Abstract
Sodium glucose cotransporter 2 inhibitors are unique antihyperglycemic agents that cause osmotic diuresis and calorie loss to urine. We previously reported that administration of tofogliflozin, a sodium glucose cotransporter 2 inhibitor, for 8 weeks decreased fat-free mass without affecting fat mass. We thus investigated the impact of tofogliflozin on metabolic parameters and body composition for 48 weeks in Japanese patients with type 2 diabetes mellitus. This single-arm open-label study enrolled 20 patients. Patients received tofogliflozin 20 mg once daily for 48 weeks. At week 48, changes in metabolic parameters and body composition from baseline were evaluated. Two patients discontinued administration due to adverse events during the first 8 weeks; however, no other adverse events occurred after that period. Seventeen patients completed the 48 weeks of administration of tofogliflodin. Body weight and body mass index decreased during the treatment period. Hemoglobin A1c decreased from 7.8% to 7.1%. The degree of improvement in hemoglobin A1c was correlated with body mass index, fat mass, and plasma glucose level at baseline. As for body composition, fat mass decreased without any change in fat-free mass (including total body water, extracellular water, and intracellular water). Red blood cell count and hematocrit increased, while the estimated glomerular filtration rate decreased. ALT and γ-GTP decreased and the decrease in γ-GTP was correlated with the loss of fat mass. In conclusion, our study clearly suggests that the body weight reduction caused by tofogliflozin administration for 48 weeks was almost entirely due to fat mass dissipation.
Keywords: Tofogliflozin, Body weight, Body composition, Body water, Fat mass
Introduction
Type 2 diabetes mellitus is mainly characterized by chronic hyperglycemia caused by increased insulin resistance and decreased insulin secretion. This metabolic disease is closely associated with obesity and its incidence is increasing all around the world [1]. In response to this recent increase in type 2 diabetes, new types of antihyperglycemic agents such as dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and sodium glucose cotransporter 2 (SGLT2) inhibitors have been developed and placed on the market. SGLT2 is distributed at proximal tubules and reabsorbs about 90% of the glucose filtered by the glomerulus [2]. SGLT2 inhibitors improve hyperglycemia by suppressing the reabsorption of glucose at proximal tubules. Enhanced expression of SGLT2 at the proximal tubules and a resulting increase in the reabsorption of glucose are observed in patients wih type 2 diabetes [3]. Therefore, inhibiting SGLT2 is considered to be an efficient way of lowering the plasma glucose level in diabetic patients. Thus, SGLT2 inhibitors are a new class of drugs that improve glycemic control independent of the action and secretion of insulin, and consequently decrease glucose toxicity and insulin resistance [4]. In addition to their glucose-lowering effects, SGLT2 inhibitors reduce body weight, visceral fat mass, and blood pressure [5]. In fact, one such SGLT2 inhibitor, empagliflozin, was reported to lower the rate of mortality from cardiovascular causes by 38% and the any-cause mortality rate by 32% in patients with type 2 diabetes at high cardiovascular risk [6]. Furthermore, this drug also slowed the progression of kidney disease and lowered rates of clinically relevant renal events in type 2 diabetes patients [7]. However, along with these desirable effects that preexisting drugs do not exert, adverse events such as dehydration, eruption, or urinary tract infections have also been reported for SGLT2 inhibitors [8, 9].
Despite these known actions of SGLT2 inhibitors, studies in which glucose metabolism and body composition were investigated in depth sequentially during the first year of administration are rare. We recently showed that the administration of tofogliflozin for 8 weeks improves glycemic control and reduces body weight in type 2 diabetic patients by decreasing fat-free mass without affecting the fat mass [10]. Thus, in the present study, we examined the effects of tofogliflozin administration for 48 weeks on glucose metabolism and body composition and clarified the alterations caused by the long-term administration of this drug.
