Abstract
Adipose tissue not only functions as the major energy-storing tissue, but also functions as an endocrine organ that regulates systemic metabolism by releasing various hormones called adipokines. Macrophages play a critical role in maintaining adipocyte health in a lean state and in remodeling during the progression of obesity. Large numbers of classically activated (M1) macrophages accumulate in adipose tissue as adipocytes become larger because of excessive energy conditions, and they adversely affect insulin resistance by triggering local and systemic inflammation. In contrast, alternatively activated (M2) macrophages seem to maintain the health of adipose tissues in a lean state. In addition, they play a role in adapting to excess energy states, because M2 macrophage dysfunction caused by genetic disruption of the M2 gene results in metabolic disorders under high-fat-fed conditions that are probably attributable to their anti-inflammatory functions. Nonetheless, how M2 macrophages contribute to maintaining the health of adipose tissue and therefore to insulin sensitivity is largely unknown. In this article, we review the literature on the role of M1 and M2 macrophages in metabolism, with a special focus on the role of M2 macrophages in adipose tissue. Likewise, we raise topics of M2 macrophages in non-adipose tissues to expand our understanding of macrophage heterogeneity.
Keywords: Macrophage, Adipose tissue, Insulin resistance, Obesity, Beiging
Introduction
Various pathological conditions such as metabolic syndrome, atherosclerosis, and arthritis are associated with low-grade inflammation. Since inflammation is one of the characteristics of the innate immune response, much attention has been devoted to the cells that control the innate immune responses. Macrophages play a major role in the pathophysiology of low-grade inflammation. Ontogenically, the progenitors of rodent macrophages are derived from at least three sites. The first site is the yolk sac, which produces progenitors that populate all tissues that start developing at embryonic day 8. The second site is the fetal liver, which is initially seeded by hematopoietic progenitors from the yolk sac, and the fetal liver is thought to be the source of most of the circulating monocytes during the embryonic stage. The major tissue resident macrophages in the brain, epidermis, lung, spleen, pancreas, and liver are derived from the yolk sac. Langerhans cells have a mixed origin from York sac and fetal liver [1]. The third site is the bone marrow. As the bone marrow grows after birth, hematopoiesis in the fetal liver declines and is replaced by hematopoiesis in the bone marrow [2]. Schulz et al. have shown that Myb-dependent bone marrow progenitors continuously replace classical dendritic cells and F4/80 low macrophages, in particular in the kidney and lung [3]. Despite the numerous studies conducted on adipose tissue macrophages (ATMs) in the development of metabolic diseases, the origin of ATMs is still incompletely understood.
Macrophages are highly heterogeneous cells. Generally, macrophages can be broadly classified into at least two major populations by their diversity and plasticity: M1 macrophages, which are classically activated macrophages, and M2 macrophages, which are alternatively activated macrophages [4]. This classification is generated primarily by in vitro experiments. M1 macrophages are mainly induced by Th1 signaling, e.g., by lipopolysaccharide (LPS) and interferon gamma (IFNγ). They are pro-inflammatory, expressing high levels of inflammatory cytokines, including tumor necrosis factor alpha (TNFα), interleukin (IL)-6, and IL-1β, and play a crucial role in host defense against bacterial and viral infections [5, 6]. M1 macrophages play a causal role in inflammatory diseases, including rheumatoid arthritis, atherosclerosis, and inflammatory bowel disease. M2 macrophages, on the other hand, are induced by Th2 stimuli, e.g., by IL-4 and IL-13, and are associated with responses to anti-inflammatory reactions, helminth infections, wound healing, tissue remodeling, and tumor progression [7]. Recent studies have revealed that macrophage diversity correlates with various pathological conditions. Here, we will review the key properties of macrophage polarization, especially the key properties of M2 macrophage polarization in metabolic diseases.
