Abstract
Diabetic nephropathy is a major complication of diabetes and a leading cause of end-stage renal failure in many developed countries. The study aimed to evaluate the efficiency of certain drugs and melatonin in the treatment of nephropathy secondary to diabetes. Diabetes was induced in rats by a single intraperitoneal injection of streptozotocin (50 mg/kg body weight). Three days after induction of diabetes (460–500 mg/dl), rats were treated daily for 60 days with Rowatinex, melatonin, Rowatinex + melatonin, Amosar (Losartan Potassium) (LSP) and LSP + melatonin. The evaluations were made by measuring blood urea nitrogen (BUN), serum uric acid, serum creatinine, urine creatinine, creatinine clearance, nitric oxide, malondialdehyde, superoxide dismutase, glutathione, total antioxidant capacity, kidney injury molecule-1, heat shock protein-70, caspase-3, transforming growth factor β1, and DNA degradation by comet assay and total protein contents. The histopathological picture of the kidneys and pancreases was confirmed in our results. Diabetic rats showed drastic changes in all the measured parameters. Treatment with melatonin and the selected drugs revealed amelioration levels with variable degrees. In conclusion, the combination of LSP and melatonin had the most potent effect on treating the deleterious action of diabetes on rat kidney.
Keywords: Diabetic nephropathy, Melatonin, Rowatinex, Rowatinex, LSP
Introduction
Diabetic nephropathy (DN) is one of the most serious secondary consequences of diabetes, leading to end-stage renal disease [1]. Studies have shown that persistent high glucose levels in blood leads to increased oxidative stress, which is one of the causative factors of DN. Thus, a high blood glucose level is the main factor in the initiation and progression of DN [2].
DN results in the alteration of various extracellular matrix (ECM) components of the kidney such as heparan sulfate (HS) [3], laminin [4] and type IV collagen [5]. This manifests as increased or decreased levels in the serum and/or urine, which can serve as important markers for evaluating DN [6]. However, the stage at which these alterations start setting in is not known. Also, usually the question arises concerning what the duration of diabetes should be in experimental models, e.g., in rats, to observe nephropathic changes. This information is therefore essential to pinpoint the changes in relation to the duration of diabetes.
Slowing the progression of diabetic nephropathy includes optimizing glycemic control, controlling hypertension and using angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs). Because ACE inhibitors and ARBs have been shown to decrease or slow the progression of complications in diabetes, it seems reasonable to use a medication from one of these two classes of antihypertensive drugs as a first-line agent in hypertensive patients who have diabetes without microalbuminuria [7].
N-acetyl-5-methoxytryptamin, known as melatonin, is a hormone synthesized in the pineal gland [8]. Its role is mediated through the activation of melatonin receptors [9]. Also, it acts as a pervasive and powerful antioxidant [10] as well as in the protection of nuclear and mitochondrial DNA [11]. In addition, it plays an important role in maintaining cellular homeostasis against cellular stresses; it inhibits apoptosis in immune cells [12] and prevents neuronal cell death [13]. Melatonin is categorized by the US Food and Drug Administration (FDA) as a dietary supplement [14]. As melatonin may cause harm in combination with certain medications or in the case of certain disorders, a doctor or pharmacist should be consulted before making a decision to take melatonin [15].
Rowatinex is a pharmaceutical product containing seven essential amino acids, 31 % pinene, 15 % camphene, 10 % borneol, 4 % anethole, 4 % fenchone and 3 % cineole. After oral intake of Rowatinex, it is rapidly absorbed because of the presence of terpenes, which are lipid-soluble substances. Due to the pharmacologic effects of these terpenes, Rowatinex is thought to have beneficial effects on conservative stone management and to support medical expulsive therapy [16]. Antibacterial effects of Rowatinex were described by Cipriani and co-workers, with pinenes being the most potent antibacterial substance, followed by borneol and fenchone [17]. Rodent studies on pinenes have indicated that hydrocarbons in this chemical category participate in similar pathways of absorption, metabolism to polar oxygenated metabolites and excretion. As a combination of seven naturally available terpenes, Rowatinex seems to be an anti-inflammatory agent and to have the potential to promote and accelerate stone expulsion in the primary management of urolithiasis [16], where an increased prevalence of nephrolithiasis has been reported in patients with diabetes.
The antihypertensive drug losartan potassium (LSP) acts by blocking the actions of angiotensin II of the renin-angiotensin-aldosterone system. It blocks the vasoconstrictor and aldosterone secreting effects of angiotensin II by antagonizing the binding of angiotensin II with its receptors [18].
In the present study, we selected melatonin because of its role as a wide-spectrum antioxidant that can easily cross cell membranes of different organs. Although Rowatinex is also considered an antioxidant, its action as an anti-inflammatory agent and kidney stone breaker is well documented. The LSP drug seems to relieve the high blood pressure of the kidney, and its combination with Rowatinex and melatonin may attenuate the adverse side effects of diabetes on the kidney. Therefore, the focus of this article is to evaluate the therapeutic effect of these drugs and melatonin on improving renal disorders in diabetic rats.
Materials and methods
Chemicals
All chemicals used were of high analytical grade, products of Merck, Germany, and Sigma, USA. Amosar is the trade name of losartan potassium (LSP) (Amuon Pharamacoceutical Co., SAE, Egypt).
Animals and ethics
Seventy male Wistar strain albino rats (150–200 g) were obtained from the animal house of the National Research Centre, Dokki, Giza, Egypt. Rats were fed on a standard diet and had free access to tap water. They were kept for 2 weeks to become acclimatized to the environmental conditions. Anesthetic procedures and handling of animals complied with the ethical guidelines of the Medical Ethics Committee of the National Research Center in Egypt (approval no. 11063).
Experimental design
The rats were divided into seven groups (10 rats each). Group I served as control rats, group 2 was the diabetic rats, group 3 was diabetic rats treated with Rowatinex, group 4 was the diabetic rats treated with melatonin, group 5 was the diabetic rats treated with a combination of Rowatinex and melatonin, group 6 was the diabetic rats treated with LSP, and group 7 was the diabetic rats treated with LSP and melatonin.
Administration regimens
Diabetic rats were injected intraperitoneally with a single dose of streptozotocin (STZ) (50 mg/kg body weight) dissolved in 0.01 M citrate buffer immediately before use [19]. After injection, animals had free access to food and water and were given 5 % glucose solution to drink overnight to counter hypoglycemic shock [20].
After 3 days of hyperglycemia (460–500 mg/dl), rats were daily injected with melatonin (200 µg/kg b.wt., ip) [21], Rowatinex (300 mg/kg b wt., orally) [22] and its combination as well as LSP (2 mg/kg b. wt. orally) [23] and its combination with melatonin for 60 days.
Sample preparations
After 60 days of drug treatment, the animals were fasted overnight; urine samples were collected and centrifuged at 3000 rpm for 10 min, and the supernatants were stored at −80 °C for the further determinations.
Blood samples were taken from each animal by puncture of the sublingual vein into sterilized tubes, and they stood for 10 min to clot. Serum was separated by centrifugation at 3000 rpm for 10 min and used for the biochemical analysis.