Materials and methods
Patients and study design
Japanese patients with type 2 diabetes mellitus were enrolled in this study at Chibune General Hospital. The inclusion criteria for this study were as follows: age >20 years old, body mass index (BMI) ≥18.5, hemoglobin A1c (HbA1c) level >6.2%, estimated glomerular filtration rate (eGFR) >45 ml/min. A C-peptide immunoreactivity (CPR) index (a predictive marker of residual β-cell function [11]) value of >1.5 was also added to the inclusion criteria when the duration of diabetes was >10 years. The exclusion criteria were as follows: patients using insulin, patients with severe hepatic, renal, and cardiac diseases, patients with severe infection, patients with severe trauma, patients with cancer, and pregnant or breastfeeding females. Eligible participants were enrolled in the study from August to October in 2014 and received a single daily dose of 20 mg tofogliflozin for 48 weeks. They continued their pre-study treatment without any change at the beginning of the study. For this period, they also received standard care, including general diet and exercise advice. The treatment was not changed until 8 weeks from the start of administration. Thereafter, antihyperglycemic agents except for tofogliflozin were adjusted according to the glycemic control of each patient, aiming at HbA1c <7.0%.
Measurements
Venous blood was drawn from all participants every 4 weeks, and serum biochemical and metabolic markers were examined. CPR index was determined by calculating the ratio of the serum CPR level (ng/mL) to the plasma glucose concentration (mg/dL) multiplied by 100. Height and body weight were determined in order to calculate body mass index (BMI). Body composition was evaluated by bioelectrical impedance analysis (BIA) using a body composition analyzer (In-Body S20, Biospace Co., Seoul, Korea), as described previously [10]. Briefly, body weight, total body water, and extracellular body water were measured by segmental bioelectric impedance using eight tactile electrodes [12–15]. Fat-free mass (i.e., total body water, protein, and minerals) and fat mass (i.e., fat-free mass subtracted from body weight) were calculated from total body water. Adverse effects were checked for at visits that occurred every 2 weeks between 0 and 8 weeks from the start, and every 4 weeks after that. This study was approved by the Institutional Review Board of Chibune General Hospital and was performed in accordance with the Declaration of Helsinki.
Statistical analysis
Values are shown as the mean ± SD. Student’s t test and Wilcoxon’s signed-rank test were used to evaluate the significance of differences in the presence and absence of normal distributions of variables, respectively. A p value of <0.05 was taken to indicate a significant difference. Correlations among variables were tested using Pearson’s correlation coefficient. All calculations were performed using the software package JMP version 8 (SAS Institute Inc., Cary, NC, USA).
Results
The baseline characteristics of the participants are shown in Table 1. The mean age and duration of diabetes were 52.1 years old and 6.4 years, respectively. The mean body weight, BMI, HbA1c, and casual plasma glucose levels at baseline were 76.6 kg, 28.9 kg/m2, 7.8%, and 166 mg/dL, respectively. Seventeen of the 20 patients completed this study; the other 3 patients dropped out in the first 8 weeks [10]. One patient discontinued administration due to the use of stagger, decreased blood pressure, and dehydration. Another patient discontinued it due to eruptions and itching of the abdomen. Their symptoms improved after drug discontinuation. The third patient stopped visiting the hospital. The other 17 patients (male/female = 7/10) completed the 48-week period of tofogliflozin administration. No episodes of severe hypoglycemia occurred in this study; indeed, no other severe adverse events were observed during the entire administration period.
Table 1.
