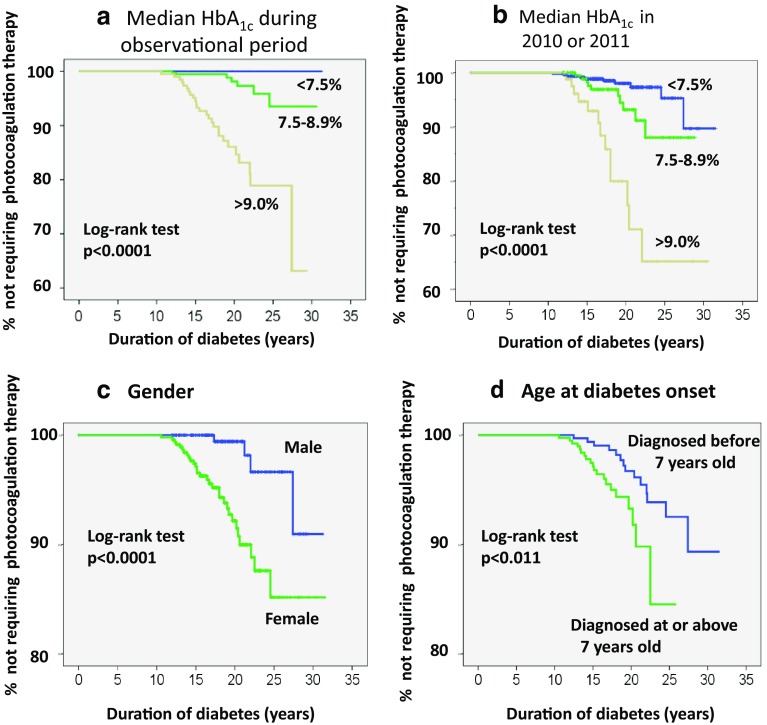
To evaluate the influence of past blood glucose control, we divided the patients into three groups according to the value of HbA1c: HbA1c < 7.5%, 7.5% ≤ HbA1c < 9%, and HbA1c ≥ 9%. None of the 152 patients with a median HbA1c during the observational period of <7.5% underwent laser photocoagulation. Both the median HbA1c during the observational period and the median HbA1c in 2010 or 2011 significantly influenced the cumulative need for laser photocoagulation (Fig. 2a, b). The median HbA1c during the observational period and the median HbA1c in 2010 or 2011 were significantly higher in patients who had undergone photocoagulation therapy than in patients who had not (Table 2).
Fig. 2.
Charts showing the cumulative incidence of photocoagulation therapy as a function of duration of diabetes. a Comparison of patients with different median HbA1c values during the observational period. b Comparison of patients with different median HbA1c values during 2010 or 2011. c Comparison of male and female patients. d Comparison of patients based on age at diagnosis (younger or older than 7 years). The data were obtained by the Kaplan–Meier method
Table 2.
Comparison of clinical characteristics between patients who did and those who did not undergo photocoagulation therapy
| Variable | PC (+) | PC (−) | p |
|---|---|---|---|
| n | 34 | 722 | |
| Gender (male/female) | 4/30 | 286/436 | <0.001* |
| Age at onset (years) | 8 (6–11) | 7 (4–10) | 0.33** |
| Median HbA1c during the observational period (%) | 10.5 (9.5–11.8) | 8.1 (7.6-9.0) | <0.0001** |
| Age in 2010 or 2011 (years) | 29 (26–31) | 27 (23–29) | |
| Duration of diabetes in 2010 or 2011 (years) | 21 (17–24) | 18 (15–21) | <0.001** |
| Median HbA1c in 2010 or 2011 (%) | 8.4 (7.7–9.6) | 7.4 (6.7–8.3) | <0.0001** |
Median (interquartile range)
PC photocoagulation therapy
** Comparison with participants using the Kruskal–Wallis test
Influence of gender on the cumulative incidence of laser photocoagulation
Four of the 290 males (1.4%) and 30 of the 466 females (6.4%) underwent laser photocoagulation. The cumulative incidence of laser photocoagulation was significantly higher in females than males (p < 0.0001) (Fig. 1c). Age, duration of diabetes, and age at diabetes onset did not differ significantly between genders. However, the median HbA1c during the observational period was significantly higher in females than in males (8.3 (7.7–9.2) % vs. 8.0 (7.4–8.8) %, respectively; p = 0.0001).