After blood collection, all rats were killed under ether anesthesia. The kidneys were immediately removed, weighed, homogenized in 0.9 N sodium chloride (1:10 w/v) and centrifuged at 4000 rpm for 15 min. The supernatant was collected and stored at −20 °C for different biochemical determinations.
Biochemical analysis
The kidney function indices, blood urea nitrogen (BUN), uric acid, creatinine and creatinine clearance, were estimated using biodiagnostic kits (Biogamma, Stanbio, West Germany).
The nephropathy index, kidney injury molecule (KIM-1), heat shock protein 70 (HSP 70), caspase-3 and transforming growth factor-β1 (TGF-β1), were determined by using an ELISA kit (R&D, Minneapolis, MN).
The oxidative stress markers, malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD) and nitric oxide (NO), were evaluated using the methods of Buege and Aust [24], Moron et al. [25], Nishikimi et al. [26] and Moshage et al. [27], respectively. The total antioxidant capacity (TAC) was estimated by using biodiagnostic kits (Biogamma, Stanbio, West Germany).
Serum and kidney total protein contents were estimated by the method of Bradford [28].
The DNA fragmentation index was evaluateded using the comet assay technique as described by Karimi et al. [29].
Histopathological analysis
Kidney and pancreas sections of all groups were stained with hematoxylin and eosin (H&E) to detect changes in cell architecture [30].
Statistical analysis
All data were expressed as mean ± SD of ten rats in each group. Statistical analysis was carried out by one-way analysis of variance (ANOVA), using the Costat software computer program, accompanied by the least significance difference (LSD) between groups at p < 0.05.
Results
The glucose level in diabetic rats was significantly increased by 528.00 % compared with the control group. Rats treated with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed significant decreases in blood glucose by 56.80, 67.40, 65.40, 66.50 and 69.90 %, respectively. Hence, treatment showed improvement levels of 357.00, 423.00, 411.00, 417.00 and 439.00 %, respectively (Table 1).
Table 1.
Therapeutic effects of different drugs on blood glucose level and body weight at the end of the experiment on rat diabetic nephropathy
| Groups | Blood glucose (mg/dl) | Body weight (g) |
|---|---|---|
| Control | 89.00d ± 12.00 | 206.00ab ± 13.00 |
| Diabetic | 559.00a ± 48.00 (+528.0) | 170.00d ± 10.00 (−17.40) |
| Treated (Rowatinex) | 241.00b ± 31.00 [−56.80] | 189.00c ± 12.00 [+16.47] |
| Treated (melatonin) | 182.00c ± 9.00 [−67.40] | 200.00abc ± 25.00 [+11.17] |
| Treated (Rowatinex + melatonin) | 193.00c ± 10.00 [−65.40] | 205.00abc ± 10.00 [+17.64] |
| LSP | 187.00c ± 17.00 [−66.50] | 193.00bc ± 10.00 [+20.50] |
| LSP + melatonin | 168.00c ± 20.00 [−69.90] | 194.00bc ± 11.00 [+13.50] |
Data are mean ± SD of ten rats in each group
Statistical analysis was done using one-way analysis of variance (ANOVA) using the CoStat computer program accompanied by the least significant level (LSD) between groups at p < 0.05
Unshared superscript letters are significant values between groups at p < 0.0001
Values between brackets are % changes over control group
Values between parentheses are % changes over the diabetic group
Concerning the body weight, the diabetic rats showed a significant decrease of 17.40 % compared with the control group. Rats treated with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed a significant increase in body weight of 11.17, 17.64, 20.50, 13.50 and 14.10 %, respectively. Hence, treatment showed improvement levels of 9.22, 14.56, 16.90, 11.16 and 11.65 % (Table 1).
Blood urea nitrogen (BUN) in diabetic rats showed a significant increase of 133.00 % compared with the control group. In the treated groups, rats treated with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed a significant decrease in BUN levels of 54.10, 53.50, 50.10, 42.00 and 51.20 %, respectively. Hence, treatment showed improvement levels of 126.00, 125.00, 117.00, 98.00 and 119.00 %.
Regarding the serum uric acid level, diabetic rats showed a significant increase of 196.00 % compared with the control group. Treatment of diabetic rats with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination significantly decreased the uric acid level by 51.60, 41.20, 36.70, 51.40 and 54.70 %, respectively, compared with the diabetic group. Therefore, we observed amelioration levels of 153.00, 122.40, 109.00, 152.60 and 162.50 %, respectively.
Concerning with serum creatinine level, the diabetic rats showed a significant increase of 535.00 %. Diabetic rats treated with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination had significantly increased serum creatinine levels of 202.00, 187.00, 211.00, 187.00 and 214.00 % compared with the rats in the diabetic group with percentages of improvement reaching to 65.90, 60.90, 69.00, 61.00 and 69.50 %.
Regarding the creatinine level in urine, diabetic rats showed a significant decrease of 67.40 %. Treatment of rats with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination significantly increases the creatinine levels by 2020.00, 187.00, 211.00, 187.87 and 214.00 %. Therefore, we observed amelioration levels of 65.90, 60.90, 69.00, 61.00 and 69.50 %, respectively.
Concerning the creatinine clearance level, diabetic rats showed a significant decrease of 88.40 %. Treatment of diabetic rats with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination significantly increased the creatinine clearance levels by 706.80, 472.60, 477.00, 520.50, 485.00 and 475.30 %, respectively, with improvement levels reaching 54.70, 55.20, 61.00, 56.00 and 55.00 % (Table 2).
Table 2.
