Abstract
Worldwide, the number of patients with diabetes is increasing. Adults with diabetes have a two- to threefold increased risk of heart attack and stroke, and diabetic nephropathy is a leading cause of end-stage renal failure. Salt sensitivity of blood pressure is reported to be elevated in patients with diabetes. Hyperinsulinemia, hyperglycemia, and an activated sympathetic nervous system play key roles in the genesis of salt-sensitive blood pressure in individuals who are obese and/or have type 2 diabetes. In this review, I summarize previous research performed to improve our understanding of the relationship between salt and hypertension in diabetic patients.
Keywords: Type 2 diabetes, Hyperinsulinemia, Sympathetic nervous system, Incretin-related drugs, Sodium–glucose transporter 2
Introduction
Diabetes mellitus and hypertension are common age-associated diseases. The National Nutrition Survey in Japan showed that in 2014 the overall prevalence of hypertension and that of diabetes among Japanese adults ≥70 years of age were 71.4 and 20.9%, respectively. An elevated blood pressure markedly increases the risks and accelerates the courses of cardiovascular diseases, stroke, kidney disease, and retinopathy in diabetic patients [1]. In addition, the presence of diabetes is strongly and independently associated with hypertension [2]. There is increasing evidence to suggest that insulin resistance (hyperinsulinemia) and hyperglycemia stimulate renal sodium absorption and play key roles in the pathogenesis of hypertension in patients with type 2 diabetes [3, 4]. Thus, an improved understanding of the relationship between salt and hypertension in diabetic patients may help to reduce the complications associated with diabetes.
Type 2 diabetes and the salt sensitivity of blood pressure
It is well established that there is a positive correlation between dietary salt intake and blood pressure [5]. However, the response of blood pressure to a changing salt intake—known as “sodium sensitivity”—is variable and can be affected by a number of factors, including race, age, sex, central obesity, and chronic renal disease [6, 7]. Such heterogeneity of blood pressure responsiveness has been demonstrated in both normotensive and hypertensive subjects [8]. In addition, animal experiments have shown that the sodium sensitivity of blood pressure is associated with a decrease in glomerular filtration or an increase in the rate of sodium reabsorption in the tubules [6]. The sodium sensitivity of blood pressure is reported to be enhanced in patients with type 2 diabetes and/or metabolic syndrome, regardless of their underlying renal function. Rocchini et al. found that a high salt intake caused a substantial increase in blood pressure in obese adolescents and that the effect of salt on blood pressure was diminished following successful weight reduction [3]. We also found that central obesity was independently associated with an increase in the sodium sensitivity of blood pressure [9]. The potential mechanisms linking type 2 diabetes and the salt sensitivity of blood pressure are discussed below.
Hyperinsulinemia
The effects of insulin or insulin resistance on renal sodium reabsorption have been studied since the 1970s. Strazzullo et al. estimated the proximal and distal fractional sodium reabsorption based on exogenous lithium clearance and found that sodium reabsorption in the proximal tubules was associated with the body mass index, waist circumference, and blood pressure [10]. Barbato et al. reported that the proximal sodium re-absorption rate was inversely associated with an insulin resistance index, HOMA-IR [11]. The effect of insulin infusion on the excretion of sodium—but not on the peripheral glucose uptake—was reported to be similar in obese (insulin-resistant) and non-obese subjects [12]. In animal models, insulin has been shown to have stimulatory effects on the sodium reabsorption of the proximal tubules, the loop of Henle, and the distal tubules. Insulin treatment was reported to cause greater epithelial sodium channel (ENaC) activity in wild-type mice, but not mice that specifically lacked insulin receptors in the principal cells of the collecting duct [13]. These findings indicate that in patients with type 2 diabetes—despite the presence of insulin resistance to carbohydrate metabolism—the response to insulin is not impaired upon the excretion of sodium, and renal sodium reabsorption is enhanced by hyperinsulinemia.