Macrophages and adipose tissue physiology: a phenotypic switch model
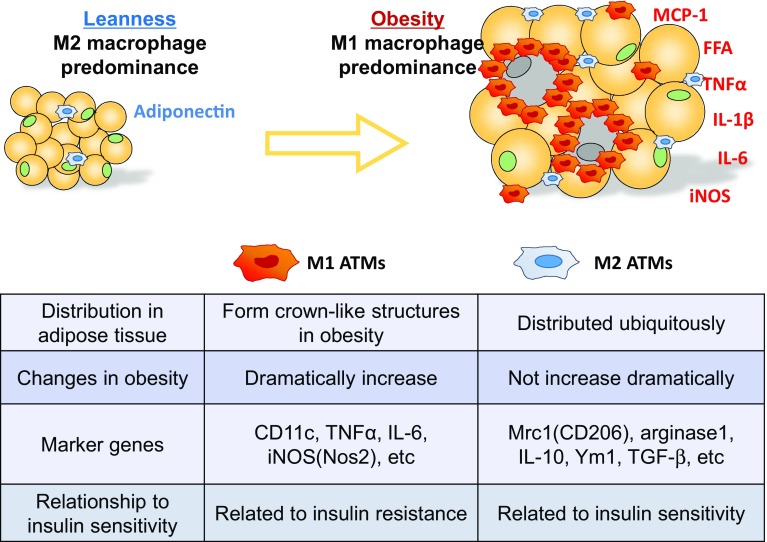
Interpretation of the phenotypic switch in ATMs during the progression of obesity is important to understand metabolic diseases [8, 9]. ATMs also consist of two subclasses: M1 ATMs and M2 ATMs. M1 ATMs highly express genes related to pro-inflammatory cytokines or oxidative stress, including genes related to TNFα, IL-6, MCP-1, and iNOS, whereas M2 ATMs highly express other genes that encode arginase-1, Mrc1(CD206), Ym1, and IL-10, an anti-inflammatory cytokine (Fig. 1).
Fig. 1.
The heterogeneity of macrophages in the development of adipose tissue inflammation. In the lean state, M2 ATMs predominate. As obesity progresses, monocytes accumulate in adipose tissue and differentiate into inflammatory M1 or anti-inflammatory M2 macrophages. Since the number of M1 ATMs increases dramatically, whereas there is only a modest increase in the number of M2 ATMs, M1 ATMs predominate in obese adipose tissue. M1 ATMs produce inflammatory cytokines and promote insulin resistance. M2 ATMs, on the other hand, secrete anti-inflammatory cytokines, including IL-10 and TGF-β, and suppress inflammation, suggesting that M2 ATMs are associated with insulin sensitivity
Ly6c+ inflammatory monocytes recruited into the adipose tissue of obese mice [10] differentiated into M1 ATMs and cluster around necrotic adipocytes to form characteristic structures, called crown-like structures (CLSs) [11], where they promote a low-grade inflammatory milieu and insulin resistance. M2 ATMs, on the other hand, are distributed evenly in the white adipose tissue (WAT) of lean mice.
There are standard markers to distinguish M1 and M2 macrophages. In a mouse model, CD11c is widely used as an M1 marker; CD206, CD209, CD163, Arg1, Mgl2, and IL-10 are used as M2 markers [12]. However, the markers and characters of M1/M2 ATMs are different between species. Not only CD206, CD163, and CD 209, but also CD14 are highly expressed in human M2 ATMs. It has been reported that human M2 ATM has a potential to express M1 markers [13].
Under lean conditions, more than 90% of ATMs are the M2 phenotype, while only around 1% of ATMs are the M1 phenotype, and the rest exhibit a mixed M1-M2 phenotype. Although the number of both M1 and M2 ATMs is markedly increased in the adipose tissue of obese mice, M1 ATM recruitment is more striking (Fig. 1) [14, 15], and the proportion of M1 ATMs increases to more than 50% as obesity progresses. Interestingly, when obesity in mice improves as a result of a restricted caloric intake or when the mice are treated with thiazolidinediones, which are an anti-insulin resistance drug, the number of M1 ATMs decreases. Moreover, even when large numbers of M1 ATMs are recruited to epididymal adipose tissue, only a few M1 ATMs are observed in subcutaneous adipose tissue (subQ) [16].