Therapeutic effects of different drugs on kidney function indices in diabetic rats
| Groups | BUN (mg/dl) | Serum uric acid (mg/dl) | Serum creatinine (mg/dl) | Urine creatinine (mg/dl) | Creatinine clearance (ml/min) |
|---|---|---|---|---|---|
| Control | 26.48e ± 2.07 | 1.31d ± 0.070 | 0.42e ± 0.041 | 42.52a ± 8.91 | 1.26a ± 0.23 |
| Diabetic | 61.86a ± 2.02 (+133.00) | 3.89a ± 0.26 (+196.00) | 2.67a ± 0.18 (+535.00) | 13.86b ± 8.98 (−67.40) | 0.14c ± 0.04 (−88.40) |
| Treated (Rowatinex) | 28.36de ± 1.28 [−54.10] | 1.88c ± 0.13 [−51.60] | 0.75b ± 0.06 [−71.90] | 41.92a ± 6.28 [+202.00] | 0.83b ± 0.08 [+472.60] |
| Treated (melatonin) | 28.72cde ± 1.65 [−53.50] | 2.28b ± 0.24 [−41.20] | 0.508d ± 0.10 [−81.20] | 39.78a ± 7.56 [+187.00] | 0.84b ± 0.14 [+477.00] |
| Treated (Rowatinex + melatonin) | 30.84c ± 0.92 [−50.10] | 2.46b ± 0.12 [−36.70] | 0.552d ± 0.06 [−76.70] | 43.22a ± 6.63 [+211.00] | 0.90b ± 0.13 [+520.50] |
| LSP | 35.86b ± 1.78 [−42.00] | 1.89c ± 0.11 [−51.40] | 0.528d ± 0.06 [−80.50] | 39.90a ± 6.26 [+187.87] | 0.85b ± 0.15 [+485.00] |
| LSP + melatonin | 30.18cd ± 2.72 [−51.20] | 1.76c ± 0.09 [−54.70] | 0.642c ± 0.06 [−75.90] | 43.53a ± 7.98 [+214.00] | 0.84b ± 0.17 [+475.30] |
Data are mean ± SD of ten rats in each group
Statistical analysis was done using one-way analysis of variance (ANOVA) using the CoStat computer program accompanied by the least significance level (LSD) between groups at p < 0.05
Unshared superscript letters are significant values between groups at p < 0.0001
Values between brackets are % changes over the control group
Values between parentheses are % changes over the diabetic group
Regarding the oxidative stress markers, the nitric oxide level in diabetic rats showed a significant increase of 127.00 %. Treatment of diabetic rats with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination significantly decreased the nitric oxide levels by 25.50, 37.47, 34.00, 44.70 and 51.00 %. Therefore, we observed amelioration levels of 58.00, 85.00, 77.00, 101.00 and 116.00 %, respectively. In addition, malondialdehyde showed a significant increase of 55.20 % in diabetic rats. Treatment with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination significantly decreased the MDA level by 20.60, 15.50, 18.00, 17.00 and 21.00 %, respectively, compared with the diabetic group. Therefore, treatment with these selected drugs improved the MDA level by 32.00, 24.00, 28.00, 26.40 and 32.00 %, respectively. Contradictorily, the total superoxide dismutase level significantly decreased by 35.00 % in diabetic rats compared with the control group. Diabetic rats treated with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination significantly increased the SOD level by 27.00, 32.50, 32.40, 43.00 and 35.50 % with ameliorating levels reaching 17.60, 17.40, 17.70, 27.70 and 19.00 %, respectively. In addition, the glutathione level showed a significant decrease of 31.00 % in diabetic rats. In diabetic rats treated with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination, the glutathione level increased significantly by 16.60, 7.60, 34.00, 21.75 and 31.00 % with amelioration levels reaching 11.50, 5.50, 23.20, 14.60 and 21.51 %, respectively. Moreover, the total antioxidant capacity level showed a significant decrease of 46.40 % in diabetic rats. Diabetic rats treated with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed a significant increase in the total antioxidant capacity level of 67.00, 62.50, 68.50, 57.00 and 46.00 % with enhancement levels reaching 35.80, 33.00, 36.70, 30.53 and 24.80 %, respectively (Table 3).
Table 3.
Therapeutic effects of different drugs on oxidative stress markers in diabetic rats
| Groups | NO (µmol/g tissue) | MDA (µg/mg protein) | SOD (µg/mg protein) | GSH (µg/mg protein) | TAC (mM/l) |
|---|---|---|---|---|---|
| Control | 39.18f ± 3.95 | 70.68d ± 2.89 | 0.82a ± 0.07 | 2.21a ± 0.12 | 2.26a ± 0.17 |
| Diabetic | 88.92a ± 6.59 (+127.00) | 109.65a ± 7.50 (+55.20) | 0.53d ± 0.06 (−35.00) | 1.52e ± 0.057 (−31.00) | 1.21e ± 0.06 (−46.40) |
| Treated (Rowatinex) | 66.24b ± 6.19 [−25.50] | 87.04c ± 5.9 [−20.60] | 0.68a ± 0.06 [+27.00] | 1.78c ± 0.12 [+16.60] | 2.02b ± 0.12 [+67.00] |
| Treated (melatonin) | 55.60c ± 5.65 [−37.47] | 92.66b ± 5.48 [−15.50] | 0.71c ± 0.07 [+32.50] | 1.64d ± 0.05 [+7.60] | 1.96bc ± 0.11 [+62.50] |
| Treated (Rowatinex + melatonin) | 58.8c ± 6.23 [−34.00] | 90.06bc ± 9.96 [−18.00] | 0.71c ± 0.13 [+32.40] | 2.04b ± 0.16 [+34.00] | 2.04b ± 0.14 [+68.50] |
| LSP | 49.16g ± 3.85 [−44.70] | 90.98bc ± 8.13 [−17.00] | 0.766b ± 0.06 [+43.00] | 1.85c ± 0.28 [+21.75] | 1.90c ± 0.14 [+57.00] |
| LSP + melatonin | 43.52e ± 0.84 [−51.00] | 86.84c ± 7.38 [−21.00] | 0.72bc ± 0.11 [+35.50] | 2.00b ± 0.14 [+31.00] | 1.77d ± 0.16 [+46.00] |
Data are mean ± SD of ten rats in each group
Statistical analysis was done using one-way analysis of variance (ANOVA) using the CoStat computer program accompanied by the least significance level (LSD) between groups at p < 0.05
Unshared superscript letters are significant values between groups at p < 0.0001
Values between brackets are % changes over the control group
Values between parentheses are % changes over the diabetic group
With respect to the nephropathy index, the kidney injury molecule-1 (KIM-1) level in diabetic rats showed a significant increase of 1156.00 %. Treatment of diabetic rats with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination led to a significant decrease in the KIM-1 level of 41.40, 20.30, 42.00, 58.50 and 64.20 % with enhancement levels reaching 520.00, 256.00, 529.00, 734.70 and 807.00 %, respectively. For the heat shock protein-70 (HSP70) level, the diabetic rats showed a significant increase of 282.70 %. Treatment of diabetic rats with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed a significant decrease in the HSP70 level by 24.50, 16.50, 29.50, 53.40 and 60.00 % with enhancement levels reaching 93.30, 63.30, 112.20, 205.00 and 228.30 %, respectively. The serum caspase-3 level in diabetic rats showed a significant increase of 667.00 %. Diabetic rats treated with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed a significant decrease in the caspase-3 level by 17.50, 23.84, 19.75, 33.29 and 54.50 % with amelioration levels reaching 135.00, 183.00, 151.400, 255.00 and 418.00 %, respectively. In addition, the serum tumor growth factor (TGF) showed a significant increase of 403.00 %. Treatment of diabetic rats with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed a significant decrease in the TGF level by 14.28, 21.38, 18.00, 61.00 and 68.00 % with amelioration levels reaching 72.00, 107.50, 90.20, 308.00 and 342.00 %, respectively (Table 4).
Table 4.