Hyperglycemia
In addition to hyperinsulinemia, hyperglycemia stimulates the reabsorption of sodium by the tubules. In normal conditions, the kidneys filter 140–160 g of glucose and re-absorb the same amount of glucose each day. Since the tubular uptake of glucose occurs in the proximal tubules via sodium/glucose cotransporters, a large amount of sodium is reabsorbed with glucose in the proximal tubules. Hyperglycemia enhances the amount of filtered glucose and is reported to increase the capacity for glucose reabsorption. In diabetes, the daily tubular reabsorption of glucose is elevated to 500–600 g and sodium reabsorption in the proximal tubules is enhanced.
Sympathetic nervous system activity
The sympathetic nervous system (SNS) contributes to sodium retention and hypertension because adrenergic blockade or renal denervation markedly attenuates these changes. Even in normotensive subjects, muscle sympathetic nerve activity [14] and the SNS activity of the kidneys [15] of obese individuals are reported to be higher than in non-obese individuals. Several mediators of SNS activation in obese individuals and/or type 2 diabetes patients have been suggested, including (1) hyperleptinemia, (2) the activation of the renin–angiotensin–aldosterone system (RAAS), (3) impaired baroreceptor reflexes, and (4) sleep-apnea-induced hypoxia.
Reduced renal blood flow and increased renal tubular reabsorption of sodium are the major mechanisms of the anti-natriuretic effects of the SNS. Fujita et al. reported that with-no-lysine kinase 4 (WNK4), a negative regulator of the thiazide-sensitive sodium chloride cotransporter (NCC), was a key regulator of the renal SNS and the tubular reabsorption of sodium [16, 17]. They found that continuous infusion of norepinephrine downregulates the expression of WNK4 and upregulates NCC in mice, leading to salt-induced BP elevation. These findings indicate that the β-adrenergic receptor plays a key role, via the SNS, in the genesis of salt-sensitive hypertension.
Glomerular ultrafiltration coefficient
A decrease in the whole-kidney ultrafiltration coefficient could increase the sodium sensitivity of blood pressure in black and glomerulonephritic patients with essential hypertension. Glomerular basement membrane thickening, the first measurable change, has been detected at as early as 1.5–2.5 years after the onset of type 1 diabetes [18]. Glomerular basement membrane thickening has also been found in type 2 diabetes patients with preserved renal function [18]. This ultrastructural change could alter filtration and hydraulic conductivity and reduce the whole-kidney ultrafiltration coefficient. Previous studies have shown that hydraulic conductivity was progressively decreased in model animals and patients with diabetic nephropathy [19, 20]. These findings indicate that the whole-kidney ultrafiltration coefficient is reduced, even in the early phase of diabetes.
Circadian rhythm and the salt sensitivity of blood pressure in type 2 diabetes
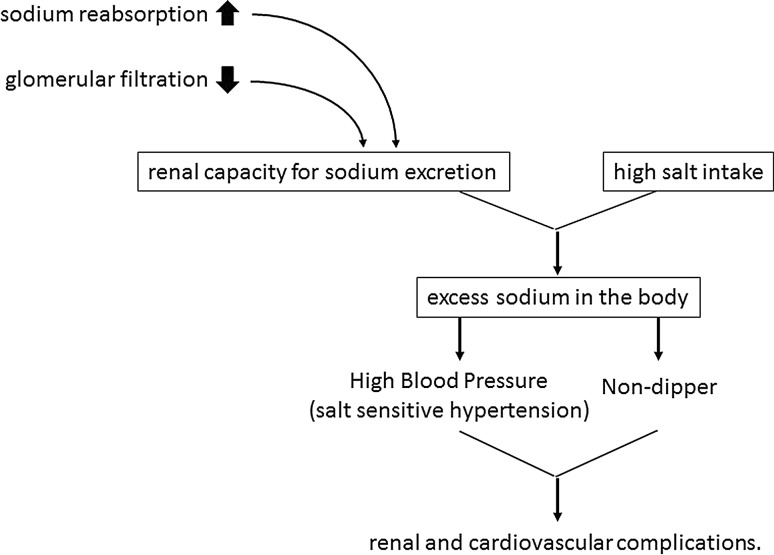
In patients with essential hypertension, it has been hypothesized that the lack of a nocturnal fall in blood pressure (non-dippers) is associated with cardiovascular damage that is more serious than that observed in patients in whom the blood pressure falls during the night (dippers). We found that the blood pressure of patients with sodium-sensitive hypertension failed to fall during the night, and that both sodium restriction and diuretics shifted the circadian rhythm of the blood pressure, resulting in non-dippers becoming dippers [21, 22]. Insulin resistance was reported to be independently associated with a smaller fall in nocturnal blood pressure in patients with essential hypertension [23]. Even in normotensive individuals, a non-dipper status was common and glucose abnormality was associated with the impairment of the nocturnal fall in blood pressure [24]. We also reported that excessive intake of salt causes non-dipping and that the administration of diuretics restored the nocturnal fall in the BP of type 2 diabetic patients who were treated with angiotensin 2 receptor blockers [7]. Furthermore, we reported that masked hypertension was common in patients with type 2 diabetes and that excessive sodium intake was an independent risk factor for masked hypertension [25]. These findings indicate that excessive sodium intake may increase cardiovascular risk through several mechanisms, and that an elevated blood pressure disturbs the circadian blood pressure rhythm. In patients with sodium-sensitive hypertension, elevated blood pressure and a diminished nocturnal blood pressure decline (non-dipper) can be rectified by sodium restriction (Fig. 1). Therefore, sodium restriction may have an additional therapeutic advantage in that it reduces the risk for renal and/or cardiovascular complications.
