Abstract
Objective
To develop both a revised version of the Diabetes Diet-Related Quality of Life (DDRQOL-R) scale that can be applied to patients with nephropathy and a short form of the DDRQOL-R.
Method
A total of 184 outpatients with type 2 diabetes were asked to complete the self-administered DDRQOL-R scale to confirm its psychometric properties. A short-form version was developed, based on two methods: the result of the developed DDRQOL-R scale and consensus using the Delphi method among medical experts.
Results
Correlations were generally strong between the DDRQOL-R factors extracted by factor analysis and each SF-36 subscale. Cronbach’s α coefficients were at least 0.7, and intraclass correlation coefficients were between 0.59 and 0.78. The nine items that showed high factor loadings were also assessed as important by the medical experts and were selected for the short form of the scale. The reliability and validity of the short form were found to be similar to those of the DDRQOL-R scale.
Discussion
Our findings indicate that the DDRQOL-R scale and its short form have acceptable reliability and validity. The revised version is highly versatile, and the short form can be conveniently administered.
Keywords: Diet-related quality of life, Diabetes, Diet therapy, Scale development
Introduction
Globally, the number of people with diabetes is increasing and is projected by the International Diabetes Federation to exceed 642 million by 2040 [1]. This trend also exists in Japan, and in 2012 the Ministry of Health, Labour and Welfare reported that the suspected number of people with diabetes was 9.5 million [2].
There are three main methods of treatment for type 2 diabetes: dietary therapy, physical activity and medications. Each treatment method involves self-management by the patient [3] that requires daily adjustments of the amount and types of food consumed, maintaining time for physical activity, and taking oral antidiabetic drugs or insulin injections and responding to hypoglycemia, all of which affect various aspects of the patient’s lifestyle, which place a physical and psychological burden on them [4–8]. Therefore, treatment evaluation should not only assess the prevention of complications but should also consider quality of life (QOL) as a subjective indicator for the patient [9]. The diabetes treatment guidelines of the Japan Diabetes Society state that the objectives of diabetes management are to reduce symptoms of diabetes, prevent the development or progression of diabetic complications and of diseases associated with diabetes, and to enable affected individuals to maintain their QOL and life expectancy comparable to healthy individuals [10]. Thus, treatment evaluation that takes QOL into consideration in addition to complication prevention and progression is necessary for maintaining or improving patient’s QOL.
Various diabetes studies of patients have been conducted in Western countries from the 1980s regarding QOL. The first recorded study to use a QOL index to assess intervention outcomes was the Diabetes Control and Complications Trial (DCCT) [11], which investigated the complication preventive effects of intensive treatment. In the DCCT, QOL evaluation was considered crucial because of the risk that the number of insulin injections would be a burden to patients and would lead to a greater risk of hypoglycemia. Therefore, the diabetes quality-of-life (DQOL) measure, the first diabetes-specific QOL scale, was developed [12]. Since then, numerous diabetes-specific QOL scales have been developed with the aim of evaluating the effects of medication and comprehensive educational programs for diabetes [12–19].
However, problems exist with many of the diabetes-specific QOL scales when evaluating patient education conducted in routine clinical practice. Patient education for dietary therapy, physical activity and diabetes medication does not offer support that directly affects QOL related to health and illness, although it does offer support for patients’ perceptions and behavior regarding diet, physical activity, taking medication and injections. Moreover, treatment support for diabetes patients in Japan is often performed by different specialists for each type of treatment. To evaluate patient education for each type of treatment and to measure the effects of each treatment on patients, QOL is considered to be the method consistent with the daily status of patient education. However, this is difficult because existing diabetes-specific QOL scales were not constructed for the evaluation of each type of treatment, and so they are also insufficient for evaluating the effects of each treatment on QOL. Therefore, patient-reported outcome (PRO) measure with which the patients themselves evaluate the effects of patient education regarding, for example, diet, physical activity or insulin injections, is required. Recently, PROs have been used to measure QOL in the field of medicine [20].
Because of the need to develop PRO measures for diabetes treatment, QOL scales have been developed to measure the physical, psychological and social effects of treatment on patients [21–24]. Previously, we focused on QOL and developed the diabetes diet-related quality of life (DDRQOL) scale [22]. We believe that the development of a QOL scale focusing on the effect of dietary therapy on daily lifestyle would be highly significant from the viewpoint of short- to medium-term evaluation of patient dietary therapy education. However, the DDRQOL scale has various weaknesses, including the burden of answering a large number of questions and being limited to patients with no protein intake restrictions. Therefore, a more suitable measurement scale that is easier to use needs to be developed.
The main aim of the present study was to improve the DDRQOL scale and to develop a DDRQOL revised (DDRQOL-R) version with high versatility that could also be used for diabetes nephropathy patients. Additionally, a simple scale with a reduced questionnaire burden and yet highly convenient in the fields of clinical treatment and research is needed to measure QOL. Therefore, the second aim of this study was to develop a short-form version of the DDRQOL-R.
Methods
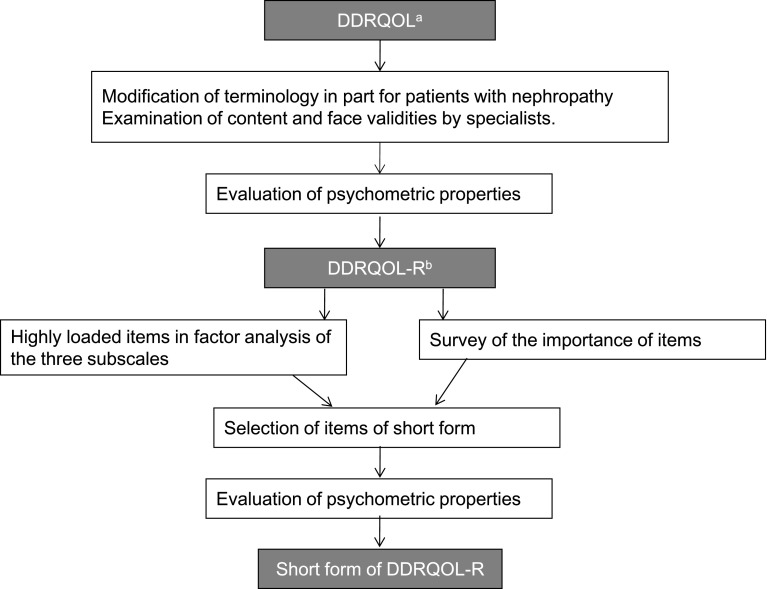
A summary of the procedures used to develop the DDRQOL-R scale and its short form is shown in Fig. 1.