Characteristics of the study participants
| Number of patients | 20 |
| Sex [n (%)] | |
| Men | 9 (45.0) |
| Women | 11 (55.0) |
| Age (years) | 52.1 ± 9.8 |
| Height (cm) | 161.6 ± 9.4 |
| Body weight (kg) | 76.6 ± 15.8 |
| BMI (kg/m2) | 28.9 ± 4.6 |
| Duration of type 2 diabetes mellitus (years) | 6.4 ± 5.8 |
| Casual plasma glucose (mg/dL) | 166 ± 60 |
| HbA1c (%) | 7.8 ± 1.5 |
| eGFR (mL/min/1.73 m2) | 80.7 ± 21.5 |
| Antidiabetic treatment | |
| Sulfonylurea (%) | 25 |
| Glinide (%) | 0 |
| DPP-4 inhibitor (%) | 65 |
| GLP-1 receptor agonist (%) | 10 |
| α-Glucosidase inhibitor (%) | 15 |
| Biguanide (%) | 75 |
| Thiazolidine (%) | 20 |
| Insulin (%) | 0 |
Values are the mean ± SD
After 48 weeks of administration of tofogliflozin, HbA1c and glycoalbumin (GA) had decreased significantly from 7.8% to 7.1% and from 17.2% to 15.4%, respectively (Table 2). There was no significant difference in CPR index or in the concentrations of glucagon and total ketone bodies. Since casual blood collection was carried out in this study, we also analyzed twelve patients for whom blood sampling was performed in the fasting state in weeks 0 and 48. We confirmed that there were no significant differences in these parameters (CPR index, glucagon, and total ketone bodies), even in the fasting state (data not shown). Blood urea nitrogen (BUN), creatinine (Cr), red blood cells (RBC), and hematocrit were all increased. Estimated glomerular filtration rate (eGFR) was decreased. In addition, both ALT and γ-GTP were lower (Table 2), although they had not changed significantly at 8 weeks of administration [10].
Table 2.
Laboratory findings before and after administration of tofogliflozin for 48 weeks
| n = 17 | Week 0 | Week 48 | p value |
|---|---|---|---|
| Blood cell counts | |||
| WBC (×104/μL) | 0.68 ± 0.18 | 0.64 ± 0.18 | 0.20 |
| RBC (×104/μL) | 458 ± 37 | 488 ± 45 | 0.006 |
| Hct (%) | 40.3 ± 3.4 | 43.2 ± 3.5 | 0.002 |
| Plt (×104/μL) | 25.4 ± 6.3 | 24.5 ± 5.8 | 0.26 |
| Biochemistry | |||
| Total protein (g/dL) | 7.0 ± 0.4 | 7.1 ± 0.5 | 0.38 |
| Albumin (mg/dL) | 4.2 ± 0.4 | 4.3 ± 0.4 | 0.52 |
| Triglyceride (mg/dL) | 190 ± 101 | 184 ± 152 | 0.85 |
| HDL cholesterol (mg/dL) | 52.7 ± 12.3 | 56.8 ± 14.3 | 0.22 |
| LDL cholesterol (mg/dL) | 111 ± 18 | 114 ± 21 | 0.45 |
| AST (IU/L) | 22.7 ± 6.7 | 21.1 ± 8.8 | 0.31 |
| ALT (IU/L) | 32.5 ± 19.0 | 25.9 ± 18.5 | 0.007 |
| LDH (IU/L) | 175 ± 25 | 173 ± 36 | 0.80 |
| ALP (IU/L) | 226 ± 83 | 235 ± 84 | 0.42 |
| γ-GTP (IU/L) | 33.8 ± 15.3 | 25.6 ± 10.8 | 0.004 |
| BUN (mg/dL) | 12.2 ± 3.5 | 15.0 ± 4.2 | <0.001 |
| Creatinine (mg/dL) | 0.75 ± 0.25 | 0.80 ± 0.28 | 0.01 |
| eGFR (ml/min/1.73 m2) | 78.9 ± 20.0 | 72.3 ± 17.5 | 0.01 |
| Uric acid (mg/dL) | 5.5 ± 1.7 | 6.4 ± 5.4 | 0.48 |
| Plasma osmolality (mOsm) | 283.5 ± 4.26 | 287.1 ± 23.7 | 0.56 |
| Glucose metabolism | |||
| HbA1c (%) | 7.8 ± 1.6 | 7.1 ± 1.2 | 0.005 |
| GA (%) | 17.2 ± 3.5 | 15.4 ± 2.7 | 0.02 |
| Casual plasma glucose (mg/dL) | 161 ± 60 | 124 ± 33 | 0.009 |
| CPR index | 2.05 ± 0.9 | 2.42 ± 1.40 | 0.10 |
| Glucagon (pg/mL) | 195 ± 41 | 188 ± 49 | 0.49 |
| Total ketone bodies (μmol/L) | 91.3 ± 56.7 | 136.4 ± 157.1 | 0.14 |
Values are the mean ± SD
WBC white blood cells, RBC red blood cells, Hct hematocrit, Plt platelets, HDL high-density lipoprotein, LDL low-density lipoprotein, AST aspartate aminotransferase, ALT alanine aminotransferase, LDH lactate dehydrogenase, ALP alkaline phosphatase, γ-GTP γ-glutamyl transpeptidase, BUN blood urea nitrogen, eGFR estimated glomerular filtration rate, HbA1c hemoglobin A1c, GA glycoalbumin, CPR index C-peptide immunoreactivity index
In terms of body composition, tofogliflozin significantly reduced body weight by 2.3 kg and fat mass by 2.6 kg after treatment for 48 weeks (Table 3), although the fat-free mass did not change. Neither total body water, extracellular water, nor intracellular water changed significantly. These results suggest that the body weight reduction induced by 48 weeks of tofogliflozin treatment was almost entirely caused by a decrease in fat.