Influence of age at diabetes onset on cumulative incidence of laser photocoagulation
Since the median age at diabetes onset was 7 years, we divided patients into two groups according to the age at diabetes onset: those diagnosed at age ≥7 years and those diagnosed at <7 years. Cumulative performance rates of laser photocoagulation were significantly higher among patients diagnosed with type 1 diabetes at 7 years or older compared with those diagnosed at younger than 7 years (p < 0.05) (Fig. 2d). The clinical characteristics of patients diagnosed at younger than 7 years vs. 7 years or older were as follows: duration of diabetes: 20 (16–23) years vs. 17 (15–19) years, respectively (p < 0.0001); HbA1c during the observational period: 8.2 (7.7–9.0) % vs. 8.1 (7.5–9.1) %, respectively (p = 0.513).
Risk for severe retinopathy requiring laser photocoagulation
Based on a Cox proportional hazards model, the risk for severe retinopathy requiring laser photocoagulation significantly increased by 1.15 (95% confidence interval [CI] 1.03–1.29, p = 0.012) with each increase of a year in the age at onset, by 4.03 (95% CI 1.20–13.5, p = 0.024 in females, and by 2.05 (95% CI 1.69–2.49, p = 0.0005) with each 1% increase in HbA1c during the observational period (Table 3).
Table 3.
Analysis of the risk factors for severe retinopathy requiring laser photocoagulation using multivariate Cox proportional hazards models
| Variable | Hazard ratio (95% CI) | p value |
|---|---|---|
| Older age at onset | 1.15 (1.03–1.29) (per year) | 0.012 |
| Female gender | 4.03 (1.20–13.5) | 0.024 |
| Higher median HbA1c during the observational period | 2.05 (1.69–2.49) (per %) | <0.0001 |
Laser photocoagulation was analyzed as a time-dependent covariant
Risks for microalbuminuria and macroalbuminuria
Information on albuminuria was available for 605 patients. The median HbA1c was significantly higher in patients who developed albuminuria than in those who developed microalbuminuria or had normoalbuminuria (Table 4). The risk for albuminuria significantly increased by 2.38 (95% CI 1.93–2.97, p < 0.0001) with a 1% increase in HbA1c during the observational period, and by 1.09 (95% CI 1.01–1.18, p = 0.025) with each increase of a year in the age at onset (Table 5).
Table 4.
Comparison of clinical characteristics between patients with and without microalbuminuria or macroalbuminuria
| Variable | Patients with normoalbuminuria | Patients with microalbuminuria | Patients with macroalbuminuria | p |
|---|---|---|---|---|
| n | 548 | 43 | 14 | |
| Gender(male/female) | 205/343 | 18/25 | 4/10 | |
| Age at onset (years) | 8 (4–10) | 9 (6–13) | 8 (5–10) | |
| Median HbA1c during observational period (%) | 8.1 (7.5–8.9) | 9.1 (8.2–10.6) | 11.5 (10.3–12.8) | <0.0001** |
| Age in 2010 or 2011 (years) | 27 (24–29) | 28 (24–30) | 28 (26–29) | 0.375 |
| Duration of diabetes in 2010 or 2011 (years) | 18 (16–21) | 17 (14–22) | 20 (16–24) | 0.171 |
| Median HbA1c in 2010 or 2011 (%) | 7.3 (6.7–8.1) | 8.4 (7.5–9.7) | 8.6 (8.1–11.1) | <0.0001** |
Values in the table are generally median (interquartile range) values
** Comparison with participants using the Kruskal–Wallis test
Table 5.
Logistic regression analysis of risk factors for albuminuria N = 605
| Variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Older age at onset | 1.09 (1.01–1.18) (per year) | 0.037 |
| Higher median HbA1c during the observational period | 2.38 (1.93–2.97) (per %) | <0.0001 |
The multivariate model included diabetes duration, age at onset, gender, and mean HbA1c during the observational period. Both microalbuminuria and macroalbuminuria were included in the albuminuria data
Discussion
In this multicenter observational study of Japanese children with type 1 diabetes, past hyperglycemia (as assessed via the median HbA1c) and older age at onset of diabetes significantly increased the risks for severe retinopathy requiring photocoagulation and the development of albuminuria. No patients who had HbA1c < 7.5% during the observational period developed severe retinopathy requiring photocoagulation therapy. On the other hand, female gender was a strong risk factor for severe retinopathy requiring photocoagulation, but not for albuminuria. The HbA1c during the observational period was significantly higher in females than in males. Thus, there was a prominent gender difference, but the reason for this is unclear.