Fig. 1.
Relationship between high salt intake and target organ damage in salt-sensitive hypertension. Sodium restriction can result in a reduction in blood pressure and restore the disturbed blood pressure rhythm (non-dipper)
Sodium restriction in type 2 diabetes
Observational studies have indicated that excessive salt intake is associated with increased blood pressure. In addition, many interventional studies have shown the hypotensive effects of salt reduction. In the TONE study, salt restriction to 5.6 g per day was required to maintain normal blood pressure after the discontinuation of antihypertensive drugs [26]. The American Diabetes Association recommends that people with diabetes should limit sodium consumption to 2300 mg/day (5.84 g NaCl), and it states that lowering the sodium intake (i.e., to 1500 mg/day) may improve blood pressure in certain circumstances [27] because The American Heart Association recommends an intake of 1500 mg/day (3.8 g NaCl/day) for African Americans, people who are diagnosed with hypertension, diabetes, or chronic kidney disease, and people who are older than 51 years of age. In Japan, the guidelines on the management of hypertension with diabetes mellitus recommend strict blood pressure control to maintain a blood pressure of below 130/80 mmHg; nondrug therapies such as weight control, exercise therapy, and salt restriction (<6 g per day) are the principal treatment [1].
However, several prospective cohort studies have found that lower urinary sodium excretion is not associated with decreased mortality [28, 29]. The activation of the RAAS or SNS and increased levels of catecholamine under the condition of strict sodium restriction may be associated with this adverse outcome. In a recent randomized double-blind trial [30], modest salt restriction (from 9.7 to 6.8 g NaCl) led to a reduction in blood pressure and urinary albumin excretion without a change in insulin sensitivity in subjects with impaired glucose tolerance or type 2 diabetes. Although there is a lack of solid evidence regarding the recommended daily sodium intake for diabetics, reducing the sodium intake to the recommended level of 6 g/day can be expected to achieve a reduction in blood pressure and to slow the progression of nephropathy.
Antidiabetic agents and the salt sensitivity of blood pressure
Incretin-related drugs
It is well established that the glucagon-like peptide 1 (GLP-1) receptor is expressed not only in β cells but also in numerous tissues, including kidney tissue. Furthermore, GLP-1 has been reported to have a natriuretic effect in obese human subjects and animal models [31]. We previously reported that db/db mice, a mouse model of obesity and type 2 diabetes, showed a significant increase in the sodium sensitivity of blood pressure, and that a GLP-1 receptor agonist could change the slopes of their pressure–natriuresis curves [32].
Dipeptidyl peptidase-4 (DPP-4) is an enzyme that is responsible for cleaving and inactivating incretins, including GLP-1. Treatment with DPP-4 inhibitors has also been reported to exert BP-lowering effects in both spontaneously hypertensive rats and hypertensive patients [33, 34]. It is also reported that, in Dahl salt-sensitive rats (a model of nondiabetic salt-sensitive hypertension), a DPP-4 inhibitor significantly attenuated high-salt-induced blood pressure elevation and improved the circadian rhythms of blood pressure such that non-dippers became dippers through the enhanced excretion of urinary sodium [35]. In recent trials, the administration of liraglutide or lixisenatide (GLP-1 receptor agonists) was reported to lead to a small but significant reduction in blood pressure [36, 37].
