Abstract
We herein report a case involving a woman with type 1 diabetes and a history of metal allergy who developed a local delayed-type (type IV) allergy to zinc-containing insulin. She had been treated by continuous subcutaneous insulin infusion, but her glycemic control was poor, and she developed diabetic ketoacidosis. Her plasma insulin concentration was unexpectedly low during use of insulin lispro, but it was recovered by changing from the zinc-containing insulin lispro to the zinc-free insulin glulisine. Intradermal tests showed no reactions to various insulins except for zinc chloride. A skin biopsy at the injection site of insulin lispro showed invasion of lymphocytes, neutrophils, and eosinophils, but a skin biopsy at the injection site of insulin glulisine showed invasion of only lymphocytes. A drug lymphocyte stimulation test against polaprezinc, an antiulcer drug containing zinc, was positive. Therefore, we diagnosed the patient with local delayed allergy to zinc-containing insulin. Insulin allergy should be considered as a possible cause of poor glycemic control and diabetic ketoacidosis in patients with type 1 diabetes.
Keywords: Insulin allergy, Zinc allergy, Type IV allergy, Diabetic ketoacidosis, Continuous subcutaneous insulin infusion
Introduction
Insulin allergy has been rare since the development of recombinant human insulin and its analogs. Type I (immediate type), III (Arthus type), and IV (delayed type) insulin allergies are induced by insulin itself, while protamine, zinc, and other components contained in insulin can cause local or systemic allergies [1, 2]. Only a few reports have addressed type IV insulin allergy [3, 4]. We herein report a case involving a patient with type 1 diabetes who developed poor glycemic control during treatment with continuous subcutaneous insulin infusion (CSII) because of a local delayed hypersensitivity reaction to zinc-containing insulin.
Case report
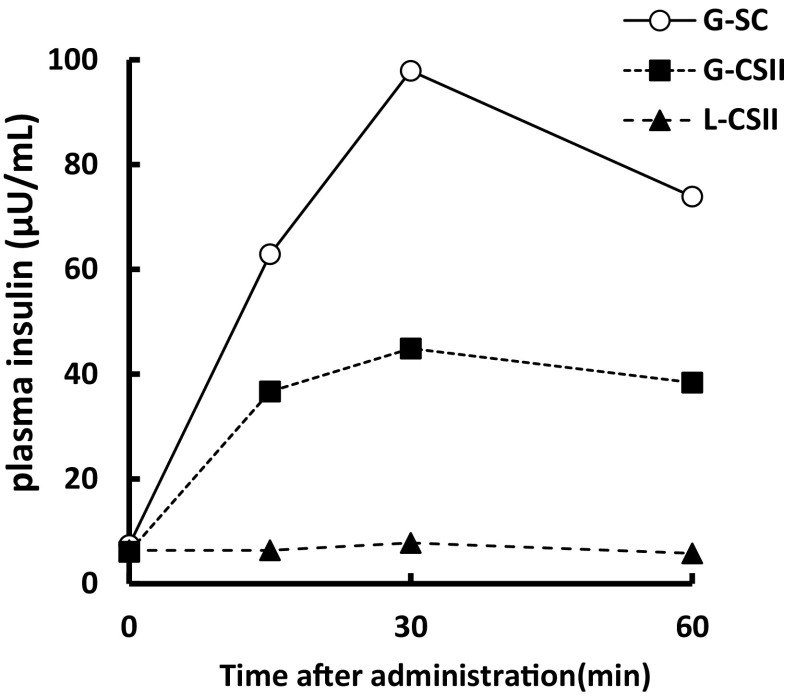
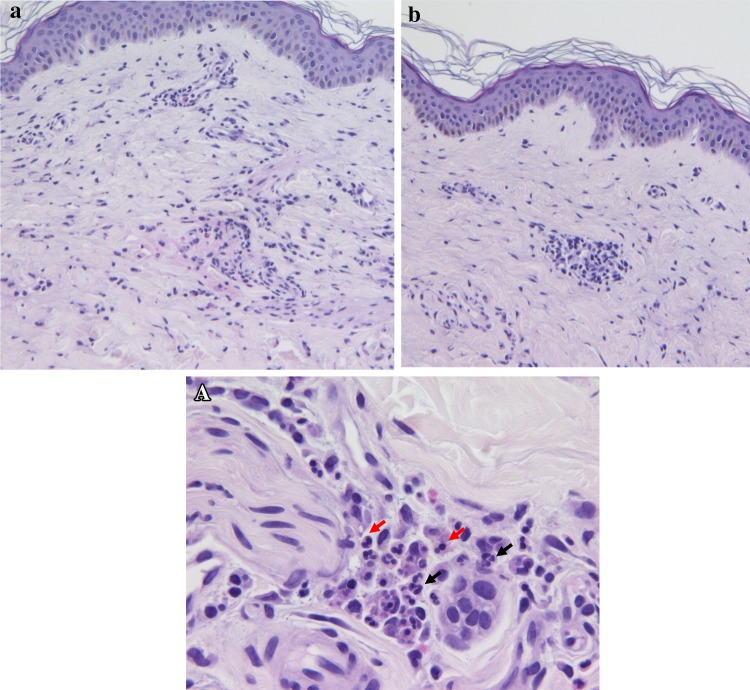
A 39-year-old female returned for a follow-up visit to our clinic for evaluation of her 9-year history of type 1 diabetes mellitus. Her medical history included the development of a metal allergy in her teens, schizophrenia at the age of 29 years, asthma at the age of 31 years, and myocardial infarction at the age of 34 years. Subcutaneous insulin injection was difficult because she had experienced needle phobia since childhood. She had therefore undergone treatment with CSII but experienced intermittent hypoglycemia and hyperglycemia. The patient was admitted to our hospital because of pinna cellulitis and was diagnosed with diabetic ketoacidosis (arterial blood gas analysis: pH, 7.215; pO2, 109 mmHg; pCO2, 14 mmol/l; HCO3−, 