Therapeutic effects of different drugs on the nephropathy index in diabetic rats
| Groups | KIM-1 (pg/ml) | HSP 70 (pg/100 mg) | Caspase-3 (pg/ml) | TGF-β1 (pg/ml) |
|---|---|---|---|---|
| Control | 83.39f ± 22.5 | 1.80g ± 0.35 | 47.31f ± 11.60 | 115.24g ± 23.58 |
| Diabetic | 1047.50a ± 96.72 (+1156.00) | 6.89a ± 0.46 (+282.70) | 362.76a ± 30.48 (+667.00) | 579.82a ± 5.09 (+403.00) |
| Treated (Rowatinex) | 613.19c ± 52.95 [−41.40] | 5.21c ± 0.08 [−24.50] | 298.98b ± 4.80 [−17.50] | 497.0b ± 9.22 [−14.28] |
| Treated (melatonin) | 834.05b ± 44.11 [−20.30] | 5.75b ± 0.17 [−16.50] | 276.26c ± 8.11 [−23.84] | 455.84d ± 11.30 [−21.38] |
| Treated (Rowatinex + melatonin) | 606.25c ± 42.36 [−42.00] | 4.87d ± 0.21 [−29.50] | 291.15b ± 8.01 [−19.75] | 475.86c ± 9.97 [−18.00] |
| LSP | 434.85d ± 66.40 [−58.50] | 3.21e ± 0.09 [−53.40] | 241.97d ± 21.45 [−33.29] | 224.85e ± 28.50 [−61.00] |
| LSP + melatonin | 374.64e ± 45.09 [−64.20] | 2.78f ± 0.25 [−60.00] | 164.92e ± 30.28 [−54.50] | 185.22f ± 41.29 [−68.00] |
Data are mean ± SD of ten rats in each group
Statistical analysis was done using one-way analysis of variance (ANOVA) using the CoStat computer program accompanied by the least significance level (LSD) between groups at p < 0.05
Unshared superscript letters are significant values between groups at p < 0.0001
Values between brackets are % changes over the control group
Values between parentheses are % changes over the diabetic group
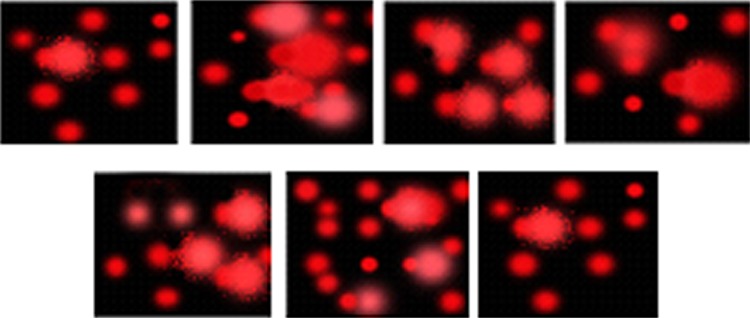
Regarding the comet assay, the DNA tail length in diabetic kidneys showed a significant increase of 25.30 %. Treatment of diabetic rats with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed a significant decrease in the DNA tail length of 25.47, 25.00, 37.00, 52.00 and 54.00 % with amelioration levels reaching 90.00, 88.00, 130.00, 184.00 and 190.00 %, respectively. The percentages of tailed DNA numbers in diabetic rats reached 245.00 %. Treatment with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed a significant decrease in the tailed DNA by 22.37, 19.26, 32.60, 46.00 and 48.00 % with enhancement levels reaching 77.00, 66.40, 112.70, 159.00 and 166.00 %, respectively. A significant increase in the tail moment (1069.00 %) in diabetic rats was observed. Treatment with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed a significant decrease in the tail moment by 42.50, 39.50, 58.30, 73.70 and 76.20 %, respectively, with the enhancement level amounting to 497.00, 463.00, 681.00, 862.00 and 892.00 % (Table 5; Fig. 1).
Table 5.
Therapeutic effects of different drugs on the DNA fragmentation index in rats with diabetic nephropathy
| Groups | DNA tail length (µm) | % of tailed DNA | Tail moment |
|---|---|---|---|
| Control | 1.50e ± 0.49 | 1.49f ± 0.33 | 2.35e ± 1.22 |
| Diabetic | 5.30a ± 0.21 (+25.30) | 5.14a ± 0.31 (+245.00) | 27.47a ± 2.74 (+1069.00) |
| Treated (Rowatinex) | 3.95b ± 0.20 [−25.47] | 3.99c ± 0.05 [−22.37] | 15.78b ± 0.99 [−42.50] |
| Treated (melatonin) | 3.98b ± 0.26 [−25.00] | 4.15b ± 0.19 [−19.26] | 16.6b ± 1.87 [−39.50] |
| Treated (Rowatinex + melatonin) | 3.35c ± 0.14 [−37.00] | 3.468d ± 0.10 [−32.60] | 11.46c ± 0.61 [−58.30] |
| LSP | 2.54d ± 0.19 [−52.00] | 2.77e ± 0.09 [−46.00] | 7.21d ± 0.30 [−73.70] |
| LSP + melatonin | 2.45d ± 0.19 [−54.00] | 2.67e ± 0.11 [−48.00] | 6.52d ± 0.53 [−76.20] |
Data are mean ± SD of ten rats in each group
Tail moment = tail length × tail % DNA
Statistical analysis was done using one-way analysis of variance (ANOVA) using the CoStat computer program accompanied by the least significance level (LSD) between groups at p < 0.05
Unshared superscript letters are significant values between groups at p < 0.0001
Values between brackets are % changes over the control group
Values between parentheses are % changes over the diabetic group
Fig. 1.
DNA fragmentations by comet assay technique. Groups from left to right: control, diabetic, treated (Rowatinex), treated (melatonin), treated (Rowatinex + melatonin), treated (LSP) and treated (LSP + melatonin). Minimal breakage and movement of DNA in the diabetic group treated with the combination of LSP and melatonin were observed
Regarding the protein contents in diabetic rat kidneys, they significantly decreased by 37.30 %. Treatment of diabetic rats with Rowatinex, melatonin, its combination, LSP, and the Amosar and melatonin combination showed a significant increase in the total protein contents by 64.70, 68.00, 44.00, 18.80 and 38.80 % with amelioration levels reaching 40.50, 42.60, 27.50, 11.80 and 24.32 %, respectively. Contradictorily, the total serum protein contents in diabetic rats showed a significant increase of 71.40 %. Treatment of diabetic rats with Rowatinex, melatonin, its combination, LSP, and the LSP and melatonin combination showed significant decreases of 50.00, 46.57, 40.30, 44.16 and 28.83 %. Therefore, the serum total protein contents had amelioration levels of 85.60, 79.80, 69.00, 75.70 and 49.00 % (Table 6).
Table 6.