Fig. 1.
Developing procedures of the DDRQOL-R scale and its short form. a Diabetes diet-related quality of life. b Diabetes diet-related quality of life revised version
DDRQOL-R validity and reliability
Subjects and procedure
From the patients with type 2 diabetes attending two clinics in central Tokyo, we selected 202 subjects who satisfied the following conditions: age 20–69 years, at least 3 months following the diagnosis of diabetes, not on dialysis, no cognitive or similar disorders and having the ability to fill out the self-administered questionnaire as judged by a doctor. The two clinics, A and B, which participated in this study, were located within an inner-city area. Clinic A specialized in diabetes and offers dietary guidance by a nutritionist when necessary. Clinic B mainly focused on health checkups for working individuals and treatment for lifestyle-related diseases. It did not offer any diabetes-specific guidance.
Each patient who satisfied the inclusion criteria attended a clinic and was seen by a doctor or nurse who explained a summary of the study, requested their cooperation and orally confirmed their participation. If agreeable, an investigator in another room then explained the purpose of the study, obtained written informed consent and asked the patient to fill in the self-administered questionnaire. After informed consent had been obtained, detailed information was collected from their medical records. The study period was from March to June 2008. Two weeks after the first survey, another copy of the questionnaire was sent by mail to the 131 patients who had given consent to evaluate the reproducibility of the scale.
Measures
The DDRQOL scale [22] was designed to determine quantitative and qualitative satisfaction with diet and the degree of restriction on daily and social life functions due to dietary changes for patients with diabetes. It consists of the following seven subscales including a total of 31 items: (1) satisfaction with diet; (2) burden of diet therapy; (3) perceived merits of diet therapy; (4) general perception of diet; (5) restriction on social functions; (6) vitality; (7) mental health. Subscales 1–3 consist of 17 items in total and were designed to determine QOL specific to diet therapy for diabetes. Subscales 5–7 were modified and applied from the Short Form Health Survey 36 (SF-36) [25–27], which is a comprehensive health-related QOL scale reflecting restriction of daily life functions. The DDRQOL scale also includes two items exploring any changes in diet during the previous year and dietary therapy adherence, but these items do not contribute to the subscale scores.
When creating the DDRQOL-R, our research members first considered the scope of the revised version while receiving advice from a specialist from the Japan Diabetes Society. Because the diabetes treatment guidelines edited by the Japan Diabetes Society state that dietary therapy for stage 5 patients with diabetic nephropathy is based on dietary therapy for dialysis patients, we limited the scope of our scale to stage from one to four patients. We also investigated whether any of the questions in the DDRQOL required revision because of expanding the scope of the scale. Because protein intake restrictions are required for patients with diabetic nephropathy of stage 3 or higher, we revised the item regarding the burden caused by calorie restrictions to “Do you find it hard to restrict energy (calories) and protein intake to a certain level?”
Investigation of the content validity of the revised draft was conducted by three specialists from the Japan Diabetes Society, two certified diabetes nurses and two dietitians qualified as certified diabetes educators. We asked for their opinions regarding whether our expansion of the scope of the scale had led to any inconsistencies, excesses or deficiencies in the defined compositional concept and scale item details. We also asked whether any parts of the proposed revision were difficult to understand or had inappropriate expressions. The opinions that we received indicated that questions were required regarding not only protein restrictions, but also salt restrictions. No problems regarding any other items were indicated.
Face validity was also investigated with a mail survey incorporating the Delphi method [28] regarding the importance of scale items used in the development of the DDRQOL-R-short form version. The Delphi method is a widely used consensus method in which opinions of a panel of experts is collected and converged to achieve consensus on issues for which the evidence is unclear. We questioned a total of 62 subjects including 21 Japan Diabetes Society specialists, 20 certified diabetes nurses and 21 dietitians qualified as certified diabetes educators recruited by means of snowball sampling regarding the face validity of the DDRQOL-R. Various respondents suggested that the expression “to a certain level” in the question, “Do you find it hard to restrict energy (calories), protein and salt intake to a certain level?”, made it seem that there was a requirement to have a designated daily intake level, but this was not appropriate because individuals had only been instructed to control their intake to a certain extent. Therefore, we once again conferred with research members and ultimately decided to revise the question to “Do you find it hard to control energy, protein and salt intake?”
We also investigated the face validity according to three diabetes patients with diabetic nephropathy of stage 3 or higher who were being treated at inner-city Clinic A. Because no problems such as meanings being difficult to understand or questions being difficult to answer were indicated, we adopted the created scale as the DDRQOL-R. Answers to each subscale of the DDRQOL-R converted to subscale scores ranging from 0 to 100 points, with higher scores indicating higher QOL.
The Japanese version of the SF-36 [25–27] was used to investigate criterion validity of the DDRQOL-R. The SF-36 is a comprehensive scale for health-related QOL that is widely used throughout the world and has currently been translated into over 170 languages. It is composed of eight subscales: physical functioning, role physical, role emotional, general health, social functioning, bodily pain, vitality and mental health. In SF-36, a higher subscale score reflects a better QOL. In the DDRQOL-R, dietary therapy adherence was questioned with the item, “In the past week, to what extent have you followed your instructed dietary restrictions?”
The background information of subjects was collected from the self-administered questionnaire and from their medical records and included: sex, age, living status (alone or with family members), occupation, height, weight, duration of diabetes, diabetes treatment, HbA1c level and diabetic nephropathy status.