Table 3.
Physical findings and body composition before and after treatment with tofogliflozin
| n = 17 | Week 0 | Week 48 | p value |
|---|---|---|---|
| Physical findings | |||
| Body weight (kg) | 75.5 ± 13.8 | 73.2 ± 15.4 | <0.001 |
| Body mass index (kg/m2) | 28.8 ± 4.0 | 27.7 ± 4.8 | 0.002 |
| Waist circumference (cm) | 97.2 ± 9.9 | 96.5 ± 13.7 | 0.44 |
| Systolic blood pressure (mmHg) | 130 ± 18.7 | 123.4 ± 15.0 | 0.31 |
| Diastolic blood pressure (mmHg) | 89.6 ± 14.7 | 74.5 ± 8.2 | 0.41 |
| Body composition | |||
| Waist to hip ratio | 0.96 ± 0.04 | 0.94 ± 0.04 | 0.06 |
| Body fat percentage (%) | 37.5 ± 6.1 | 34.5 ± 8.5 | 0.03 |
| Fat mass (kg) | 28.3 ± 6.7 | 25.7 ± 9.1 | 0.02 |
| Fat-free mass (kg) | 47.3 ± 10.3 | 47.5 ± 10.3 | 0.82 |
| Total body water (kg) | 34.9 ± 7.6 | 34.6 ± 7.3 | 0.66 |
| Extracellular water (kg) | 13.6 ± 2.8 | 13.0 ± 3.0 | 0.24 |
| Intracellular water (kg) | 21.3 ± 4.8 | 21.5 ± 4.9 | 0.54 |
Values are the mean ± SD
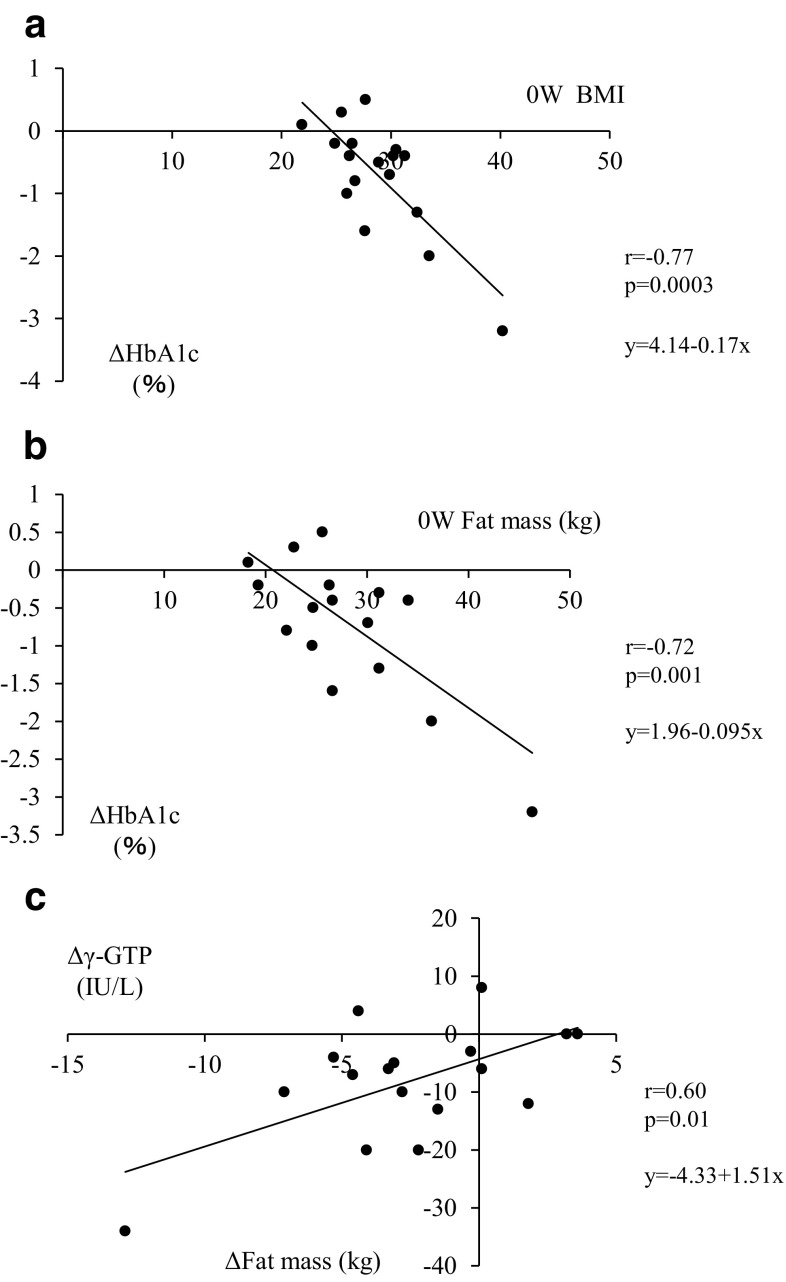
Correlation analysis revealed that the variations in HbA1c from baseline to 48 weeks (ΔHbA1c) were correlated with both BMI and fat mass at baseline (Fig. 1a, b). ΔHbA1c was also correlated with the plasma glucose level at baseline (r = −0.66, p = 0.015). However, ΔHbA1c was not correlated with the changes in body weight or fat mass from