Sodium–glucose transporter 2 (SGLT-2) inhibitors
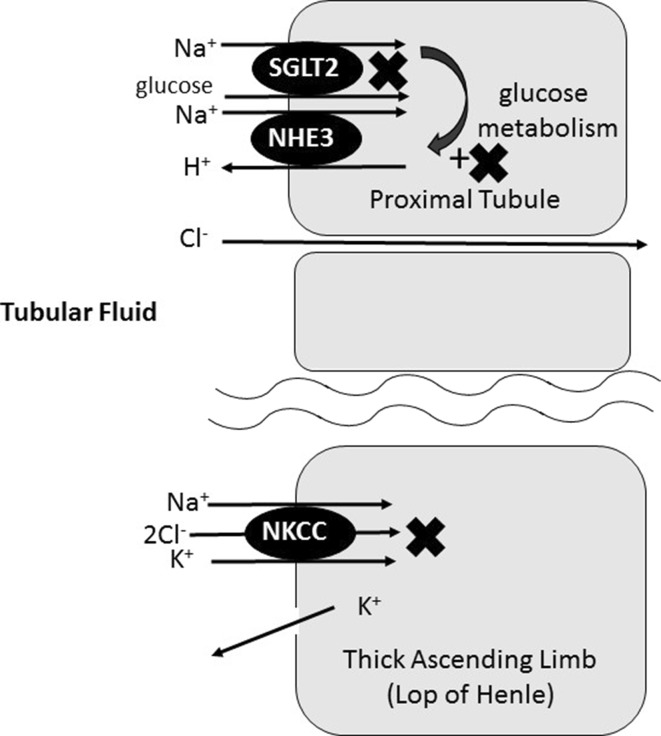
SGLT-2 inhibitors suppress the cotransport of glucose coupled with sodium from the lumen of the proximal tubules into the blood and induce both glycosuria and natriuresis, reducing the blood glucose concentration, body weight, and blood pressure. The proximal tubules are highly permeable and have multiple options for sodium reabsorption; however, glucose reabsorption is mainly dependent on SGLT-2. Thus, under the inhibition of SGLT-2, sodium and chloride are reabsorbed and the absolute amount of glucose in the proximal tubules is maintained. Since the total solute concentration in the proximal tubules remains constant, the concentrations of sodium and chloride are decreased by SGLT-2 inhibitors [29]. The loop of Henle, which extends from the proximal tubule, is responsible for the reabsorption of approximately 30–40% of the filtered sodium chloride. The reduction in the chloride concentration in the proximal tubules leads to a decrease in the chloride concentration in the fluid in the loop of Henle and inhibits sodium chloride (NaCl) reabsorption along the loop of Henle [38, 39]. Thus, SGLT-2 inhibitors my act as loop diuretics (Fig. 2) [40]. It is known that SGLT1-mediated glucose uptake leads to the activation of Na+–H+ exchanger 3 (NHE3) in the intestine. In the renal proximal tubule, NHE3 co-localizes with SGLT2 but not SGLT1, and the physiological glucose concentration in the proximal tubules activates NHE3 [41]. SGLT-2 inhibitors reduce the glucose concentration in the proximal tubular cells and inhibit NHE3 activity. Thus, SGLT-2 inhibitors may act in a similar manner to acetazolamide (Fig. 2).
Fig. 2.