Poor blood glucose control in adolescent female patients with type 1 diabetes was reported by the Hvidore study group [12] and others [13]. Yokoyama et al. reported that female gender was associated with the development of proliferative diabetic retinopathy in Japanese patients with type 1 diabetes [14, 15]. In females, insulin sensitivity periodically changes due to the menstrual cycle [16]. This phenomenon may make it more difficult for females to control blood glucose levels and could worsen retinopathy. However, we did not track how the blood glucose level changed during the menstrual cycle and thus cannot confirm the reasons for the gender differences observed in our study.
In contrast to our study, a Danish study reported that the two-step progression of retinopathy was more closely associated with the male gender [17]. Some studies have reported an influence of gender on nephropathy, with males showing a higher complication rate [18, 19]. In adolescents, female gender was shown to increase the risk of microalbuminuria, while adult males were shown to be at a higher risk of developing advanced nephropathy than adult females.
In this study, higher age at onset of type 1 diabetes was an independent risk factor for both severe retinopathy requiring photocoagulation and the development of albuminuria. The cumulative use of laser photocoagulation was significantly higher in patients diagnosed with diabetes at age 7 years or older compared with those diagnosed at <7 years old. The mean age of the former group was 10 (9–12) years, and patients with pubertal onset type 1 diabetes were included in this group. Some studies have reported that microvascular complications are worse for patients with pubertal onset type 1 diabetes than for those with prepubertal onset type 1 diabetes [20–22]. In Japan, pubertal onset patients were found to be at higher risk for developing blindness and for requiring renal replacement therapy [23]. However, there are no data on glycemic control, so a large-scale longitudinal prospective study is needed to fully investigate the differences in risk between prepubertal and pubertal onset patients.
Adolescence is the transitional phase of development between childhood and adulthood that incorporates the biological and psychosocial changes associated with puberty [24]. Young people with pubertal onset type 1 diabetes may face greater psychological distress and adjustment difficulties compared with those with prepubertal onset type 1 diabetes. When psychological adjustment problems persist into late adolescence, there is evidence of a greater risk of poor diabetes management during early adulthood [25, 26]. In addition, insulin resistance during puberty leads to poor metabolic control [27]. In this study, glycemic control did not differ significantly between those diagnosed at age ≥7 years and those diagnosed at age <7 years, and most of the patients were diagnosed before the age of pubertal onset.
This study has several limitations. First, we were only able to collect data on 61.1% of the subjects in this cohort. Second, the information evaluated in this study was limited to the use of photocoagulation and the presence of albuminuria. We did not determine the stage of retinopathy, the type of laser photocoagulation therapy used, or whether vitreous surgery had been performed. Third, we did not collect enough baseline information on the risk factors for microvascular complications, such as hypertension, dyslipidemia, smoking, and so on. Finally, obtaining HbA1c readings every 4 months for a period of 6 years was insufficient to allow the relationship between blood glucose control and microvascular complications to be determined [28].
In conclusion, diabetes care must continue to focus on long-term metabolic control, and it is desirable to follow females and patients with later-onset type 1 diabetes carefully.