5.5 mmol/l; anion gap, 31.8; glucose, 479 mg/dl; lactate, 24 mg/dl; 3-hydroxybutyrate, 6.2 mmol/l). Laboratory data on admission are shown in Table 1. The patient had iron deficiency anemia and subclinical hypothyroidism due to Hashimoto’s disease, indicating autoimmune polyglandular syndrome type 3. Four days after she received continuous intravenous insulin infusion (CVII) using regular insulin to reach euglycemia, she developed hyperglycemia that did not resolve with CSII. We were therefore unable to stop the CVII. On day 12, the patient was determined to be positive for anti-insulin antibodies (35.7 U/ml). We doubted that she had insulin resistance because of the formation of insulin lispro-specific antibodies; therefore, we changed the insulin lispro to regular insulin following insulin aspart. However, her hyperglycemia persisted, and CVII could not be withdrawn. On day 24, redness, swelling, and pain developed at the insulin injection site. Patch tests against zinc chloride were performed because these symptoms were speculated to be due to an insulin allergy based on the patient’s history of metal allergy. The results were positive against zinc chloride but not against regular insulin or cannula tubes of CSII (data not shown). After injection of various insulins, we measured the plasma insulin concentration using the ST AIA-PACK IRI (Tosoh Bioscience, Tokyo, Japan). Six units of insulin lispro or insulin glulisine were administered subcutaneously or via CSII (Fig. 1) combined with CVII with regular insulin to avoid diabetic ketoacidosis. The plasma insulin concentration was not elevated after insulin lispro injection via CSII, but that after insulin glulisine injection via CSII was not elevated as much as that via subcutaneous bolus injection. The patient’s glycemic control improved, and CVII could be withdrawn after the insulin was changed to insulin glulisine, which does not contain zinc. The patient’s clinical course of glycemic control is shown in Fig. 2. Intradermal tests were performed against regular insulin, insulin lispro, insulin aspart, and insulin glulisine. All results were negative after 15 min (early response) and 48 h (delayed response). A skin biopsy at the site of insulin lispro injection showed infiltration of lymphocytes, neutrophils, and eosinophils in the dermal lesion, but a skin biopsy at the site of glulisine injection showed infiltration of only lymphocytes (Fig. 3). Insulin-specific IgE antibody was negative (<0.1 U/ml). A drug lymphocyte stimulation test against polaprezinc, an antiulcer drug containing zinc, was positive (216 %; reference range, 0–200 %). Based on these results, we diagnosed the patient with a local delayed allergy to zinc-containing insulin.
Table 1.