Data analysis
The distribution of responses to each item of the DDRQOL-R scale was examined to check if there was any marked skewness or lack of response for any of the items. Exploratory factor analysis (maximum likelihood method, promax rotation) of the 17 items reflecting QOL specific to diet therapy was performed to evaluate the factor validity of these items. Cronbach’s α coefficient was calculated for each subscale to evaluate its internal consistency.
To evaluate the criterion validity, Spearman’s rank correlation coefficients between each of the DDRQOL-R and SF-36 subscale scores were calculated. The following hypotheses were then examined: (1) “satisfaction with diet” is strongly correlated with “vitality” and “mental health;” (2) “burden of diet therapy” is strongly correlated with “social functioning” and “mental health;” (3) “restriction of social functions” is strongly correlated with “social functioning” and “role emotional;” (4) “perceived merits of diet therapy” is strongly correlated with dietary therapy adherence. The DDRQOL-R subscale scores were compared by HbA1c, nephropathy stage and the extent of implementation of adherence to diet therapy using the Mann-Whitney U test.
The intraclass correlation coefficient (ICC) was calculated for each subscale, and the weighted k coefficient for each item was calculated to evaluate reproducibility. IBM SPSS Statistics 22 (IBM Corp., Armonk, NY) was used for the analyses, and the level of significance was set at 5% (two-tailed).
Creation of DDRQOL-R-short form version
The candidate items included in the short form were selected from items in the three subscales reflecting QOL specific to diabetes diet therapy, namely, “satisfaction with diet,” “burden of diet therapy” and “perceived merits of diet therapy.”
Investigation of item importance (qualitative analysis)
The importance of each item was qualitatively investigated. We used the Delphi method [28] to gather the opinions of 62 medical specialists, as described previously in this article, regarding the importance of each item in the DDRQOL-R, ranked the items and selected candidate items for the short form of the scale. During recruitment, we set conditions not limited to individuals with the aforementioned qualifications, but to people interested in patient QOL and asked for subjects to be referred. The study period was from August to October 2007.
The first round of the survey asked the participants to rate, on a 6-point scale ranging from “very important” to “not important at all,” the 17 items in the three subscales of the DDRQOL-R. When an item was rated as “not very important” to “not important at all,” respondents were asked to give the reason, and the reasons were categorized according to their content. After compiling the results of the first round of the survey, we conducted the second round of the survey, in which the participants were provided with the response ratios for each item, the reasons for having judged items as unimportant and the respondent’s responses to the first round of the survey and were asked to fill out the same questionnaire again as in the first round of the survey. It was clearly stated on the questionnaire that the participants were allowed to revise the answers they gave in the first round of the survey. The subjects were asked their profession, years of experience, gender and age as their basic attributes.
The participants’ selection of each item were calculated as percentages. Items for which over 70% of responses were “very important” or “important” according to the results of the second survey were set as medical specialist opinion consolidation results and were used as candidate items for the short form of the scale based on qualitative analysis.
Item selection and data analysis (quantitative analysis)
Items with high factor loading according to factor analysis of DDRQOL-R were used as candidate items for the short form of the scale based on quantitative analysis. Items given high ranking for both of these two methods were selected to create the short form of the scale.
The validity and reliability of the DDRQOL-R-short form version were investigated by calculating Spearman’s rank correlation coefficient between the DDRQOL-R short-form version and DDRQOL-R subscales. In addition, validity and reliability were also investigated with the same analysis used for the DDRQOL-R.
Ethical considerations
This study was conducted following approval by the Research Ethics Committee, Faculty of Medicine and Graduate School of Medicine, University of Tokyo (approval no. 1754-1 and 1965).
Results
Psychometric properties of the DDRQOL-R scale
Collection of the questionnaires and subjects’ backgrounds
Of the 202 patients who gave their informed consent for the study, questionnaires were collected from 189. Five patients were excluded from the analysis because they had left 10% or more of the questions blank. Thus, 184 patients (91.1% effective response rate) were included in the analysis. Of the 184 subjects, 142 were from Clinic A and 42 from Clinic B. In the retest, the questionnaire was collected from 118 of the 131 patients who gave their consent. Three patients had left 10% or more of the questions blank. Thus, 115 patients (87.8% effective response rate) were included in the analysis.
The subjects’ background characteristics are presented in Table 1. Concerning the diabetes treatment method, 15.8% of the patients were undertaking only diet and physical activity, 67.4% were taking oral hypoglycemic agents, and 16.8% were on insulin therapy. Duration of diabetes for subjects was 12.0 ± 8.6 years on average. A total of 11.4% of the subjects were patients with stage 3 diabetic nephropathy according to the Joint Committee on Diabetic Nephropathy’s classification [29], and they required a restricted protein intake.
Table 1.
Characteristics of the participants (n = 184)
| n (%) or mean ± SD | |
|---|---|
| Sex | |
| Male | 146 (79.3) |
| Female | 38 (20.7) |
| Age (years) | 57.3 ± 5.8 |
| Employment status | |
| Full- or part-time | 140 (76.1) |
| Unemployed | 44 (23.9) |
| Education | |
| High school or less | 62 (33.7) |
| College or more | 121 (65.2) |
| No answer | 2 (1.1) |
| Living situation | |
| Living with family | 154 (83.7) |
| Living alone | 30 (16.3) |
| Treatment | |
| Diet and physical activity | 29 (15.8) |
| Tablets | 124 (67.4) |
| Insulin | 31 (16.8) |
| Duration of disease (years) | 12.0 ± 8.6 |
| BMI (kg/m2) | 25.3 ± 4.4 |
| HbA1c | 7.4 ± 1.2 |
| Diabetic nephropathya | |
| Stage 1 (pre-nephropathy) | 127 (69.0) |
| Stage 2 (incipient nephropathy) | 36 (19.6) |
| Stage 3 (overt nephropathy) | 21 (11.4) |
SD standard deviation
aAccording to the Joint Committee on Diabetic Nephropathy
Validity
The distribution of responses to each item was examined, and no marked skewness was found. There were missing responses for 5 of the 31 items, and for each only one response was missing.