The marked increase in M1 ATMs in obesity is mediated by the monocyte chemotactic protein-1 (MCP-1)/C-C chemokine receptor type2 (CCR2) system. MCP-1 is recognized by Ly6C-positive monocytes, promotes the migration of Ly6C+ inflammatory monocytes into adipose tissue, and differentiates into M1 ATMs. The number of the ATMs decreases and insulin resistance improves in MCP-1- or CCR2-knockout mice [10, 17], whereas when MCP-1 is overexpressed in the adipocytes of mice, the number of ATMs increases, and the mice become insulin-resistant [17, 18]. Suganami et al. have reported the existence of a paracrine loop formed by adipocytes and macrophages mediated by saturated free fatty acids and the TLR4 receptor system, respectively, in the process of M1 ATM polarization [19].
We recently reported that a hypoxia signal also participates in the polarization of M1 ATMs to the adipose tissue of obese mice. CLSs are the most hypoxic sites in the adipose tissue of obese mice, and the hypoxia accelerates further polarization of macrophages to proinflammatory M1 state. Strong uptake of the hypoxia probe pimonidazole has been observed in CLSs. In addition, a macrophage-specific HIF-1α deletion decreased the number of ATMs recruited into the adipose tissue of high-fat diet-fed mice [16], suggesting that adipose tissue hypoxia participates in the recruitment of M1 ATMs through activation of the macrophage HIF-1α signal.
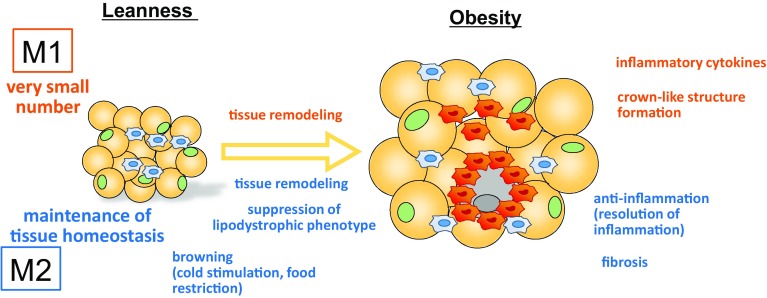
In 2008, Patsouris et al. clearly reported that M1 ATMs that express CD11c, an M1 marker, play important roles in the pathogenesis of obesity-associated insulin resistance. To study the effects of depletion of CD11c-positive cells, they generated a mouse model by using a conditional cell ablation system based on transgenic expression of the diphtheria toxin receptor under the control of the CD11c promoter, and the results showed rapid normalization of insulin sensitivity and a marked decrease in inflammatory markers in the adipose tissue of obese mice [20]. M2 ATMs, on the other hand, are thought to play roles in the maintenance or improvement of insulin sensitivity. The details about the roles are described in “Roles of M2 macrophages in adipose tissue” (Fig. 2).
Fig. 2.