baseline to 48 weeks (r = −0.48, p = 0.051 and r = −0.20, p = 0.44, respectively). In addition, variations in γ-GTP (Δγ-GTP) but not of ALT from baseline to 48 weeks were correlated with those in the fat mass (ΔFat mass) (Fig. 1c).
Fig. 1a–c.
Scatter plots of correlation analyses. a Correlation between ΔHbA1c and baseline (0 W) BMI (r = −0.77, P = 0.0003). b Correlation between ΔHbA1c and 0 W fat mass (r = −0.72, P = 0.001). c Correlation between Δγ-GTP and ΔFat mass (r = 0.60, P = 0.01). ΔHbA1c, Δγ-GTP, and ΔFat mass refer to the differences in HbA1c, γ-GTP, and fat mass obtained by subtracting the relevant values at week 48 from those at baseline, respectively
Discussion
Daily administration of 20 mg tofogliflozin for 48 weeks reduced body weight by 2.3 kg as well as fat mass by 2.6 kg and ameliorated glycemic control in type 2 diabetes patients. This implies that the body weight loss induced by tofogliflozin was almost entirely attributable to fat mass loss. Indeed, tofogliflozin administration did not affect the fat-free mass, total body water, extracellular water, or intracellular water at all. The decrease in HbA1c was significantly correlated with BMI, fat mass, and plasma glucose level at baseline. Furthermore, tofogliflozin decreased ALT and γ-GTP, and the decrease in γ-GTP was correlated with the decrease in fat mass.