The results of exploratory factor analysis of the 17 items reflecting QOL specific to diabetes diet therapy are shown in Table 2. The expected factor structure, similar to that of the original version, was obtained. All items, except Question 5, contributed to only one factor, with factor loadings of at least 0.4.
Table 2.
Factor analysis of 17 items specific to diabetes diet therapy (maximum likelihood method, promax rotation) n = 184
| Factor 1 | Factor 2 | Factor 3 | ||
|---|---|---|---|---|
| Factor 1: satisfaction with diet | ||||
| Q1 | Do your meals taste good?a | 0.72 | −0.08 | −0.07 |
| Q2 | Do you feel satisfied after having a meal?a | 0.93 | 0.07 | 0.01 |
| Q3 | Do you enjoy having meals?a | 0.77 | 0.05 | 0.05 |
| Q4 | Do you feel full after having a meal?a | 0.71 | −0.03 | 0.01 |
| Factor 2: burden of diet therapy | ||||
| Q5 | Do you find it hard to have meals at regular times? | 0.19 | 0.38 | 0.08 |
| Q6 | Do you find it hard to control energy, protein or salt intake? | −0.03 | 0.75 | 0.12 |
| Q7 | Do you find it hard to plan menus? | 0.05 | 0.70 | 0.00 |
| Q8 | Do you find it hard that you cannot eat what you like? | −0.10 | 0.82 | 0.00 |
| Q9 | Do you find it hard that you cannot eat the same foods as others on social occasions? | 0.04 | 0.80 | −0.10 |
| Q10 | Do you feel that your diet therapy imposes an economic burden? | 0.14 | 0.48 | 0.01 |
| Q11 | Do you find it hard that you cannot eat the same foods as other members of your family? | −0.05 | 0.82 | −0.03 |
| Q12 | Do you feel that preparing diabetic meals imposes a burden on you or the person who prepares them for you? | −0.09 | 0.66 | −0.03 |
| Factor 3: perceived merits of diet therapy | ||||
| Q13 | Do you feel that your diet therapy has improved your physical condition?a | −0.06 | 0.04 | 0.76 |
| Q14 | Do you feel that your diet therapy has improved your glycemic control?a | −0.03 | −0.14 | 0.78 |
| Q15 | Do you think that your diet therapy has helped you to lead a regular life?a | 0.07 | 0.04 | 0.68 |
| Q16 | Do you feel that your diet therapy has contributed to making your family closer?a | 0.13 | 0.01 | 0.56 |
| Q17 | Do you think that your diet therapy is helping to prevent the progression of your diabetes?a | −0.09 | 0.07 | 0.80 |
aReverse items
Correlations between factor 1 “satisfaction with diet,” factor 2 “burden of diet therapy” and factor 3 “perceived merits of diet therapy” were investigated. Results indicated a correlation of −0.10 between factors 1 and 2, 0.27 between factors 2 and 3 and 0.18 between factors 1 and 3.
With regard to criterion validity, Spearman’s rank correlation coefficient was calculated for DDRQOL-R and SF-36 subscale scores (Table 3). Spearman’s rank correlation coefficients for “satisfaction with diet” with “vitality” and “mental health” were 0.51 and 0.46, respectively. Spearman’s rank correlation coefficients for “restriction of social functions” with “social functioning” and “role emotional” were 0.45 and 0.35, respectively. Although the Spearman’s rank correlation coefficient for “burden of diet therapy” with “mental health” was 0.45, it was 0.17 with “social functioning,” demonstrating stronger correlations with “vitality” and “general health.” The results of subgroup analysis that compared the DDRQOL-R subscale scores by HbA1c, nephropathy stage and the extent of implementation of adherence to diet therapy are shown in Table 4. Regarding the “perceived merits of diet therapy,” patients with an HbA1c of ≥8% had a significantly lower QOL than patients with an HbA1c of <8%. In addition, patients with stage 3 diabetic nephropathy had significantly lower “perceived merits of diet therapy” than stages 1 and 2 patients. As for dietary therapy adherence, comparing patients with good adherence to diet therapy, patients with poor adherence to diet therapy had significantly lower “satisfaction with diet,” “burden of diet therapy,” “perceived merits of diet therapy,” “general perception of diet” and “vitality.”
Table 3.
Correlations among the DDRQOL-R, short form of DDRQOL-R, SF-36 (n = 184)
| Physical functioning | Role physical | Bodily pain | General health perception | Vitality | Social functioning | Role emotional | Mental health | |
|---|---|---|---|---|---|---|---|---|
| DDRQOL-R | ||||||||
| Satisfaction with diet | 0.26** | 0.24** | 0.16* | 0.40** | 0.51 ** | 0.32** | 0.35** | 0.46 ** |
| Burden of diet therapy | 0.30** | 0.26** | 0.15* | 0.41** | 0.54** | 0.17 * | 0.27** | 0.45 ** |
| Perceived merits of diet therapy | 0.12 | 0.06 | 0.05 | 0.05 | 0.15* | 0.14 | −0.01 | 0.11 |
| General perception of diet | 0.25** | 0.18* | 0.10 | 0.38** | 0.37** | 0.17* | 0.13 | 0.28** |
| Restriction of social functions | 0.13 | 0.28** | 0.17* | 0.28** | 0.33** | 0.45 ** | 0.35 ** | 0.36** |
| Vitality | 0.42** | 0.43** | 0.39** | 0.60** | 1.00** | 0.37** | 0.50** | 0.73** |
| Mental health | 0.30** | 0.32** | 0.32** | 0.48** | 0.73** | 0.40** | 0.48** | 1.00** |
| Short form of DDRQOL-R (DDRQOL-R-9) | ||||||||
| Satisfaction with diet (3 items) | 0.31** | 0.27** | 0.18* | 0.40** | 0.53 ** | 0.29** | 0.37** | 0.47 ** |
| Burden of diet therapy (3 items) | 0.26** | 0.22** | 0.16* | 0.39** | 0.43** | 0.12 | 0.27** | 0.40 ** |
| Perceived merits of diet therapy (3 items) | 0.08 | 0.03 | 0.08 | −0.03 | 0.09 | 0.11 | −0.04 | 0.03 |
Bold indicates an expected relationship
* p < 0.05, ** p < 0.01
Table 4.