Functional differences between M1 ATMs and M2 ATMs. In the lean state, the major ATMs are the M2 phenotype, and they play roles in maintaining tissue homeostasis. During cold stimulation and caloric restriction, M2 ATMs are involved in the browning of subQ. As obesity progresses both M1 and M2 ATMs become involved in tissue remodeling. M1 ATMs accumulate around necrotic adipocytes and form crown-like structures (CLSs), which further contribute to polarization into the M1 phenotype by activating HIF-1α. By contrast, M2 ATMs somehow prevent the development of the lipodystrophic phenotype, and they may play a role in suppressing inflammatory responses induced by M1 ATMs. In some situations, M2 ATMs induce fibrosis, thereby aggravating insulin resistance
Metabolic regulation of M1 and M2 phenotypes
M1 and M2 macrophages have different metabolic profiles: M1 macrophages obtain energy mainly by glycolysis, whereas M2 macrophages utilize aerobic metabolism. The difference in the metabolic regulations plays an important role in the induction of M1 and M2 macrophages to their respective polarities. For example, when oxidative metabolism in M1 macrophages is artificially increased, M2 polarity is induced [21], and when oxidative metabolism is inhibited, M1 macrophage polarity is induced [22]. Some metabolic characteristics of M1 ATMs are in close relation to an acquisition of inflammatory M1 polarity. For example, glucose uptake by M1 macrophages is greater than by M2 macrophages. Pyruvate dehydrogenase kinase-1 (PDK1) changes the lipid-dependent cell metabolism in M1 macrophages to glucose-dependent metabolism by suppressing oxidative respiration in the mitochondria. In M1 ATMs, the liver form of 6-phosphofructose-2-kinase/fructose-2,6-bisphosphatase (L-PFK2) is switched to the more activate ubiquitous form of PFK2 (u-PFK2), and anaerobic glycolysis is increased [21]. When the glycolytic flow is led to the pentose phosphate pathway with downregulation of carbohydrate kinase-like protein (CARKL) expression, the respiratory chain is attenuated and reactive oxygen species (ROS) production is promoted [23]. When succinate accumulates in the TCA cycle, production of IL-1β increases as a result of stabilization of HIF-1α [24]. Moreover, when an arginosuccinate shunt is formed, nitric oxide (NO) and IL-6 as well as fumarate and malate are produced [25]. The gene that encodes GLUT1, a glucose transporter, is one of the target genes of HIF-1α. When GLUT1 is artificially overexpressed in macrophages, production of several inflammatory cytokines increases [26].
On the other hand, the energy requirements of M2 macrophages are met by high rates of oxidative glucose metabolism and fatty acid oxidation (FAO) [27, 28]. For example, in the downstream of STAT6, PGC-1β is involved in the M2 switch [22], and PPARγ and PPARδ are involved in maintaining the M2 phenotype. M2 macrophages take triglycerides with CD36 and hydrolyze them into fatty acids by lysosomal lipolysis, and the fatty acids are used as their energy source [29]. When oxidative phosphorylation is impaired in M2 macrophages by deleting CD36 or liposomal acid lipase (LAL), M2 marker expression is suppressed [29]. Thus, there is a close causal relationship between the intracellular metabolism of macrophages and their acquisition of M2 polarity as well as M1 polarity.
Roles of M2 macrophages in adipose tissue
Tissue remodeling
M2 polarized macrophages have been implicated in the tissue remodeling that occurs during development, in response to injury, and in the general regulation of tissue homeostasis [7]. Macrophages in the M2 configuration typically upregulate arginase-1 and downregulate inducible NOS (iNOS) while expressing inflammation-suppressive factors (e.g., IL-10) and matrix metalloproteinases (MMPs). Scherer’s group reported that induction of adipocyte apoptosis in a lean state resulted in a large flux of M2 ATMs, and they described the influx as a healthy process that contributes to normal tissue homeostasis without causing a pro-inflammatory response [30]. Of note, it is notabe that they did not find any CLSs when they induced apoptosis of adipocytes in the lean state. Their report indicated that adipocyte apoptosis is a strong inducer of M2 ATMs.
Anti-inflammatory role
Although the mechanism of M2 macrophage regulation in obesity is not fully understood, various studies have shown a potential correlation between M2 ATMs and insulin sensitivity through the anti-inflammatory effects. Most ATMs in lean mice are M2-like. Using a mouse model (∆db1GATA mice), Wu et al. demonstrated that eosinophils are the major IL-4-expressing cells in the WAT of mice and that M2 ATMs are greatly attenuated in the absence of eosinophils, resulting in obesity-insulin resistance and glucose intolerance when fed on a high-fat diet [31]. Adipose tissue-derived IL-4 and IL-13 activate STAT6 through the IRF/STAT pathway in M2 macrophages [32–34]. STAT6 induces expression of transcriptional regulators, including peroxisome proliferator-activated receptor-γ (PPAR-γ). PPAR-γ promotes oxidative metabolism and M2 gene expression. Another member of the PPAR family, PPARβ/δ appears to differentially influence macrophage activation, along with IL-4 and IL-13, and they promote an alternative M2 macrophage phenotype. When PPARγ in myeloid cells is deleted or PPARδ−/− bone marrow is adoptively transferred into irradiated wild-type mice to generate mice whose hematopoietic cells lack PPARδ, M2 polarization is impaired. Such mice exhibit elevated inflammation, increased weight gain, and reduced insulin sensitivity on a high-fat diet, suggesting the existence of a correlation between M2 activation and insulin sensitivity [32, 33, 35, 36].