Effects of SGLT2 inhibitors on sodium reabsorption. SGLT2 inhibitors reduce sodium reabsorption from SGLT2 and NHE3 in the proximal tubules. Furthermore, the low concentration of chloride in tubular fluid caused by SGLT-2 inhibition suppresses sodium reabsorption by Na–K–Cl cotransporters. SGLT2 sodium–glucose transporter 2, NHE3 Na+–H+ exchanger 3, NKC Na–K–Cl cotransporter
The NaCl concentration at the site of the macula densa cells also controls the glomerular filtration rate (GFR). An increase in NaCl concentration in the distal nephron due to SGLT-2 inhibition reduces the GFR and the excretion of urinary albumin. The recently published EMPA-REG OUTCOME study demonstrated that sodium–glucose cotransporter 2 inhibition with empagliflozin achieved significant cardiovascular benefits and improved the mortality of patients with type 2 diabetes and established cardiovascular disease [42, 43]. Those results may suggest that sodium–glucose cotransporter 2 inhibitors play a broader role in preventing diabetic complications by both reducing the plasma glucose level and by implementing changes in relation to renal sodium handling.
Conclusion
Worldwide, the number of patients with diabetes is predicted to increase from 415 million in 2015 to 642 million in 2040. Adults with diabetes have a two- to threefold increase in the risk of heart attack and that of stroke, and diabetic nephropathy is a leading cause of end-stage renal failure. It is well established that reducing the intake of sodium can reduce blood pressure. A reduction in blood pressure can also reduce the risks of developing cardiovascular diseases and renal failure. Although the evidence to support the recommendation is insufficient, it is nevertheless recommended that patients with diabetes should reduce their sodium intake to <6 g/day. In addition to salt restriction, several antidiabetic agents such as DPP-4 inhibitors, GLP-1 receptor agonists, and SGLT-2 inhibitors may help to reduce renal and/or cardiovascular events by rectifying the elevated salt sensitivity of blood pressure in patients with type 2 diabetes.
Compliance with ethical standards
Conflict of interest
Author TU received research grants from Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Daiichi Sankyo Company Limited, Shionogi & Co., Ltd., and Takeda Pharmaceutical Company Ltd. Author TU received lecture fees from Kyowa Hakko Kirin Co. and Nippon Boehringer Ingelheim Co., Ltd.
References
- 1.Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014) Hypertens Res. 2014;37(4):253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 2.Izzo R, de Simone G, Chinali M, Iaccarino G, Trimarco V, Rozza F, Giudice R, Trimarco B, De Luca N. Insufficient control of blood pressure and incident diabetes. Diabetes Care. 2009;32(5):845–850. doi: 10.2337/dc08-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;321(9):580–585. doi: 10.1056/NEJM198908313210905. [DOI] [PubMed] [Google Scholar]
- 4.Rocchini AP. Obesity hypertension, salt sensitivity and insulin resistance. Utr Metab Cardiovasc Dis. 2000;10(5):287–94. [PubMed]
- 5.Law MR, Frost CD, Wald NJ. Dietary salt and blood pressure. J Hypertens Suppl. 1991;9(6):S37–S41. doi: 10.1097/00004872-199112000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kimura G, Frem GJ. Brenner BM Renal mechanisms of salt sensitivity in hypertension. Curr Opin Nephrol Hypertens. 1994;3(1):1–12. doi: 10.1097/00041552-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Uzu T, Sakaguchi M, Yokomaku Y, Kume S, Kanasaki M, Isshiki K, Araki S, Sugiomoto T, Koya D, Haneda M, Kashiwagi A. Effects of high sodium intake and diuretics on the circadian rhythm of blood pressure in type 2 diabetic patients treated with an angiotensin II receptor blocker. Clin Exp Nephrol. 2009;13(4):300–6. [DOI] [PubMed]
- 8.Miller JZ, Weinberger MH, Daugherty SA, Fineberg NS, Christian JC, Grim CE. Heterogeneity of blood pressure response to dietary sodium restriction in normotensive adults. J Chronic Dis. 1987;40(3):245–250. doi: 10.1016/0021-9681(87)90160-3. [DOI] [PubMed] [Google Scholar]