Acknowledgements
This study was supported by a grant-in-aid from the Japan Diabetes Foundation. We would like to thank the following doctors who were members of the Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT): Masanori Adachi, M.D., and Katsuhiko Tachibana, M.D., Kanagawa Children’s Medical Center; Taiji Aso, M.D., and Rika Kizu, M.D., Department of Pediatrics, Yokosuka Kyousai Hospital; Masatoshi Fujimoto, M.D., Department of Pediatrics, St. Marianna University; Satoshi Fujitsuka, M.D., Akira Motegi, M.D., and Yoichi Morinishi, M.D., Department of Pediatrics, National Defence Medical Collage; Ikuma Fujiwara, M.D., and Eishin Ogawa, M.D., Department of Pediatrics, Tohoku University Hospital; Naoki Fukushima, M.D., Department of Pediatrics, Sapporo City General Hospital; Keiichi Hanaki, M.D., and Susumu Kanzaki, M.D., Department of Pediatrics, Tottori University; Yukihiro Hasegawa, M.D., Tokyo Kiyose Metropolitan Children’s Hospital; Takeki Hirano, M.D., Ibaragi Children’s Hospital; Reiko Horikawa, M.D., National Center for Child Health and Development; Tomoyuki Hotsubo, M.D., Department of Pediatrics, Tonan Hospital; Yutaka Igarashi, M.D., Igarashi Children’s Clinic; Masaru Inoue, M.D., and Susumu Kanzaki, M.D., Department of Pediatrics, Tokai University; Gen Isshiki, M.D., and Tomomi Hashimoto, M.D., Department of Pediatrics, Osaka City University Graduate School of Medicine; Yoshiya Ito, M.D., and Tokuo Mukai, M.D., Department of Pediatrics, Asahikawa Medical University; Kazuhiko Jinno, M.D., Department of Pediatrics, Hiroshima General Hospital of West Japan Railway Company; Sachiko Kanematsu, M.D., and Kaori Sasaki, M.D., Department of Pediatrics, Tokyo Women’s Medical University; Yoshihito Kasahara, M.D., Department of Pediatrics, Kanazawa University; Kaichi Kida, M.D., and Koji Takemoto, M.D., Department of Pediatrics, Ehime University Graduate School of Medicine; Nobuyuki Kikuchi, M.D., and Kentaro Shiga, M.D., Department of Pediatrics, Yokohama City University Medical Center; Hitoshi Kohno, M.D., and Saori Kinjo, M.D., Fukuoka Children’s Hospital; Masanori Minagawa, M.D., and Kaori Kinoshita, M.D., Department of Pediatrics, Chiba University; Akihiko Kinugasa, M.D., Department of Pediatrics, Kyoto Prefectural University of Medicine; Yukashi Ohki, M.D., and Megumi Kishi, M.D., Department of Pediatrics, Nippon Medical School; Koji Kobayashi, M.D., and Mie Mochizuki, M.D., Department of Pediatrics, Yamanashi University; Akemi Koike, M.D., Koike Children’s Clinic; Susumu Konda, M.D., Konda Children’s Clinic; Kazutaka Konishi, M.D., Abuyama Children’s Clinic; Ichiro Yokota, M.D., and Yumiko Kotani, M.D., Department of Pediatrics, Tokushima Univeisity Graduate School of Medicine; Kazumichi Onigata, M.D., Takanori Kowase, M.D., and Fumitake Mizoguchi, M.D., Department of Pediatrics, Gunma University; Hidenari Masuda, M.D., Department of Pediatrics, National Mie Hospital; Hisafumi Matsuoka, M.D., Department of Pediatrics, Tokyo Women’s Medical University Medical Center East; Yuko Miki, M.D., and Utako Sato, M.D., Department of Pediatrics, Tokyo University; Shigeki Miyamoto, M.D., and Rieko Takatani, M.D., Chiba Children’s Hospital; Tetsuo Mori, M.D., Department of Pediatrics, Nagano National Hospital; Yoshikazu Nishi, M.D., Department of Pediatrics, Hiroshima Red Cross Hospital; Osamu Nukada, M.D., Department of Pediatrics, Kobe City West Municipal Hospital; Haruo Ogawa, M.D., Kaoru Nasuda, M.D., and Kazuhiko Toya, M.D., Department of Pediatrics, Hamamatsu University School of Medicine; Taisuke Okada, M.D., Department of Pediatrics, Mominoki Hospital; Soroku Nishiyama, M.D., and Toshihisa Okada, M.D., Department of Pediatrics, Kumamoto University; Toshikazu Takahashi, M.D., Takahashi Clinic; Masakuni Tokuda, M.D., and Ryuzo Takaya, M.D., Department of Pediatrics, Osaka Medical College; Masaro Takesue, M.D., Department of Pediatrics, Japan Red Cross Musashino Hospital; Tokuo Taketani, M.D., Department of Pediatrics, Kurobe City Hospital; Yukifumi Yokota, M.D., and Noriyuki Takubo, M.D., Department of Pediatrics, Kitasato University; Masakuni Tokuda, M.D., Tokuda Children’s Clinic; Makoto Uchiyama, M.D., Department of Pediatrics, Niigata University.