M2 macrophages also strongly express another transcriptional factor, PPARγ-coactivator 1β (PGC-1β). PGC-1β activates mitochondrial respiration and biogenesis, and their activation drives the metabolic switch of macrophages from the M1 phenotype to the M2 phenotype. Inhibition of PGC-1β results in impaired M2 metabolism and functions [22, 28]. When PGC-1β is activated downstream of STAT6, PGC-1β reinforces the activation of STAT6 itself or PPARγ and PPARδ, which leads to the increased gene expression related to the M2 property such as anti-inflammation and fatty acid oxidation.
Browning
Several pieces of evidence have suggested that the browning of WAT is a key factor in combating obesity and insulin resistance. M2 macrophages play a key role in shifting WAT into the beiging phenotype via activation of type 2 cytokine production during cold exposure and caloric restriction via β3 adrenergic receptor (ADRB3) activation [37–39]. This role correlates with better metabolic profile.
In response to cold stimulation, M2 macrophages release various cytokines in WAT and brown adipose tissue (BAT) that are crucially important to energy expenditure and to the regulation of fatty acid metabolism. During prolonged cold stress, WAT is able to maintain a positive energy balance by upregulation of M2 macrophages. Qiu et al. have shown that genetic disruption of IL-4/13 signaling or tyrosine hydroxylase (Th) prevents the cold-induced biogenesis of beige fat and that this effect in thermoneutral obese mice was reversed by IL-4 treatment [39]. There have been several reports that M2 macrophage polarization regulates thermogenesis and browning of WAT by increasing expression of UCP1, PGC-1α, and other browning genes [39–41]. Chronic cold exposure also increases the percentage of M2 macrophages in WAT as well as EdU uptake [40], suggesting that cold stimulation modulates M2 macrophages within WAT and promotes the browning of WAT.
Some studies have reported that activation of ADRB3, which upregulates the M2 macrophage phenotype, plays key roles in remodeling of WAT as well as the browning phenomenon [37, 42]. Lee et al. reported that induction of brown adipogenesis by (ADRB3) activation involves the death of white adipocytes and their removal by M2-polarized macrophages [37]. ADRB3 remodeling of WAT is associated with tissue restoration, upregulation of ALOX15 in M2 macrophages that correlate with phagocytosis of dead adipocytes and recruitment of these macrophages [41].