Subgroup analysis of the DDRQOL-R scale
| Category | n | Satisfaction with diet | Burden of diet therapy | Perceived merits of diet therapy | General perception of diet | Restriction of social functions | Vitality | Mental health | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SDa | p | Mean ± SDa | p | Mean ± SDa | p | Mean ± SDa | p | Mean ± SDa | p | Mean ± SDa | p | Mean ± SDa | p | ||
| HbA1c | |||||||||||||||
| <8% | 147 | 72.2 ± 18.9 | 0.067 | 71.3 ± 18.9 | 0.177 | 49.5 ± 22.0 | 0.028 | 54.9 ± 25.4 | 0.970 | 87.2 ± 17.0 | 0.440 | 65.2 ± 19.7 | 0.067 | 73.3 ± 15.8 | 0.423 |
| 8%≤ | 37 | 78.6 ± 16.8 | 64.4 ± 25.3 | 39.2 ± 24.3 | 54.7 ± 28.8 | 84.1 ± 19.2 | 59.0 ± 21.2 | 68.8 ± 23.4 | |||||||
| Diabetic nephropathy | |||||||||||||||
| Stage 1, 2 | 163 | 73.9 ± 18.7 | 0.157 | 70.7 ± 19.8 | 0.161 | 48.7 ± 22.4 | 0.033 | 55.4 ± 26.0 | 0.534 | 87.0 ± 17.0 | 0.531 | 65.0 ± 20.0 | 0.124 | 72.4 ± 17.0 | 0.713 |
| Stage 3 | 21 | 69.7 ± 17.8 | 63.1 ± 24.3 | 37.9 ± 23.6 | 51.2 ± 26.8 | 83.3 ± 21.1 | 56.0 ± 22.7 | 72.4 ± 22.5 | |||||||
| Dietary therapy adherence | |||||||||||||||
| Always Usually |
79 | 76.6 ± 17.5 | 0.031 | 76.0 ± 18.3 | <0.0001 | 54.4 ± 24.2 | <0.0001 | 66.5 ± 21.9 | <0.0001 | 86.7 ± 19.1 | 0.565 | 68.9 ± 18.2 | 0.002 | 74.4 ± 17.4 | 0.126 |
| Often Sometimes Never |
104 | 70.8 ± 19.0 | 65.0 ± 20.7 | 42.5 ± 19.8 | 45.9 ± 25.6 | 86.3 ± 16.2 | 59.9 ± 20.4 | 70.7 ± 17.8 | |||||||
Mann-Whitney U test
SD standard deviation
The mean, standard deviation, and Cronbach’s α coefficient for each of the DDRQOL-R subscales are shown in Table 5. Cronbach’s α coefficient for “restriction of social functions” was 0.73 and that for the other subscales was between 0.81 and 0.87.
Table 5.
Internal consistency and reproducibility of subscales in DDRQOL-R and the short form
| Subscale | DDRQOL-R | Short form of DDRQOL-R (DDRQOL-R-9) | ||||
|---|---|---|---|---|---|---|
| Mean ± SDa | α b | ICCc | Mean ± SDa | α b | ICCc | |
| Satisfaction with diet | 73.5 ± 18.6 | 0.85 | 0.67 | 75.4 ± 19.2 | 0.86 | 0.61 |
| Burden of diet therapy | 69.9 ± 20.4 | 0.87 | 0.78 | 68.1 ± 24.7 | 0.86 | 0.66 |
| Perceived merits of diet therapy | 47.4 ± 22.8 | 0.84 | 0.66 | 52.9 ± 24.9 | 0.82 | 0.55 |
| General perception of diet | 54.9 ± 26.1 | – | – | – | – | – |
| Restriction of social functions | 86.5 ± 17.5 | 0.73 | 0.59 | – | – | – |
| Vitality | 64.0 ± 20.1 | 0.81 | 0.77 | – | – | – |
| Mental health | 72.4 ± 17.6 | 0.81 | 0.73 | – | – | – |
SD standard deviation
aAll measures are scored from 0 to 100
bCronbach’s α
cInternal correlation coefficient
Test-retest reliability
The ICC for each subscale, calculated using the data from the 115 subjects who provided retest responses, was between 0.59 and 0.78 (Table 5). The weighted k coefficient for each item was between 0.38 and 0.78.
Items and psychometric properties of the short form of the DDRQOL-R scale
Survey regarding the importance of DDRQOL-R items using the Delphi method
The questionnaires were filled in by 56 experts (90.3% response rate) for both the first and second rounds. The subjects’ background characteristics were: male 32.1%; average age 42.3 ± 7.7 years; average work experience 17.2 ± 6.1 years. The percentage of men varied by profession, accounting for 88.2% of Japan Diabetes Society specialists, 0% of certified diabetes nurses and 15.8% of dietitians qualified as certified diabetes educators. The mean overall age was 42.3 ± 7.7 years, the mean number of years of experience in a specialized position was 17.2 ± 6.1 years, and the mean number of years specializing in the field of diabetes was 12.8 ± 6.4 years. Mean values for age, number of years of experience in a specialized position and number of years specializing in the field of diabetes were all higher in the Japan Diabetes Society specialists.
With regard to selection percentages for each item in the first survey, over 70% of respondents answered “very important” or “important” for a total of ten items. These comprised three items (Q1, Q2, Q3) for “satisfaction with diet,” four items (Q8, Q9, Q11, Q12) for “burden of diet therapy” and three items (Q13, Q14, Q17) for “perceived merits of diet therapy.”
The response distribution for each item in the second survey is shown in Fig. 2. Of the items for which over 70% of respondents answered “very important” or “important” in the first survey, the percentage was somewhat low for Q12, “burden of diet therapy,” falling below 70%. For the other items, the number of “very important” or “important” responses increased or hardly changed. Therefore, the remaining nine items were implemented as medical specialist opinion consensus results used as candidate items for the short form of the scale based on qualitative analysis.