Conflict of interest
Tatsuhiko Urakami has served in advisory panels for Novo Nordisk and Sanofi, and has served on speakers’ bureaus for Sanofi.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients before they were included in the study.
Contributor Information
Yasuko Uchigata, Phone: +81-03-3353-8111, Email: uchigata@dmc.twmu.ac.jp.
The Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT):
Masanori Adachi, Katsuhiko Tachibana, Taiji Aso, Rika Kizu, Masatoshi Fujimoto, Satoshi Fujitsuka, Akira Motegi, Yoichi Morinishi, Ikuma Fujiwara, Eishin Ogawa, Naoki Fukushima, Keiichi Hanaki, Susumu Kanzaki, Yukihiro Hasegawa, Takeki Hirano, Reiko Horikawa, Tomoyuki Hotsubo, Yutaka Igarashi, Masaru Inoue, Susumu Kanzaki, Gen Isshiki, Tomomi Hashimoto, Yoshiya Ito, Tokuo Mukai, Kazuhiko Jinno, Sachiko Kanematsu, Kaori Sasaki, Yoshihito Kasahara, Kaichi Kida, Koji Takemoto, Nobuyuki Kikuchi, Kentaro Shiga, Hitoshi Kohno, Saori Kinjo, Masanori Minagawa, Kaori Kinoshita, Akihiko Kinugasa, Yukashi Ohki, Megumi Kishi, Koji Kobayashi, Mie Mochizuki, Akemi Koike, Susumu Konda, Kazutaka Konishi, Ichiro Yokota, Yumiko Kotani, Kazumichi Onigata, Takanori Kowase, Fumitake Mizoguchi, Hidenari Masuda, Hisafumi Matsuoka, Yuko Miki, Utako Sato, Shigeki Miyamoto, Rieko Takatani, Tetsuo Mori, Yoshikazu Nishi, Osamu Nukada, Haruo Ogawa, Kaoru Nasuda, Kazuhiko Toya, Taisuke Okada, Soroku Nishiyama, Toshihisa Okada, Toshikazu Takahashi, Masakuni Tokuda, Ryuzo Takaya, Masaro Takesue, Tokuo Taketani, Yukifumi Yokota, Noriyuki Takubo, Masakuni Tokuda, and Makoto Uchiyama
References
- 1.Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25:2238–2243. doi: 10.2337/diacare.25.12.2238. [DOI] [PubMed] [Google Scholar]
- 2.Nordwall M, Bojestig M, Arnqvist HJ, Ludvigsson J. Declining incidence of severe retinopathy and persisting decrease of nephropathy in an unselected population of type 1 diabetes: the Linkoping Diabetes Complications Study. Diabetologia. 2004;47:1266–1272. doi: 10.1007/s00125-004-1431-6. [DOI] [PubMed] [Google Scholar]
- 3.Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 4.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.The DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–66. [DOI] [PubMed]
- 7.Diabetes Epidemiology Research International Mortality Study Group Major cross-country differences in risk of dying for people with IDDM. Diabetes Care. 1991;14:49–54. doi: 10.2337/diacare.14.1.49. [DOI] [PubMed] [Google Scholar]
- 8.Matsuura N, Yokota Y, Kazahari K, et al. The Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT): initial aims and impact of the family history of type 1 diabetes mellitus in Japanese children. Pediatr Diabetes. 2001;2:160–9. [DOI] [PubMed]
- 9.World Health Organization. Diabetes mellitus: report of a WHO study group. Technical report, series 727. Geneva: WHO; 1985. [PubMed]
- 10.Shima K, Endo J, Oimomi M, Omori Y, Katayama Y, Kanazawa Y, et al. Interlaboratory difference in GHb measurement in Japan: the fifth report of the GHb Standardization Committee, the Japan Diabetes Society (in Japanese). J Jpn Diabetes Soc. 1998;41:317–23.