Therapeutic effects of different drugs on total protein contents in rats with diabetic nephropathy
| Groups | Tissue total protein (mg/g tissue) | Serum total protein (g/dl) |
|---|---|---|
| Control | 8.14cd ± 0.81 | 6.05ab ± 0.44 |
| Diabetic | 5.10a ± 0.48 (−37.30) | 10.37e ± 3.20 (+71.40) |
| Treated (Rowatinex) | 8.4d ± 0.66 [+64.70] | 5.19ab ± 1.19 [−50.00] |
| Treated (melatonin) | 8.57cd ± 0.56 [+68.00] | 5.54a ± 1.04 [−46.57] |
| Treated (Rowatinex + melatonin) | 7.34c ± 0.67 [+44.00] | 6.19c ± 0.25 [−40.30] |
| LSP | 6.06cd ± 0.52 [+18.80] | 5.79bc ± 0.68 [−44.16] |
| LSP + melatonin | 7.08b ± 0.95 [+38.80] | 7.38d ± 1.47 [−28.83] |
Data are mean ± SD of ten rats in each group
Statistical analysis was done using one-way analysis of variance (ANOVA) using the CoStat computer program accompanied by the least significance level (LSD) between groups at p < 0.05
Unshared superscript letters are significant values between groups at p < 0.0001
Values between brackets are % changes over the control group
Values between parentheses are % changes over the diabetic group
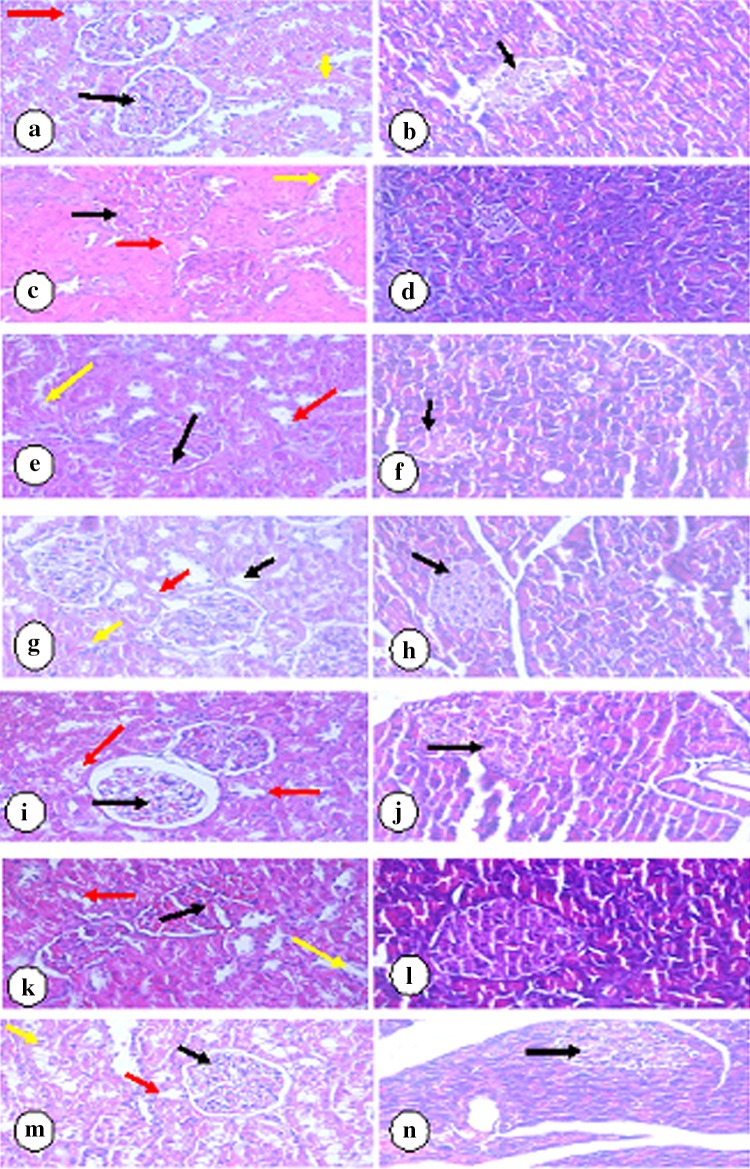
Regarding the histopathological analysis of the kidneys and pancreases in the present study, control rats showed more or less normal kidney and pancreatic architectures (Fig. 2a, b).
Fig. 2.
a Kidney section of normal rats showed renal cortex with normal corpuscles, a normal glomerulus (black arrow), and a normal pattern of proximal convoluted (red arrow) and distal convoluted (yellow arrow) tubules. b Pancreatic section of normal rats showed atrophic pancreatic islets of Langhans (arrow). c Kidney section of diabetic rats showed renal cortex with corpuscles of high cellularity and obliterated capsular space (black arrow), proximal convoluted tubules (red arrow) and distal convoluted tubules (yellow arrow) with destructed epithelial lining cells. d Pancreatic section from diabetic rats showed atrophic pancreatic islets of Langhans (arrow). e Kidney section from diabetic rats treated with Rowatinex showed renal cortex with corpuscles of high cellularity and obliterated capsular space (black arrow), proximal convoluted tubules (red arrow) and distal convoluted tubules (yellow arrow) showing destructed epithelial lining cells. f Pancreatic section from the diabetic group treated with Rowatinex showed small-size pancreatic islets of Langerhans with regular and arranged shape (arrow). g Kidney section from the diabetic group treated with melatonin showed renal cortex of a renal corpuscle with normal glomerulus (black arrow) and normal pattern of proximal convoluted (red arrow) and distal convoluted (yellow arrow) tubules. h Pancreatic section from the diabetic group treated with melatonin showed almost normal sized pancreatic islets of Langhans with regular shape (arrow). i Kidney section from the diabetic group treated with Rowatinex and melatonin showed renal cortex of a renal corpuscle with normal glomerulus (black arrow) and a normal pattern of proximal convoluted (red arrow) and distal convoluted (yellow arrow) tubules. j Pancreatic section from the diabetic group treated with Rowatinex and melatonin showed vacuolations and necrosis of β-cells of islets of Langhans (arrow). k Kidney section from the diabetic group treated with LSP showed renal cortex with corpuscles of high cellularity and obliterated capsular space (black arrow) and proximal convoluted tubules (red arrow), and distal convoluted tubules (yellow arrow) showed destructed epithelial lining cells. l Pancreatic section from the diabetic group treated with LSP showed pancreatic islets of Langerhans with regular and arranged shape (arrow). m Kidney section from diabetic rats treated with LSP and melatonin showed renal cortex of normal corpuscles with mild cellularity and mildly obliterated capsular space (black arrow), normal proximal convoluted tubules (red arrow) and normal distal convoluted (yellow arrow) tubules. n Pancreatic section from diabetic rats treated with LSP and melatonin showed mildly atrophic pancreatic islets of Langhans (arrow). (H&E, 400×)
Diabetic rats showed the renal cortex with high cellularity and obliterated the capsular space of most corpuscles. Proximal convoluted tubules showed destructed epithelial lining of cells. Obliterated and destructed epithelial lining of distal convoluted tubules was also seen. Pancreatic section from diabetic rats showed atrophic pancreatic islets of Langhans (Fig. 2c, d).
Diabetic rats treated with Rowatinex showed high atrophy of the kidney and pancreatic architecture (Fig. 2e, f). Treatment with melatonin and the compilation of melatonin and Rowatinex showed enhancement of the normal shapes (Fig. 2g–j). Treatment with LSP showed high atrophy of kidney and pancreatic structures (Fig. 2k, l), while treatment with LSP and melatonin showed mild enhancement of kidney and pancreatic structures (Fig. 2m, n).
The detailed histological findings of both the kidney and pancreas are shown in Fig. 2a–n, which represents the histopathological features of all the experimental groups under investigation.
Discussion
Diabetes mellitus remains one of the age-old chronic diseases of the human race, and its frontiers are expanding daily [31].