- 9.Uzu T, Kimura G, Yamauchi A, Kanasaki M, Isshiki K, Araki S, Sugiomoto T, Nishio Y, Maegawa H, Koya D, Haneda M, Kashiwagi A. Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertension. J Hypertens. 2006;24(8):1627–1632. doi: 10.1097/01.hjh.0000239299.71001.77. [DOI] [PubMed] [Google Scholar]
- 10.Strazzullo P, Barbato A, Galletti F, Barba G, Siani A, Iacone R, D’Elia L, Russo O, Versiero M, Farinaro E, Cappuccio FP. Abnormalities of renal sodium handling in the metabolic syndrome. Results of the Olivetti Heart Study. J Hypertens. 2006;24(8):1633–1639. doi: 10.1097/01.hjh.0000239300.48130.07. [DOI] [PubMed] [Google Scholar]
- 11.Barbato A, Cappuccio FP, Folkerd EJ, Strazzullo P, Sampson B, Cook DG, Alberti KG. Metabolic syndrome and renal sodium handling in three ethnic groups living in England. Diabetologia. 2004;47(1):40–46. doi: 10.1007/s00125-003-1260-z. [DOI] [PubMed] [Google Scholar]
- 12.Rocchini AP, Katch V, Kveselis D, Moorehead C, Martin M, Lampman R, Gregory M. Insulin and renal sodium retention in obese adolescents. Hypertension. 1989;14(4):367–374. doi: 10.1161/01.HYP.14.4.367. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari S, Sharma N, Gill PS, Igarashi P, Kahn CR, Wade JB, Ecelbarger CM. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci USA. 2008;105(17):6469–6474. doi: 10.1073/pnas.0711283105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grassi G, Colombo M, Seravalle G, Spaziani D, Mancia G. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity, and congestive heart failure. Hypertension. 1998;31(1):64–67. doi: 10.1161/01.HYP.31.1.64. [DOI] [PubMed] [Google Scholar]
- 15.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96(10):3423–3429. doi: 10.1161/01.CIR.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 16.Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T. Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med. 2011;17(5):573–580. doi: 10.1038/nm.2337. [DOI] [PubMed] [Google Scholar]
- 17.Fujita T. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol. 2014;25(6):1148–1155. doi: 10.1681/ASN.2013121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalla Vestra M, Saller A, Bortoloso E, Mauer M, Fioretto P. Structural involvement in type 1 and type 2 diabetic nephropathy. Diabetes Metab. 2000;26(Suppl 4):8–14. [PubMed] [Google Scholar]
- 19.Ellis EN, Wiegmann TB, Savin VJ. Diminished glomerular capillary hydraulic conductivity precedes morphologic changes in experimental diabetes mellitus in the rat. Diabetes. 1992;41(9):1106–1112. doi: 10.2337/diab.41.9.1106. [DOI] [PubMed] [Google Scholar]
- 20.Tomlanovich S, Deen WM, Jones HW, 3rd, Schwartz HC, Myers BD. Functional nature of glomerular injury in progressive diabetic glomerulopathy. Diabetes. 1987;36(5):556–565. doi: 10.2337/diab.36.5.556. [DOI] [PubMed] [Google Scholar]
- 21.Uzu T, Kazembe FS, Ishikawa K, Nakamura S, Inenaga T, Kimura G. High sodium sensitivity implicates nocturnal hypertension in essential hypertension. Hypertension. 1996;28(1):139–142. doi: 10.1161/01.HYP.28.1.139. [DOI] [PubMed] [Google Scholar]
- 22.Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997;96(6):1859–1862. doi: 10.1161/01.CIR.96.6.1859. [DOI] [PubMed] [Google Scholar]
- 23.Anan F, Takahashi N, Ooie T, Yufu K, Saikawa T, Yoshimatsu H. Role of insulin resistance in nondipper essential hypertensive patients. Hypertens Res. 2003;26(9):669–676. doi: 10.1291/hypres.26.669. [DOI] [PubMed] [Google Scholar]
- 24.Flores L, Janka M, Canivell S, Jiménez A, Vidal J. Glucose abnormalities associated with impaired nocturnal fall in blood pressure in normotensive severely obese patients. Diabetes Res Clin Pract. 2013;101(2):153–158. doi: 10.1016/j.diabres.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Uzu T, Nakao K, Kume S, Araki H, Isshiki K, Araki S, Kawai H, Ugi S, Kashiwagi A, Maegawa H. High sodium intake is associated with masked hypertension in Japanese patients with type 2 diabetes and treated hypertension. Am J Hypertens. 2012;25(11):1170–1174. doi: 10.1038/ajh.2012.102. [DOI] [PubMed] [Google Scholar]
- 26.Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH, Jr, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, Cutler JA, TONE, Collaborative Research Group Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE) JAMA. 1998;279:839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2017;40(Suppl 1).