- 11.Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, et al. Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes Society: international clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol Int. 2012;3:8–10. [DOI] [PMC free article] [PubMed]
- 12.Mortensen HB, Robertson KJ, Aanstoot HJ, Danne T, Holl RW, Hougaard P, et al. Insulin management and metabolic control of type 1 diabetes mellitus in childhood and adolescence in 18 countries. Diabetes Med. 1998;15:752–759. doi: 10.1002/(SICI)1096-9136(199809)15:9<752::AID-DIA678>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Holl RW, Lang GE, Grabert M, Heinze E, Lang GK, Debatin KM, et al. Diabetic retinopathy in pediatric patients with type 1 diabetes: effect of diabetes duration, prepubertal and pubertal onset of diabetes, and metabolic control. J Pediatr. 1998;132:790–794. doi: 10.1016/S0022-3476(98)70305-1. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama H, Uchigata Y, Otani T, Saeki A, Aoki K, Kasahara T, Omori Y. Metabolic regulation and microangiopathy in a cohort of Japanese IDDM patients. Diabetes Res Clin Pract. 1995;29:203–9. [DOI] [PubMed]
- 15.Yokoyama H, Uchigata Y, Otani T, Aoki K, Maruyama A, Maruyama H, et al. Development of proliferative retinopathy in Japanese patients with IDDM: Tokyo Women’s Medical College Epidemiologic Study. Diabetes Res Clin Pract. 1994;24:113–119. doi: 10.1016/0168-8227(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 16.Cawood EH, Bancroft J, Steel JM. Perimenstrual symptoms in women with diabetes mellitus and the relationship to diabetic control. Diabet Med. 1993;10:444–448. doi: 10.1111/j.1464-5491.1993.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 17.Broe R, Rasmussen ML, Frydkjaer-Olsen U, Olsen BS, Mortensen HB, Peto T, et al. The 16-year incidence, progression and regression of diabetic retinopathy in young population-based Danish cohort with type 1 diabetes mellitus: the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987). Acta Diabetol. 2014;51:413–20. [DOI] [PubMed]
- 18.Schultz CJ, Konopelska-Bahu T, Dalton RN, Carrokk TA, Stratton I, Gale EA, et al. Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study: Oxford Regional Prospective Study Group. Diabetes Care. 1999;22:495–502. [DOI] [PubMed]
- 19.Hovind P, Tarnow L, Rossing P, Jensen BR, Graue M, Torp I, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes. BMJ. 2004;328:1105–1110. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sano H, Nishimura R, Asao K, Matsudaira T, Morimoto A, Agata T, et al. Blindness and laser photocoagulation in patients with childhood-onset type 1 diabetes in Japan. Br J Ophthalmol. 2009;93:726–730. doi: 10.1136/bjo.2008.149534. [DOI] [PubMed] [Google Scholar]
- 21.Kostraba JN, Dorman JS, Orchard TJ, Becker DJ, Ohki Y, Ellis D, et al. Contribution of diabetes duration before puberty to development of microvascular complications in IDDM subjects. Diabetes Care. 1989;12:686–693. doi: 10.2337/diacare.12.10.686. [DOI] [PubMed] [Google Scholar]
- 22.Acerini CL, Williams RM, Dunger DB. Metabolic impact of puberty on the course of type 1 diabetes. Diabetes Metab. 2001;27:S19–S25. [PubMed] [Google Scholar]
- 23.Morimoto A, Nishimura R, Matsudaira T, Sano H, Tajima N. Diabetes Epidemiology Research International Study Group. Is pubertal onset a risk factor for blindness and renal replacement therapy in childhood-onset type 1 diabetes in Japan? Diabetes Care. 2007;30:2338–40. [DOI] [PubMed]
- 24.Cameron FJ, Amin R, de Beaufort C, Codner R, Acerini CL. ISPAD clinical practice consensus guidelines 2014: diabetes in adolescence. Pediatric Diabetes. 2014;15(20):245–56. [DOI] [PubMed]
- 25.Bryden KS, Peveler RC, Stein A, Neil A, Mayou RA, Dunger DB. Clinical and psychological course of diabetes from adolescence to young adulthood: a longitudinal cohort study. Diabetes Care. 2001;24:1536–1540. doi: 10.2337/diacare.24.9.1536. [DOI] [PubMed] [Google Scholar]
- 26.Wysocki T, Hough BS, Ward KM, Green LB. Diabetes mellitus in the transition to adulthood: adjustment, self-care, and health status. J Dev Behav Pediatr. 1992;13:194–201. doi: 10.1097/00004703-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 28.Hirose A, Furushima D, Yamaguchi N, Kitano S, Uchigata Y. Prediction of retinopathy at 20 years after onset in younger-onset type 1 diabetes using mean metabolic memory-free HbA1c values. Diabetes Care. 2013;36:3812–3814. doi: 10.2337/dc13-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]