Fig. 2.
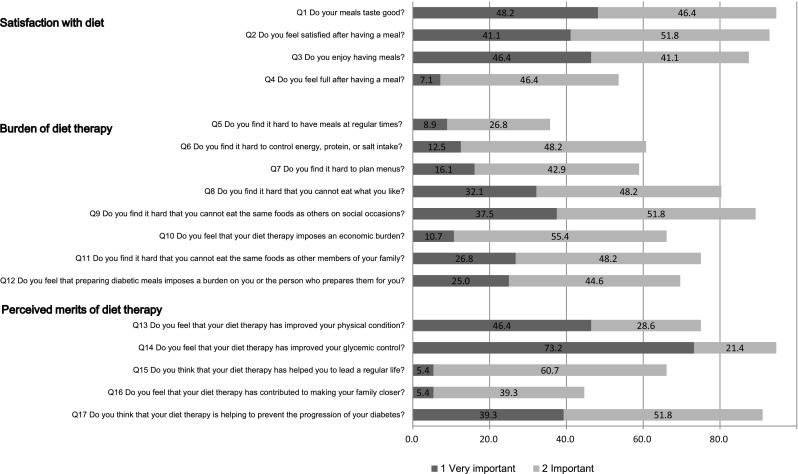
Medical experts’ ratings of the importance of the 17 items reflecting QOL specific to diet therapy (second-round survey results according to the Delphi method). Sum of the proportions of “very important” and “important” for each item
Selected items
Table 2 shows results of the exploratory factor analysis for the DDRQOL-R. The top three items for factor loading of the three subscales specific to dietary therapy for diabetes were Q1, Q2 and Q3 for “satisfaction with diet,” Q8, Q9 and Q11 for “burden of diet therapy” and Q13, Q14 and Q17 for “perceived merits of diet therapy.” These nine items that were rated as “very important” or “important” by over 70% of respondents using the Delphi method were completely consistent. Accordingly, we used these as items for the DDRQOL-R-short form version (DDRQOL-R-9).
Psychometric properties of the short form of the DDRQOL-R scale
Spearman’s rank correlation coefficient between the DDRQOL-R-9 and DDRQOL-R subscales was 0.97 for “satisfaction with diet,” 0.90 for “burden of diet therapy” and 0.93 for “perceived merits of diet therapy.”
For criterion validity, we calculated Spearman’s rank correlation coefficient between the DDRQOL-R-9 and SF-36 subscale scores (Table 3). The correlation coefficients for “satisfaction with diet” with “vitality” and “mental health” were 0.53 and 0.47, respectively. For “burden of diet therapy” with “mental health,” the result was 0.40, but there was no significant correlation with “social functioning” (0.12). For “perceived merits of diet therapy” and “dietary therapy adherence,” the correlation coefficient was 0.26. No significant correlation was observed between “perceived merits of diet therapy” and SF-36 subscales.
The mean, standard deviation and Cronbach’s α coefficient for each of the subscales of the short form of DDRQOL-R scale are shown in Table 5. Cronbach’s α coefficients were between 0.82 and 0.86.
The ICC for each subscale was between 0.55 and 0.66 (Table 5). The weighted k coefficient for each item was between 0.39 and 0.63.
Discussion
We have created and investigated the validity and reliability of a revised and short form version of the DDRQOL for type 2 diabetes patients being treated on an outpatient basis. When creating the DDRQOL-R, we not only investigated content validity with a limited number of specialists, but also surveyed the importance of DDRQOL-R items using the Delphi method. We sought opinions from many specialists comprising 62 Japan Diabetes Society specialists, certified diabetes nurses and dietitians qualified as certified diabetes educators to examine face validity. The survey specialists had on average over 10 years specializing in the field of diabetes and had a strong interest in QOL. By involving many opinions and carefully revising any indicated problematic points, we believe that we were able to improve the validity of the scale.
Exploratory factor analysis of the 17 items reflecting the QOL directly related to diabetes diet therapy in the DDRQOL-R scale showed that all items, except Question 5, contributed to only one factor, with factor loadings greater or equal to 0.4 [30]. The expected three-factor structure, as seen in the DDRQOL scale, was obtained for the DDRQOL-R scale. As in the development of the DDRQOL, there is no appropriate scale for criterion validity so SF-36 was used to evaluate the revised version of the scale, producing results very similar to those hypothesized. However, although a moderate correlation was seen for “burden of diet therapy” with “mental health,” the Spearman’s rank correlation coefficient with “social functioning” was 0.17. This was low even when compared with the DDRQOL development. Compared with the DDRQOL development survey, subjects for this survey had good blood sugar control, were highly educated and included patients with diabetic nephropathy stage 3 or higher. The clinics that participated in this study were in the inner city, but for development of the DDRQOL, we targeted regional hospitals. Therefore, the different clinics may also have affected the results. The results of subgroup analysis of DDRQOL-R showed that QOL was lower in cases of a high HbA1c, severe diabetic nephropathy and a lack of implementation of diet therapy. This finding is consistent with previous studies [9, 13, 31] and is thought to support criterion validity.
Concerning the reproducibility of the DDRQOL-R scale, the weighted k coefficients for two items were slightly below the standard value of 0.4 [32]. Not including “general perception of diet,” which includes only one item, ICCs were greater than or equal to the standard value of 0.7 for three of the remaining six subscales. However, those for the three subscales, “satisfaction with diet,” “perceived merits of diet therapy” and “restriction of social functions” were slightly lower than 0.7. Nonetheless, the standard value of 0.7 is not absolute, and some researchers consider an ICC of 0.5–0.6 to be acceptable [33]. Moreover, considering that only two items are included in the “restriction of social functions” subscale, the results suggest that the DDRQOL-R scale exhibits acceptable reproducibility.