Interestingly, our previous work showed that 8 weeks of administration of 20 mg tofogliflozin led to a decrease in total body water of about 1 kg without affecting fat mass [10]. In contrast, the present study clarified that the body weight reduction after 48 weeks of administration was entirely attributable to a loss of fat mass. This means that the body weight loss during the initial period of tofogliflozin administration is caused by body water depletion, whereas the body weight loss resulting from a prolonged period of administration is due to fat mass dissipation. It was previously reported that administration of 100 mg and 300 mg canagliflozin for 52 weeks decreased the body weight by 3.3 kg and 3.7 kg, respectively [16]. In that study, the body weight loss was rapid until 6 weeks after the start of administration, but was rather more gradual after that until 52 weeks of administration [16]. Considering our results, the rapid body weight loss until 6 weeks may correspond to a decrease in body water and the subsequent gradual body weight loss may correspond to a decrease in fat. In addition, dapagliflozin took 24 weeks to decrease the visceral and subcutaneous fat mass by 258.4 cm3 and 184.9 cm3, respectively [17]. These results are compatible with our data, which indicate that the SGLT2 inhibitor-mediated loss of fat mass occurs over a long period [18]. Therefore, it is important to sustain diet and exercise therapy even when the body weight decreases rapidly during the initial stage of SGLT2-inhibitor administration, as water, not fat, is lost during this period. In addition, reports suggest that the administration of an SGLT2 inhibitor enhances appetite [19, 20].
Our study revealed that the degree of improvement in HbA1c was closely associated with BMI and fat mass at baseline. This indicates that tofogliflozin should be more effective in obese patients. On the other hand, the degree of improvement in HbA1c was not significantly associated with the reduction in body weight or fat mass. This may suggest that the fat mass reduction is not the main reason for the improvement in glycemic control upon tofogliflozin administration for at least 48 weeks. In addition, 2 patients discontinued administration due to adverse events during the first 8 weeks of our study. However, adverse events were not observed after that in the study, suggesting that the development of adverse events is closely associated with the dehydration that occurs during the early stage of administration.
In the present study, ALT and γ-GTP significantly decreased during the administration period, and the decrease in γ-GTP was closely correlated with the fat mass loss. Given that γ-GTP is closely correlated with hepatic lipid content in normal subjects [21], the decrease in γ-GTP observed in this study is thought to reflect the decrease in hepatic lipid content. In a diabetic obese mouse model, luseogliflozin administration for 8 weeks did indeed decrease hepatic triacylglycerol, cholesterol, and type 1 collagen, leading to inhibition of nonalcoholic steatohepatitis [22]. Ipragliflozin usage for 4 weeks also decreased body weight and ALT, in addition to improving insulin resistance [23]. Furthermore, tofogliflozin administration in male mice decreased body weight, fat mass, and hepatic triacylglycerol content, and ameliorated glucose intolerance and insulin resistance [24]. These results are also consistent with the present data showing that tofogliflozin decreases body weight, fat mass, and markers of hepatic steatosis.
In this study, eGFR decreased as the BUN and Cr levels increased. Such changes were also observed during 8 weeks of tofogliflozin administration [10]. These results suggest that the osmotic diuresis-induced reduction in circulating plasma volume can persist even throughout 48 weeks of tofogliflozin administration, even though the level of extracellular water does not change. A recent study using empagliflozin also showed that the decreased eGFR level even persisted to 192 weeks after the initial administration of the drug [7]. In addition, the RBC count and hematocrit also increased over the administration period in the present study. Given that several studies have also demonstrated that dapagliflozin and ipragliflozin increase the hematocrit [25–27], the SGLT2 inhibitor-induced hemoconcentration and plasma volume reduction may be maintained for as long as the SGLT2 inhibitor is administered.
In summary, tofogliflozin treatment for 48 weeks reduced fat mass without affecting fat-free mass. Neither total body water, extracellular water, nor intracellular water varied during treatment. The improvement in glycemic control upon tofogliflozin administration was more efficient in obese patients. Limitations of our study are the small number of patients included and that it was a single-arm clinical study.