- 28.Ekinci EI, Clarke S, Thomas MC, Moran JL, Cheong K, MacIsaac RJ, Jerums G. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34(3):703–709. doi: 10.2337/dc10-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araki S, Haneda M, Koya D, Kondo K, Tanaka S, Arima H, Kume S, Nakazawa J, Chin-Kanasaki M, Ugi S, Kawai H, Araki H, Uzu T, Maegawa H. Urinary potassium excretion and renal and cardiovascular complications in patients with type 2 diabetes and normal renal function. Clin J Am Soc Nephrol. 2015;10(12):2152–2158. doi: 10.2215/CJN.00980115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suckling RJ, He FJ, Markandu ND, MacGregor GA. Modest salt reduction lowers blood pressure and albumin excretion in impaired glucose tolerance and type 2 diabetes mellitus: a randomized double-blind trial. Hypertension. 2016;67(6):1189–1195. doi: 10.1161/HYPERTENSIONAHA.115.06637. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T, Nangaku M, Nishiyama A. The role of incretins in salt-sensitive hypertension: the potential use of dipeptidyl peptidase-4 inhibitors. Curr Opin Nephrol Hypertens. 2011;2(5):476–481. doi: 10.1097/MNH.0b013e328349af9d. [DOI] [PubMed] [Google Scholar]
- 32.Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Uzu T. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun. 2009;380(1):44–49. doi: 10.1016/j.bbrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Sharkovska Y, Reichetzeder C, Alter M, Tsuprykov O, Bachmann S, Secher T, Klein T, Hocher B. Blood pressure and glucose independent renoprotective effects of dipeptidyl peptidase-4 inhibition in a mouse model of type-2 diabetic nephropathy. J Hypertens. 2014;32(11):2211–2223. doi: 10.1097/HJH.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson PM, Diez J. DPP-4 inhibition and blood pressure lowering in perspective. J Hypertens. 2016;34(2):184–187. doi: 10.1097/HJH.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 35.Sufiun A, Rafiq K, Fujisawa Y, Rahman A, Mori H, Nakano D, Kobori H, Ohmori K, Masaki T, Kohno M, Nishiyama A. Effect of dipeptidyl peptidase-4 inhibition on circadian blood pressure during the development of salt-dependent hypertension in rats. Hypertens Res. 2015;38(4):237–243. doi: 10.1038/hr.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B, Zhong J, Lin H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab. 2013;15(8):737–749. doi: 10.1111/dom.12085. [DOI] [PubMed] [Google Scholar]
- 37.Meier JJ, Rosenstock J, Hincelin-Méry A, Roy-Duval C, Delfolie A, Coester HV, Menge BA, Forst T, Kapitza C. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized. Open-label trial. Diabetes Care. 2015;38(7):1263–1273. doi: 10.2337/dc14-1984. [DOI] [PubMed] [Google Scholar]
- 38.Kimura G. Diuretic action of sodium–glucose cotransporter 2 inhibitors and its importance in the management of heart failure. Circ J. 2016;80(11):2277–81. [DOI] [PubMed]
- 39.Greger R, Velázquez H. The cortical thick ascending limb and early distal convoluted tubule in the urinary concentrating mechanism. Kidney Int. 1987;31(2):590–596. doi: 10.1038/ki.1987.39. [DOI] [PubMed] [Google Scholar]
- 40.Kimura G. Importance of inhibiting sodium–glucose cotransporter and its compelling indication in type 2 diabetes: pathophysiological hypothesis. J Am Soc Hypertens. 2016;10(3):271–278. doi: 10.1016/j.jash.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Pessoa TD, Campos LC, Carraro-Lacroix L, Girardi AC, Malnic G. Functional role of glucose metabolism, osmotic stress, and sodium–glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol. 2014;25(9):2028–39. doi:10.1681/ASN.2013060588(Epub 2014 Mar 20). [DOI] [PMC free article] [PubMed]
- 42.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 43.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B, EMPA-REG OUTCOME Investigators Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]