Laboratory data on admission
| Ht | 30.8 % |
| Hb | 10.0 g/dl |
| RBC | 4.42 × 106/μl |
| WBC | 7900/μl |
| Seg./neut. | 82.1 % |
| Eos. | 1.1 % |
| Baso. | 0.4 % |
| Lymph. | 9.8 % |
| Mono. | 6.6 % |
| PLTS | 25.9 × 104/μl |
| TP | 6.6 g/dl |
| Alb | 3.8 g/dl |
| AST | 13 U/l |
| ALT | 9 U/l |
| LDH | 93 U/l |
| ALP | 218 U/l |
| γGTP | 40 U/l |
| ChE | 262 U/l |
| T-Bil | 0.54 mg/dl |
| Na | 136 mmol/l |
| Cl | 107 mmol/l |
| K | 3.3 mEq/l |
| UN | 7.9 mg/dl |
| Cre | 0.42 mg/dl |
| eGFR | 129.4 ml/min/1.73m2 |
| UA | 2.8 mg/dl |
| Ca | 8.2 mg/dl |
| P | 2.8 mg/dl |
| Amy | 42 U/l |
| Lipase | 11 U/l |
| CPK | 13 U/l |
| Ferritin | 3.3 ng/ml |
| CRP | 5.72 mg/dl |
| TSH | 4.66 μIU/ml |
| FreeT4 | 0.79 ng/dl |
| FreeT3 | 1.8 pg/ml |
| TgAb | 95 IU/ml |
| TPOAb | 81 IU/ml |
| TRAb | <1.0 IU/l |
| FPG | 295 mg/dl |
| HbA1c | 8.5 % |
| IRI | 7.4 μU/ml |
| CPR | <0.2 ng/ml |
| IgG | 1098 mg/dl |
| IgM | 247 mg/dl |
| IgA | 226 mg/dl |
| IgE | 22.9 IU/ml |
| ANA | ×40 (homogenous) |
Fig. 1.
Plasma insulin concentration after injection of two kinds of insulin. Six units of each insulin analog were administered before breakfast, combined with 0.4 U/h of regular insulin via CVII. G-SC insulin glulisine administered subcutaneously; G-CSII insulin glulisine administered via CSII; L-CSII insulin lispro administered via CSII
Fig. 2.
Clinical course of glycemic control after transfer to our department. Blood glucose was measured before and 2 h after each meal using a self-monitoring blood sugar kit. Red square means blood glucose concentration before every meal. Insulin regimens are shown as orange columns (regular insulin), pale blue columns (insulin lispro), red columns (insulin aspart), and navy columns (insulin glulisine). 3-OHBA 3-hydroxybutyrate
Fig. 3.
Histological examination of the insulin analog injection sites. a A skin biopsy at the insulin lispro injection site showed infiltration of lymphocytes, neutrophils, and eosinophils in the dermal lesion. A Magnification of a Black arrow neutrophils. Red arrow eosinophils. b A skin biopsy at the insulin glulisine injection site showed infiltration of only lymphocytes
Discussion
We concluded that the cause of the poor glycemic control in this patient was impaired insulin efficacy due to delayed hypersensitivity to zinc.
The evidence of aberrant insulin absorption due to hypersensitivity to insulin is insufficient. However, considering the lack of elevation in the plasma insulin concentration after insulin lispro injection via CSII, we developed two hypotheses. The first is that either local inflammation caused by delayed allergy prevented the absorption of insulin or local accumulation of zinc facilitated insulin degradation by activation of metalloproteases such as insulin-degrading enzymes [5]. In one study, patients reportedly had no response to subcutaneous injection of insulin but had normal responses to intravenous insulin [6]. Subcutaneous administration of a mixture of aprotinin (a protease inhibitor) and insulin resulted in euglycemia, suggesting protease-induced degradation of insulin at the site of injection [6]. It would be interesting to see whether serum insulin levels rise in response to intravenous administration of lispro. However, the intravenous administration of recombinant insulin is a contraindication in Japan. A mixture of insulin plus aprotinin, as previously described [6], may be useful to prove the first hypothesis regarding insulin degradation. The second hypothesis is that we could not measure the plasma insulin concentration accurately because of the absence of affinity of antibodies to insulin lispro. Unpublished data from Tosoh Bioscience obtained from a personal communication indicate that the company’s immunoreactive insulin enzyme immunoassay kit can measure lispro, aspart, and regular insulin equally. However, the measured concentrations of glulisine, detemir, and degludec were approximately 60, 70, and 40 % of the theoretical values, respectively. According to these data, this kit can properly measure the concentration of lispro. For this reason, the second hypothesis is unlikely.
The data in Fig. 1 were obtained during CVII using regular insulin because the patient’s glucose concentration was unstable and could not be controlled by CSII alone. Therefore, her fasting insulin concentration was thought to be equal to that of regular insulin administered by CVII.