Disclosure
None of the authors have any potential conflict of interest associated with this research.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients before they were included in the study.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Bailey CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci. 2011;2011(32):63–71. doi: 10.1016/j.tips.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 4.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endcrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 6.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 7.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B, EMPA-REG OUTCOME Investigators Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 8.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A Phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:721–728. doi: 10.1111/dom.12081. [DOI] [PubMed] [Google Scholar]
- 10.Hirose S, Nakajima S, Iwahashi Y, Seo A, Takahashi T, Tamori Y. Impact of 8-week administration of tofogliflozin for glycemic control and body composition in Japanese patients with type 2 diabetes mellitus. Intern Med. 2016;55 (in press). [DOI] [PMC free article] [PubMed]
- 11.Saisho Y, Kou K, Tanaka K, Abe T, Kurosawa H, Shimada A, Meguro S, Kawai T, Itoh H. Postprandial serum C-peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr J. 2011;58:315–322. doi: 10.1507/endocrj.K10E-399. [DOI] [PubMed] [Google Scholar]
- 12.Sartorio A, Malavolti M, Agosti F, Marinone PG, Caiti O, Batttistini N, Bedpgni G. Body water distribution in severe obesity and its assessment from eight-polar bioelectrical impedance analysis. Eur J Clin Nutr. 2005;59:155–160. doi: 10.1038/sj.ejcn.1602049. [DOI] [PubMed] [Google Scholar]
- 13.Ling CH, de Craen AJ, Slagboom PE, Gunn DA, Stokkel MP, Westendorp RG, Maier AB. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr. 2011;30:610–615. doi: 10.1016/j.clnu.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Shinkai S, Murayama H, Mori S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr Gerontol Int. 2015;15:1013–1022. doi: 10.1111/ggi.12384. [DOI] [PubMed] [Google Scholar]
- 15.Androutsos O, Gerasimidis K, Karanikolou A, Reilly JJ, Edwards CA. Impact of eating and drinking on body composition measurements by bioelectrical impedance. J Hum Nutr Diet. 2015;28:165–171. doi: 10.1111/jhn.12259. [DOI] [PubMed] [Google Scholar]
- 16.Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–2592. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effect of dapagliflozin on body weight, total fat mass, and regional adipose tissue distributeon in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 18.Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16:159–169. doi: 10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- 19.Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, Palleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity. 2012;20:1645–1652. doi: 10.1038/oby.2012.59. [DOI] [PubMed] [Google Scholar]
- 20.Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther. 2013;345:464–472. doi: 10.1124/jpet.113.203869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thamer C, Tschritter O, Haap M, Shirkavand F, Machann J, Fritsche A, Schick F, Haring H, Stumvoll M. Elevated serum GGT concentrations predict reduced insulin sensitivity and increased intrahepatic lipids. Horm Metab Res. 2015;37:246–251. doi: 10.1055/s-2005-861411. [DOI] [PubMed] [Google Scholar]
- 22.Qiang S, Nakatsu Y, Seno Y, Fujishiro M, Sakoda H, Kushiyama A, Mori K, Matsunaga Y, Yamamotoya T, Kamata H, Asano T. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetes Metab Syndr. 2015;7:104. doi: 10.1186/s13098-015-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komiya C, Tsuchiya K, Shiba K, Miyachi Y, Furuke S, Shimazu N, Yamaguchi S, Kanno K, Ogawa Y. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS One. 2016;11:e0151511. doi:10.1371/e0151511. [DOI] [PMC free article] [PubMed]
- 24.Obata A, Kubota N, Kubota T, Iwamoto M, Sato H, Sakurai Y, Takamoto I, Katsuyama H, Suzuki Y, Fukazawa M, Ikeda S, Iwayama K, Tokuyama K, Ueki K, Kadowaki T. Tofogliflozin improves insulin resistance in skeletal muscle and accelerates lipolysis in adipose tissue in male mice. Endocrinology. 2016;157:1029–1042. doi: 10.1210/en.2015-1588. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes. 2012;5:313–327. doi: 10.2147/DMSO.S22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah NK, Deeb WE, Choksi R, Epstein BJ. Dapagliflozin: a novel sodium-glucose cotransporter type 2 inhibitor for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2012;32:80–94. doi: 10.1002/PHAR.1010. [DOI] [PubMed] [Google Scholar]
- 27.Kurosaki E, Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data. Pharmacol Ther. 2013;139:51–59. doi: 10.1016/j.pharmthera.2013.04.003. [DOI] [PubMed] [Google Scholar]