STZ administration to mature rats induced a significant increase in glucose levels with a decrease in body weight. This was in accordance with the results of Haligur et al. [32] who observed a decrease in insulin levels after STZ induction that produced a cytotoxic model of diabetes very similar to type I DM. Streptozotocin damages the Î2 cells of the islets of Langerhans in the pancreas [33], where the main reason for cellular injury is hypoxia. An extracellular calcium increase in diabetes leads to cell damage and activates both plasma membrane and mitochondrial injuries, which are the main cell damage pathways. Cell death is the last stage of the cellular damage, and it can occur by apoptosis or necrosis, where caspase-3 is the main marker [34]. Treatments with different drugs ameliorate the blood glucose level by variable degrees, where the LSP and melatonin combination demonstrated the most potent effect.
It was further observed in the present study that administration of STZ (50 mg/kg body weight) caused DN with an increase in the blood urea nitrogen, serum creatinine level and serum uric acid level and reduced urine creatinine and creatinin clearance levels. Treatment with the selected drugs resulted in a reduction in blood urea nitrogen, serum creatinine level and serum uric acid, while urine creatinine and creatinine clearance levels were increased upon treatment. Parving et al. [18] showed that treatment with an antihypertensive drug provided a renoprotective effect in hypertensive patients with type 2 diabetes and microalbuminuria. In agreement with the results of Parving et al. [18], we found that the combination of LSP and melatonin greatly improved the serum uric acid and urine creatinine levels.
It is well known that inflammation and oxidative stress play important roles in podocyte injury and downregulation of nephrin and podocin [35]. MDA is a marker of lipid peroxidation induced by oxidative stress, while SOD is an important defensive metalloproteinase that catalyzes the dismutation of superoxide radicals [36, 37]. In the present study, the increased MDA and NO levels and the decreased SOD, GSH and TAC levels indicated increased oxidative stress. Increased NO production was also found during chronic inflammation, suggesting the role of NO in the kidney response to inflammatory stimuli [38, 39]. Furthermore, oxidative stress could lead to proinflammatory cytokine production, which may further increase oxidative stress, setting up a vicious cycle [36]. Treatment with the selected drugs reverses the effect of diabetes on the oxidative marker levels, where the combination of LSP and melatonin greatly improved the NO, MDA, SOD and GSH levels.
Recently, several studies described the involvement of macrophages in the pathogenesis of diabetic nephropathy [40]. In line with this observation, we can speculate that infiltrated macrophages stimulate mesangial cells to secrete TGF-β1, which induces the production of extracellular matrix (ECM) proteins in an autocrine or paracrine manner. However, macrophages can secrete TGF-β1, which can stimulate mesangial cells to produce ECM proteins [40]. Thus, macrophages could stimulate mesangial cells to produce mesangial matrix directly and/or indirectly via TGF-β1 in the development of diabetic nephropathy [41]. TGF-β1 was improved after treatment with the chosen drugs by variable degrees, where LSP and melatonin showed the highest improvement level.
The KIM-1 in diabetic rats was significantly increased. In agreement with our results, Chaudhary et al. [42] stated that early renal tubular damage biomarker levels including urinary KIM-1 are elevated in diabetes, even in those with normoalbuminuria. Carlsson et al. [43] added that insulin sensitivity was inversely and independently associated with the KIM-1 concentration. Impaired insulin sensitivity and hyperinsulinemia have been suggested to contribute to the development of renal injury by promotion of mitogenic and fibrotic processes via activation of insulin-like growth factor-1, transforming growth factor-β, endothelin-1 and the rennin-angiotensin-aldosterone system [44]. Moreover, insulin resistance is associated with oxidative stress [45], proinflammatory cytokines and adipokines [46], which lead to renal injury. Contradictorily, increased inflammatory activity due to ongoing kidney damage could also impair insulin sensitivity [47].
Tubulointerstitial injury is present in chronic kidney disease, and it seems to be a better predictor of disease progression and long-term prognosis than the glomerular damage [48]. By measuring the KIM-1 level, this “tubular phase” of renal damage could be detected before the development of albuminuria, the currently used marker of early diabetic nephropathy [44]. In the present study, treatment showed a significant decrease in the glomerular damage KIM-1 level. Vaidya et al. [49] confirmed our results by the observed regression of diabetic nephropathy after treatment due to the reduced levels of urinary KIM-1. The most pronounced level of improvement was recorded after treatment with the combination of LSP and melatonin.
Heat shock proteins (HSPs) are a family of proteins that are known to be highly conserved intracellular proteins and categorized according to their molecular weight [50]. It has been found that various sources of stress including thermal, oxidative, hemodynamic, osmotic and hypoxic stresses have the ability to induce the expression of various types of heat shock protein members including HSP70 to offer cytoprotection [51]. Barutta et al. [52] found that diabetes expressed HSP70 in the glomeruli and in the medulla, which may explain the ability of renal cells to increase the effectiveness of the cytoprotective response. Diabetes has oxidative properties, and thus HSP70 is well expressed. Treatment with the selected drugs, especially the combination of LSP and melatonin, improved the level of HSP70 through their antioxidative effects and the resulting protein depression.
Caspases are cysteine-aspartyl-specific proteases that play a key role in apoptosis. Caspase-3 is one of the effector caspases of apoptotic pathways [32]. Gene targeting strategies have provided valuable tools to study the physiologic function of individual caspases in vivo and have shown their roles not only in apoptosis but also in other fundamental cellular processes [53]. Several in vitro studies have suggested that caspase-dependent apoptotic pathways are essential for β cell apoptosis [54]. The mechanisms of tubular changes in diabetic kidneys are unclear. One attractive mechanism is apoptosis, which has been demonstrated to mediate cell death in a variety of renal diseases, including diabetic nephropathy [55]. Indeed, apoptosis was detected in renal proximal tubular cells of different species including experimental animals and patients with diabetes, suggesting that tubular apoptosis may precede tubular atrophy [56]. Frances et al. [57] demonstrated that apoptosis occurring in the diabetic state was associated by elevation of OH that contributed partially to the mitochondrial cytochrome c release and elevation of caspase-3 activation. Treatment with the selected drugs, with special attention to the LSP and melatonin combination, attenuated the elevated free radicals, which improved the apoptotic condition.
Apoptosis is also mediated by DNA fragmentation [58, 59], which is associated with a decrease in protein content. This decrease may be due to a decrease in ribosomal granules of the rough endoplasmic reticulum or to a decrease in DNA content [58]. These results fit with those of Szkudelski [59], who stated that one of the targets of the reactive oxygen species is DNA of the pancreatic islets where its fragmentation takes place in β cells. These observations were confirmed by the observed increase in DNA tail lengths, the percentages of tailed DNA and the tail moment (the product of the tail length and the fraction of total DNA in the tail) in diabetic rats.
The comet assay technique in the present study is a commonly used method to detect oxidative damage in lymphocyte DNA, and the degree of oxidative stress is related to the size of the comet tail [60]. High blood glucose levels in vitro impair cellular DNA repair and increase DNA cleavage [61]. Hyperglycemia itself contributes to increased generation of ROS, and increased oxidative stress would lead to oxidative DNA damage. The same authors added that blockade of hyperglycemia-induced ROS production would reverse the adverse effect of diabetes on DNA structure. This was confirmed by the observed improvement in DNA degradation after treatment with the LSP and melatonin combination.