Skin biopsy at the site of lispro injection, but not that at the site of glulisine injection, clearly showed a local delayed allergy response. Furthermore, both a drug lymphocyte stimulation test against polaprezinc and a zinc patch test were positive. These results suggest that the delayed allergy was against zinc. There may be a relationship between susceptibility to zinc allergy and metal allergy because the present patient had a history of metal allergy. However, other causes of hypersensitivity reactions may have existed in this patient because she had a skin induration at the site of insulin glulisine injection, and histological examination showed infiltration of lymphocytes. This is in agreement with previous studies showing that protamine or metacresol contained in insulin preparations could cause these reactions [1].
The negative result of the patch test against insulin glulisine and regular insulin might have been due to the sensitivity of the test. According to established guidelines [7], a low concentration might yield false-negative results. Drug patch tests need to be performed with high concentrations of the commercialized form of the drug when performing skin tests in the investigation of cutaneous adverse drug reactions [7]. We presume that the concentration of zinc contained in regular insulin was so much lower (21 μg/ml) than that in the zinc chloride (10 mg/ml) used in the patch test that it did not affect the lymphocyte response. A previous report showed that the zinc concentrations in insulin were correlated with the severity of skin symptoms [3]. The activation of zinc-sensitized lymphocytes may be dose-dependent.
In conclusion, this is the first reported case in which the plasma insulin concentration after subcutaneous injection of insulin was impaired because of a type IV zinc allergy. Insulin allergy should be addressed as a potential cause of poor glycemic control and diabetic ketoacidosis in patients with type 1 diabetes. Further immunological mechanisms involved in the relationship between ineffective insulin injection and delayed hypersensitivity response should also be explored.
Conflict of interest
H. Maegawa has received honoraria for manuscripts from Takeda Pharmaceutical Co., Ltd.; Merck Sharp & Dohme Corp.; Sanofi K.K.; Nippon Boehringer Ingelheim Co., Ltd.; Asteralls Pharma Inc.; Taisho Toyama Pharmaceutical Co., Ltd.; Kowa Pharmaceutical Co., Ltd.; Daiichi Sankyo Inc., Ono Pharmaceutical Co., Ltd.; and Mitsubishi Tanabe Pharma Co., Ltd. K. Nemoto, S. Ugi, S. Ogaku, N. Nakaizumi, K. Fuse, O. Sekine, K. Morino, and H. Maegawa received scholarship grants from Takeda Pharmaceutical Co., Ltd.; Merck Sharp & Dohme Corp.; Teijin Pharma Ltd.; Sanofi K.K.; Nippon Boehringer Ingelheim Co., Ltd.; and Kyowa Hakko Kirin Co., Ltd. T. Kato and T. Tanaka declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients for being included in the study.
References
- 1.Heinzerling L, et al. Insulin allergy: clinical manifestations and management strategies. Allergy. 2008;63(2):148–155. doi: 10.1111/j.1398-9995.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- 2.Radermecker RP, et al. Allergy reactions to insulin: effects of continuous subcutaneous insulin infusion and insulin analogues. Diabetes Metab Res Rev. 2007;23(5):348–355. doi: 10.1002/dmrr.714. [DOI] [PubMed] [Google Scholar]
- 3.Feinglos MN, et al. “Insulin” allergy due to zinc. Lancet. 1979;1(8108):122–124. doi: 10.1016/S0140-6736(79)90517-8. [DOI] [PubMed] [Google Scholar]
- 4.Gin H, et al. Generalized allergy due to zinc and protamine in insulin preparation treated with insulin pump. Diabetes Care. 1987;10(6):789–790. doi: 10.2337/diacare.10.6.789. [DOI] [PubMed] [Google Scholar]
- 5.Gehm BD, et al. Mutations in a zinc-binding domain of human insulin-degrading enzyme eliminate catalytic activity but not insulin binding. J Biol Chem. 1993;268(11):7943–7948. [PubMed] [Google Scholar]
- 6.Freidenberg GR, et al. Diabetes responsive to intravenous but not subcutaneous insulin: effectiveness of aprotinin. N Engl J Med. 1981;305(7):363–368. doi: 10.1056/NEJM198108133050702. [DOI] [PubMed] [Google Scholar]
- 7.Barbaud A, et al. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001;45(6):321–328. doi: 10.1034/j.1600-0536.2001.450601.x. [DOI] [PubMed] [Google Scholar]