At present, no gold standard for preparing a short form of a scale has been established. Therefore, both qualitative and quantitative methods were used to select items for the DDRQOL-R-9 scale. The Delphi method, a consensus method, was used by medical experts to rank the items in the DDRQOL-R scale according to their importance, and nine items were selected as candidate items for the short form of the scale. These items were the same as the nine items that showed high factor loadings in the exploratory factor analysis of the DDRQOL-R scale. The fact that the same results were obtained from qualitative and quantitative analyses suggests that the short form, i.e., the DDRQOL-R-9 scale, has good validity. As with the criterion validity of the DDRQOL-R scale, very similar results to the hypothesized relationships were obtained, which suggests good criterion validity. Cronbach’s α coefficients exceeded the standard value of 0.7 for all subscales, suggesting good internal consistency.
Concerning reproducibility, the weighted k coefficient for one item was slightly below the standard value of 0.4 [32], while the others satisfied the standard. The ICC for the three subscales of the DDRQOL-R-9 scale were between 0.55 and 0.66, which is slightly lower than the standard value of 0.7, but, as mentioned before, some researchers think that an ICC of 0.5–0.6 is acceptable [33]. Moreover, considering that only three items are included in each subscale of the DDRQOL-R-9, the results suggest that the scale has an acceptable level of reproducibility. Thus, these results show that the validity and reliability of the DDRQOL-R scale we developed are comparable to those of the DDRQOL scale and that the DDRQOL-R-9 scale also has an acceptable level of validity and reliability.
Because we targeted inner-city clinics for convenience, just over 10% of subjects had diabetic nephropathy requiring protein restrictions. However, the primary objective of this study was not to create a scale for patients with advanced diabetic nephropathy, but to expand the scope of application of the DDRQOL that was originally developed for patients with stage 1 and 2 diabetic nephropathy and to revise it into a scale that could also be used for patients with advanced diabetic nephropathy. Regarding the proportions of each stage of diabetic nephropathy in Japan, the Japan Diabetes Complications Study (JDDM) [34], a large-scale clinical trial, reported that the proportions at primary care facilities are as follows: stage 1, 58%; stage 2, 32%; stage 3, 7%; stage 4, 2.6%; stage 5, 0.4%. The proportion of patients in stage 3 and above, the stages in which diet therapy includes protein restrictions did not differ greatly between subjects in the JDDM (10%) and our study (11.4%). Therefore, the DDRQOL-R was deemed applicable to patients with advanced diabetic nephropathy as well. However, further investigation of populations containing many diabetic nephropathy patients is needed in the future. Also, the fact that all subjects were attending the clinics as outpatients and were able to answer a self-administered questionnaire suggests that their complications were relatively non-advanced. Therefore, a wider range of subjects needs to be investigated. Furthermore, the physician in charge obtained information about the diabetic nephropathy stage, but data on numerical values such as eGFR were not collected, which would have been more appropriate.
Our investigation of criterion validity used SF-36 and dietary adherence as indices, but problems exist for both of these. First, because there is no Japanese language scale that can measure similar concepts to the diet-related QOL for which reliability and validity were verified by the comprehensive QOL SF-36 scale, the SF-36 was only used for developing the DDRQOL as well as in other previous studies. However, criterion validity was not sufficiently investigated. Another limitation of our study was that of subject memory for accurately evaluating dietary therapy adherence. In this survey, we questioned patients regarding self-evaluation of dietary therapy adherence over the previous 1 week. However, because the “perceived merits of diet therapy” cannot be felt within a short period of time, it was difficult to detect correlations, and this may have led to underevaluation of validity. Therefore, future evaluations from a longitudinal viewpoint will be required. Additionally, evaluation of dietary therapy adherence using objective methods with verified reliability and validity is required. Finally, it would also be optimal to investigate the capacity of the DDRQOL-R and DDRQOL-R-9 from the viewpoints of sensitivity with and without dietary therapy protein restrictions and responsivity when protein restrictions are added.
The development of the DDRQOL-R expanded the scope of the DDRQOL scale, making it possible to be applied to diabetic nephropathy patients undergoing stricter dietary therapy. We also created the DDRQOL-R-9, which has the advantages of reducing the burden and simplifying the answering of questions for patients. Therefore, we believe that these scales could now be easier to apply to patients as a PRO for evaluating the quality of patient education regarding dietary therapy. The quantification of diet-related QOL and identification of related factors make it possible to investigate the details of support aimed at the maintenance and improvement of diet-related QOL. Furthermore, the sharing of diet-related QOL evaluation results between patients and medical professionals could provide a useful reference when selecting the method and details of treatment. It has also been reported that these sharing methods for determining treatment selection after a QOL appraisal increase patient satisfaction and trust of medical professionals [35]. Thus, it is anticipated that the scale developed in this study will become widely used as a PRO for QOL evaluation by patients themselves.
Acknowledgements
This study is part of a project funded by a Grant-in-Aid for Scientific Research (Scientific Research B 19390555). Our heartfelt gratitude is extended to the patients who participated in the study, to Dr. Shigeru Mashiko and the members of staff at Jinbocho Metabolism Clinic, and to everyone at Fukagawa Gatharia Clinic.
Conflict of interest
The authors have nothing to disclose.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later version.
Informed consent
Informed consent or a substitute for it was obtained from all patients included in the study.
References
- 1.International Diabetes Federation (2015) IDF Diabetes Atlas 7th edition 2015. International Diabetes Federation. http://www.diabetesatlas.org/resources/2015-atlas.html. Accessed 22 Feb 2016.