The histopathological picture of diabetic kidney in our study showed vacuolar degeneration in some of the tubular epithelial cells. There was an increase in the thickness of tubular epithelial cells with narrowing of the lumen, signs of degeneration in the form of karyolysis and karyorrhexis as well as deformation of the renal tissue architecture. These observations were in agreement with Shaffie et al. [62]. These vacuolations may be due to the altered permeability of the cell membrane, which would allow increasing the fluid uptake. Lannergren et al. [58] added that the presence of vacuoles was due to lactate accumulation in the tubules of the kidney resulting in increased osmotic pressure and subsequent water influx. In the pancreatic tissue, degeneration of islet cells was seen with moderate vacuolations. Rowatinex and LSP treatments showed mild destruction of the epithelial lining of the proximal and distal convoluted tubules, while treatment with melatonin and its combination with Rowatinex or Amosar showed a normal pattern of proximal and distal convoluted tubules. This amelioration was due to the antioxidant properties of melatonin [10] and Rowatinex [16, 17] and the renoprotective effect of LSP [18].
In conclusion, diabetes had a deleterious effect on rats' kidney functions, nephropathy index, oxidative stress markers, DNA fragmentation, protein contents and kidney histopathological features. Treatment with the selected drugs improved the biochemical parameters by variable degrees. The combination of melatonin and LSP improved most of the biochemical parameters under investigation. Further studies are needed to explore the use of melatonin as a dietary supplement in the treatment of diabetic nephropathy.
Animal rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol. 2005;16:S30–S33. doi: 10.1681/ASN.2004110970. [DOI] [PubMed] [Google Scholar]
- 2.Yoh K, Hirayama A, Ishizaki K, et al. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells. 2008;13:1159–1170. doi: 10.1111/j.1365-2443.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 3.Lewis EJ, Xu X. Abnormal glomerular permeability characteristics in diabetic nephropathy: implications for the therapeutic use of low-molecular weight heparin. Diab Care. 2008;31:S202–S207. doi: 10.2337/dc08-s251. [DOI] [PubMed] [Google Scholar]
- 4.Schaeffer V, Hansen KM, Morris DR, Abrass CK. Reductions in laminin beta2 mRNA translation are responsible for impaired IGFBP-5-mediated mesangial cell migration in the presence of high glucose. Am J Physiol Renal Physiol. 2010;298:F314–F322. doi: 10.1152/ajprenal.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yozai K, Shikata K, Sasaki M, et al. Methotrexate prevents renal injury in experimental diabetic rats via anti-inflammatory actions. J Am Soc Nephrol. 2005;16:3326–3338. doi: 10.1681/ASN.2004111011. [DOI] [PubMed] [Google Scholar]
- 6.Seon YD, Lee TH, Lee MC. Changes of glomerular basement membrane components in Vacor-induced diabetic nephropathy. Korean J Intern Med. 1999;14:77–84. doi: 10.3904/kjim.1999.14.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micah L, Thorp DO. Diabetic nephropathy: common questions. Am Fam Physician. 2005;72:96–99. [PubMed] [Google Scholar]
- 8.Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ. Phytomelatonin: a review. J Exp Botany. 2009;60:57–69. doi: 10.1093/jxb/ern284. [DOI] [PubMed] [Google Scholar]
- 9.Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–419. doi: 10.1016/j.tips.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Hardeland R, Pandi-Perumal SR. Melatonin, a potent agent in antioxidative defense: actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nut Metab. 2005;2:22. doi: 10.1186/1743-7075-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter R, Acuña-Castroviejo D, Tan DX, Burkhardt S. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann NY Acad Sci. 2001;939:200–215. doi: 10.1111/j.1749-6632.2001.tb03627.x. [DOI] [PubMed] [Google Scholar]
- 12.Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci. 2003;60:1407–1426. doi: 10.1007/s00018-003-2319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvira D, Tajes M, Verdaguer E, et al. Inhibition of the cdk5/p25 fragment formation may explain the antiapoptotic effects of melatonin in an experimental model of Parkinson’s disease. J Pineal Res. 2006;40:251–258. doi: 10.1111/j.1600-079X.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 14.FDA Tightens up Dietary Supplement Manufacturing and Labelling. Medical News Today, 2007. Retrieved 2 Sept 2013.
- 15.Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28:1271–1278. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- 16.Bach T. Preclinical and clinical overview of terpenes in the treatment of urolithiasis. European Urol. 2010;9:801–826. doi: 10.1016/j.eursup.2010.11.009. [DOI] [Google Scholar]
- 17.Cipriani P, Mancini C. Microbiological activity of a terpen product used in the treatment of urinary diseases. Gaz Int Mede Chir. 1972;77–82.
- 18.Parving H, Lehnert H, Brochner-Mortansen J, Gomis R, Anderson S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Eng J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 19.Punithavathi VR, Anuthama R, Prince SM. Combined treatment with naringin and vitamin C ameliorates streptozotocin-induced diabetes in male Wistar rats. J Appl Toxicol. 2008;28:806–813. doi: 10.1002/jat.1343. [DOI] [PubMed] [Google Scholar]
- 20.Bhandari U, Kanojia R, Pillai KK. Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J Ethnopharmacol. 2005;97:227–230. doi: 10.1016/j.jep.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Yavuz O, Camb M, Bukanc N, Guvenb A, Siland F. Protective effect of melatonin on β-cell damage in streptozotocin-induced diabetes in rats. Acta Histochem. 2003;105:261–266. doi: 10.1078/0065-1281-00711. [DOI] [PubMed] [Google Scholar]
- 22.Romics I, Siller G, Kohnen R, Mavrogenis S, Varga J, Holman E. A Special terpene combination (Rowatinex®) improves stone clearance after extracorporeal shockwave lithotripsy in Urolithiasis patients: results of a placebo-controlled randomised controlled trial. Urol Int. 2011;86:102–109. doi: 10.1159/000320999. [DOI] [PubMed] [Google Scholar]
- 23.Murali B, Goyal RK. Effect of chronic treatment with losartan on streptozotocin induced diabetic nephropathy. Clin Exp Hypertens. 2001;23:513–520. doi: 10.1081/CEH-100106822. [DOI] [PubMed] [Google Scholar]
- 24.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 25.Moron MS, Depierre JW, Mannervik B. Level of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 26.Nishikimi M, Rae NA, Yagi K. The occurrence of superoxide anion in the action of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–853. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 27.Moshage H, Kok B, Huzenge JR, Jansen PL. Nitrite and nitrate determination in plasma: a critical evaluation. Clin Chem. 1995;41:892–896. [PubMed] [Google Scholar]
- 28.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Karimi MM, Sani MJ, Mahmudabadi AZ, Sani AJ, Khatibi SR. Effect of acute toxicity of cadmium in mice kidney cells. Iran J Toxicol. 2012;6:691–798. [Google Scholar]
- 30.Suzuki H, Suzuki K. Rat hypoplastic kidney (hpk/hpk) induces renal anemia, hyperparathyroidism, and osteodystrophy at the end stage of renal failure. J Vet Med Sci. 1998;60:1051–1058. doi: 10.1292/jvms.60.1051. [DOI] [PubMed] [Google Scholar]
- 31.Atangwho IJ, Ebong PE, Eteng MU, Eyong EV, Obi AU. Effect of Vernomia amygdalina Del leaf on kidney function of diabetic rats. Int J Pharmacol. 2007;3:143–148. doi: 10.3923/ijp.2007.143.148. [DOI] [Google Scholar]
- 32.Haligur M, Topsakal S, Ozmen O. Early degenerative effects of diabetes mellitus on pancreas, liver, and kidney in rats: an immunohistochemical study. Exp Diabetes Res. 2012;2:1–10. doi: 10.1155/2012/120645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson GL, Leiter EH. Streptozotocin interactions with pancreatic β cells and the induction of insulin-dependent diabetes. Curr Topics Microbiol Immunol. 1990;156:27–54. doi: 10.1007/978-3-642-75239-1_3. [DOI] [PubMed] [Google Scholar]
- 34.Slauson DO, Cooper BJ (eds.). Pathology—the study of disease. In: Mechanisms of disease a textbook of comparative general pathology. Mosby, St. Louis; 2002, p. 1–15.
- 35.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 36.Tang J, Yan H, Zhuang S. Inflammation and oxidative stress in obesity-related glomerulopathy. Int J Nephrol. 2012;2012:e1–e11. doi: 10.1155/2012/608397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan QR, Ren YL, Zhu JJ, et al. Resveratrol increases nephrin and podocin expression and alleviates renal damage in rats fed a high-fat diet. Nutrients. 2014;6:2619–2631. doi: 10.3390/nu6072619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkali G, Gezer S, Umur N. Nitric oxide in chronic liver disease. Turk J Med Sci. 2000;30:511–515. [Google Scholar]
- 39.El-Gengaihi SE, Hamed MA, Khalaf-Allah AM, Mohammed MA. Golden berry juice attenuates the severity of hepatorenal injury. J Diet Suppl. 2013;10:357–369. doi: 10.3109/19390211.2013.830675. [DOI] [PubMed] [Google Scholar]
- 40.Leonarduzzi G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogel S, Dargel R, Poli G. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta-1- expression in the macrophage lineage. A link between oxidative energy and fibrosclerosis. FASEB J. 1997;11:851–857. doi: 10.1096/fasebj.11.11.9285483. [DOI] [PubMed] [Google Scholar]
- 41.Pawluczyk IZ, Harris KP. Macrophages promote prosclerotic responses in cultured rat mesangial cells: a mechanism for the initiation of glomerulosclerosis. J Am Soc Nephrol. 1997;8:1525–1536. doi: 10.1681/ASN.V8101525. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhary K, Phadke G, Nistala R, Weidmeyer CE, McFarlane SI, Whaley-Connell A. The emerging role of biomarkers in diabetic and hypertensive chronic kidney disease. Curr Diabetes Rep. 2010;10:37–42. doi: 10.1007/s11892-009-0080-z. [DOI] [PubMed] [Google Scholar]
- 43.Carlsson AC, Calamia M, Rise’rus U, et al. Kidney injury molecule (KIM)-1 is associated with insulin resistance: results from two community-based studies of elderly individuals. Diab Resh Clin Pract. 2014;103:516–521. doi: 10.1016/j.diabres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol. 2006;26:232–244. doi: 10.1159/000093632. [DOI] [PubMed] [Google Scholar]
- 45.Riserus U, Basu S, Jovinge S, Fredrikson GN, Arnlov J, Vessby B. Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated Creactive protein: a potential link to fatty acid-induced insulin resistance. Circulation. 2002;106:1925–1929. doi: 10.1161/01.CIR.0000033589.15413.48. [DOI] [PubMed] [Google Scholar]
- 46.Knight SF, Imig JD. Obesity, insulin resistance, and renal function. Microcirculation. 2007;14:349–362. doi: 10.1080/10739680701283018. [DOI] [PubMed] [Google Scholar]
- 47.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.CIR.0000034509.14906.AE. [DOI] [PubMed] [Google Scholar]
- 48.Peralta CA, Katz R, Bonventre JV, Sabbisetti V, Siscovick D. Kidney injury molecule 1 (KIM-1) and neutrophil gelatinase associated lipocalin (NGAL) with kidney function decline in the multi-ethnic study of atherosclerosis (MESA) Am J Kidney Dis. 2012;60:904–911. doi: 10.1053/j.ajkd.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-d-glucosaminidase. Kidney Int. 2011;79:464–470. doi: 10.1038/ki.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 51.Kim HJ, Hwang NR, Lee KJ. Heat shock response for understanding diseases of protein denaturation. Moll Cell. 2007;23:123–131. [PubMed] [Google Scholar]
- 52.Barutta F, Pinach S, Giunti S, et al. Heat shock protein expression in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1817–F1824. doi: 10.1152/ajprenal.90234.2008. [DOI] [PubMed] [Google Scholar]
- 53.Woo M, Hakem R, Soengas MS. Essential contribution of caspase 3/CPPp32 to apoptosis and its associated nuclear changes. Genes Dev. 1998;12:806–819. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maedler K, Spinas GA, Lehmann R. Glucose induces beta-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes. 2011;50:1683–1690. doi: 10.2337/diabetes.50.8.1683. [DOI] [PubMed] [Google Scholar]
- 55.Brezniceanu ML, Liu F, Wei CC. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes. 2008;57:451–459. doi: 10.2337/db07-0013. [DOI] [PubMed] [Google Scholar]
- 56.Liu F, Brezniceanu ML, Wei CC. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol. 2008;19:269–280. doi: 10.1681/ASN.2007010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frances DE, Ronco MT, Monti JA. Hyperglycemia induces apoptosis in rat liver through the increase of hydroxyl radical: new insights into the insulin effect. J Endocrinol. 2010;205:187–200. doi: 10.1677/JOE-09-0462. [DOI] [PubMed] [Google Scholar]
- 58.Lannergren J, Westerblod H, Bruton SD. Dynamic vacuolation in skeletal muscle fibers after fatigue. Cell Biol Int. 2002;26:911–920. doi: 10.1006/cbir.2002.0941. [DOI] [PubMed] [Google Scholar]
- 59.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:536–546. [PubMed] [Google Scholar]
- 60.Woods JA, Young AJ, Gilmore IT. Measurement of menadione-mediated DNA damage in human lymphocytes using the Comet assay. Free Radic Res. 1997;26:113–124. doi: 10.3109/10715769709097790. [DOI] [PubMed] [Google Scholar]
- 61.Kaneto H, Fujii J, Suzuki K. DNA cleavage induced by glycation of Cu, Zn superoxide dismutase. Biochem J. 1994;304:219–225. doi: 10.1042/bj3040219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaffie NM, Morsy FA, Ali AG, Sharaf HA. Effect of craway, coriander and fennel on the structure of kidney and islets of Langerhans in alloxan-induced diabetic rats: histological and histochemical study. Researcher. 2010;2:27–40. [Google Scholar]