- 2.Ministry of Health, Labour and Welfare (2014) The National Health and Nutrition Survey in Japan, 2012. http://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h24-houkoku.pdf. Accessed 22 Feb 2016. (in Japanese)
- 3.American Diabetes Association Foundations of care and comprehensive medical evaluation. Diabetes Care. 2016;39(Suppl 1):S23–S35. doi: 10.2337/dc16-S006. [DOI] [PubMed] [Google Scholar]
- 4.Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabet Med. 2005;22(10):1379–1385. doi: 10.1111/j.1464-5491.2005.01644.x. [DOI] [PubMed] [Google Scholar]
- 5.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 6.Wardian J, Sun F. Factors associated with diabetes-related distress: implications for diabetes self-management. Soc Work Health Care. 2014;53(4):364–381. doi: 10.1080/00981389.2014.884038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chew BH, Mohd-Sidik S, Shariff-Ghazali S. Negative effects of diabetes-related distress on health-related quality of life: an evaluation among the adult patients with type 2 diabetes mellitus in three primary healthcare clinics in Malaysia. Health Qual Life Outcomes. 2015;13:187. doi: 10.1186/s12955-015-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pintaudi B, Lucisano G, Gentile S, Bulotta A, Skovlund SE, Vespasiani G, Rossi MC, Nicolucci A, BENCH-D Study Group Correlates of diabetes-related distress in type 2 diabetes: Findings from the benchmarking network for clinical and humanistic outcomes in diabetes (BENCH-D) study. J Psychosom Res. 2015;79(5):348–354. doi: 10.1016/j.jpsychores.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15(3):205–218. doi: 10.1002/(SICI)1520-7560(199905/06)15:3<205::AID-DMRR29>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.The Japan Diabetes Society (2013) Evidence-based Practice Guideline for the Treatment for Diabetes in Japan 2013. http://www.jds.or.jp/modules/en/index.php?content_id=44. Accessed 22 Feb 2016.
- 11.The Diabetes Control and Complication Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 12.The DCCT Research Group Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT) Diabetes Care. 1988;11(9):725–732. doi: 10.2337/diacare.11.9.725. [DOI] [PubMed] [Google Scholar]
- 13.Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(5):754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the Diabetes Care Profile. Eval Health Prof. 1996;19(2):208–230. doi: 10.1177/016327879601900205. [DOI] [PubMed] [Google Scholar]
- 15.Meadows K, Steen N, McColl E, Eccles M, Shiels C, Hewison J, et al. The Diabetes Health Profile (DHP): a new instrument for assessing the psychosocial profile of insulin requiring patients–development and psychometric evaluation. Qual Life Res. 1996;5(2):242–254. doi: 10.1007/BF00434746. [DOI] [PubMed] [Google Scholar]
- 16.Bott U, Muhlhauser I, Overmann H, Berger M. Validation of a diabetes-specific quality-of-life scale for patients with type 1 diabetes. Diabetes Care. 1998;21(5):757–769. doi: 10.2337/diacare.21.5.757. [DOI] [PubMed] [Google Scholar]
- 17.Boyer JG, Earp JA. The development of an instrument for assessing the quality of life of people with diabetes. Diabetes-39. Med Care. 1997;35(5):440–453. doi: 10.1097/00005650-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res. 1999;8(1–2):79–91. doi: 10.1023/A:1026485130100. [DOI] [PubMed] [Google Scholar]
- 19.Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6(3):246–252. doi: 10.1370/afm.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services, Food and Drug Administration (2009) Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed 22 Feb 2016.
- 21.Ishii H, Yamamoto T, Ohashi Y. Development of insulin therapy related quality of life measure (ITR-QOL) J Jpn Diabetes Soc. 2001;44(1):9–15. [Google Scholar]
- 22.Sato E, Suzukamo Y, Miyashita M, Kazuma K. Development of a diabetes diet-related quality-of-life scale. Diabetes Care. 2004;27(6):1271–1275. doi: 10.2337/diacare.27.6.1271. [DOI] [PubMed] [Google Scholar]
- 23.Ahlgren SS, Shultz JA, Massey LK, Hicks BC, Wysham C. Development of a preliminary diabetes dietary satisfaction and outcomes measure for patients with type 2 diabetes. Qual Life Res. 2004;13(4):819–832. doi: 10.1023/B:QURE.0000021694.59992.a1. [DOI] [PubMed] [Google Scholar]
- 24.Ishii H, Furuya M, Iburi T, Yamagami K, Ishibashi R, Tsujii S. Insulin therapy related QOL at night (ITR-QOLN) J Jpn Diabetes Soc. 2008;51(7):593–600. [Google Scholar]
- 25.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 health survey for use in Japan. J Clin Epidemiol. 1998;51(11):1037–1044. doi: 10.1016/S0895-4356(98)00095-X. [DOI] [PubMed] [Google Scholar]
- 26.Fukuhara S, Ware JE, Jr, Kosinski M, Wada S, Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 health survey. J Clin Epidemiol. 1998;51(11):1045–1053. doi: 10.1016/S0895-4356(98)00096-1. [DOI] [PubMed] [Google Scholar]
- 27.Fukuhara S, Suzukamo Y. Manual of SF-36v2 Japanese version. Kyoto: Institute for Health Outcomes and Process Evaluation Research; 2004. [Google Scholar]
- 28.Pope C, Mays N, editors. Qualitative research in health care. 3. London: BMJ Books; 2006. [Google Scholar]
- 29.Haneda M, Utsunomiya K, Koya D, Babazono T, Moriya T, Makino H, et al. A new classification of diabetic nephropathy 2014: a report from joint committee on diabetic nephropathy. Clin Exp Nephrol. 2015;19(1):1–5. doi: 10.1007/s10157-014-1057-z. [DOI] [PubMed] [Google Scholar]
- 30.Ikegami N, Shimotuma K, Fukuhara S. Quality of life evaluation handbook for clinicians. Tokyo: Igakushoin; 2001. [Google Scholar]
- 31.Glasgow RE, Ruggiero L, Eakin EG, Dryfoos J, Chobanian L. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care. 1997;20(4):562–567. doi: 10.2337/diacare.20.4.562. [DOI] [PubMed] [Google Scholar]
- 32.Landis J, Koch G. The measurement of observer agrement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 33.Fayers P, Machin D, editors. Quality of life: assessment, analysis and interpretation. Chichester: Wiley; 2000. [Google Scholar]
- 34.Yokoyama H, Kawai K, Kobayashi M, Japan Diabetes Clinical Data Management Study Group Microalbuminuria is common in Japanese type 2 diabetic patients: a nationwide survey from the Japan Diabetes Clinical Data Management Study Group (JDDM 10) Diabetes Care. 2007;30(4):989–992. doi: 10.2337/dc06-1859. [DOI] [PubMed] [Google Scholar]
- 35.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288(23):3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]