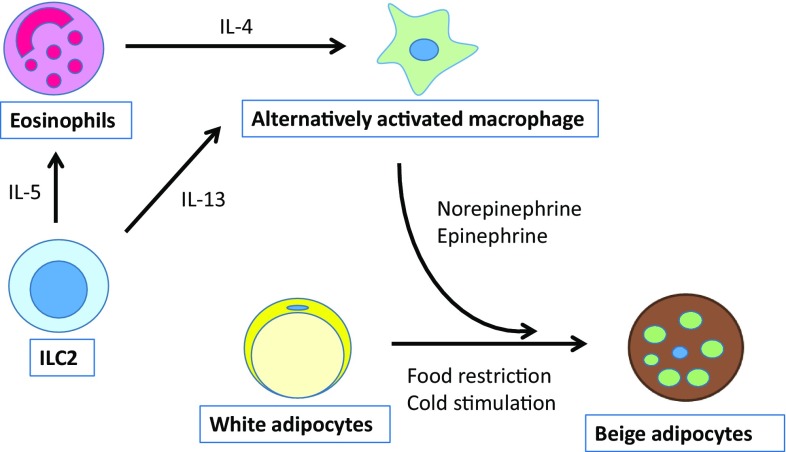
Group 2 innate lymphoid cells (ILC2s) have recently reported to promote browning of the WAT [43, 44]. ILC2s present in lymphoid and nonlymphoid tissues, promote type 2 inflammation [44, 45]. These cells were originally identified in fat-associated lymphoid clusters [46], and they have been shown to be key players in the recruitment and maintenance of eosinophils in WAT [43]. Recent studies [44, 47] have reported that ILC2s are capable of regulating the beiging of WAT. ILC2s produce the type 2 cytokines IL-5 and IL-13. ILC2-derived IL-5 stimulates eosinophils to produce IL-4, and eosinophil-derived IL-4 stimulates M2 macrophages to produce norepinephrine, which promotes beiging of WAT [39, 48]. Lee et al. demonstrated that eosinophil-derived IL-4 and ILC2-derived IL-13 stimulate signaling via the IL-4Rα in adipocyte progenitors and thereby promote their differentiation into beige adipocytes [44]. Brestoff et al. found that ILC2-derived methionine-enkephalin (MetEnk) directly binds to the δ1 opioid receptor expressed on WAT and promotes its beiging [47]. IL-33 secreted by epithelial cells acts on ILC2s to produce IL-5, IL-1 IL-5, IL-13 and MetEnk [44, 47]. It might be possible to use the ILC2/M2 ATM/beiging pathway (Fig. 3) as a new target for the treatment of obesity.
Fig. 3.
A simplified view of the relationship between M2 ATMs and other cells in beiging. ILC2s secrete IL-5, which activates eosinophils, and they produce IL-4 in response. IL-4 induces M2 polarization. M2 macrophages produce norepinephrine, which in turn promotes differentiation toward beige adipocytes
Prevention of the lipodystrophic phenotype
Several other proteins have been reported to control M2 polarity. Satoh et al. demonstrated that Trib1, an adaptor protein involved in protein degradation, is critical to the differentiation of M2 macrophages and eosinophils. They reported that disruption of this gene resulted in a lipodystrophic phenotype on a high-fat diet with hyperglycemia and insulin resistance in mice. Supplementation of M2-like macrophages rescued the pathophysiology [49]. These data suggested that M2 macrophages and eosinophils may be involved in the maintenance of adipose tissue homeostasis.
Fibrosis
Other studies have shown that M2 macrophages are associated with insulin resistance. Spencer et al. showed that M2 ATMs induce collagen VI synthesis and play an important role in adipose tissue fibrosis in obese subjects, which is associated with the development of insulin resistance [50]. The adipose tissue of insulin-resistant humans is characterized by the appearance of increased fibrosis, M2 macrophage abundance, and TGF-β activity. Broad areas of fibrosis were found in the adipose tissue of obese subjects, and they contained many macrophages, most of which were alternatively activated and expressed high levels of transforming growth factor beta (TGF-β), which promotes fibrosis [50]. TGF-β1 expressed by subcutaneous ATMs may contribute to fibrosis by inducing the differentiation of progenitor cells into myofibroblast-like cells [51]. Thus, human subQ progenitors have been identified as target cells for subQ ATM-derived TGF-β and as a potential source of fibrosis through their induction of myofibroblast-like cells.
Pro-inflammatory role of an M2 macrophage marker
Westcott et al. demonstrated the role of Mgl1, a marker of M2 macrophages, and showed that Mgl1−/− mice are protected from glucose intolerance, insulin resistance, and steatosis, despite having more adipose tissue. These effects are associated with a smaller number of inflammatory M1 ATMs, suggesting that M2 macrophages are involved in the differentiation of M1 ATMs [52]. There have been conflicting reports regarding M2 macrophage functions in WAT in relation to insulin resistance, and the role of M2 macrophages in the regulation of metabolism remains to be elucidated.
Role of M2 macrophages in the liver
The resident macrophages in the liver are called Kupffer cells, and they constitute one of the largest resident populations of macrophages. Kupffer cells are located in the liver sinusoids, and they clear microbes and apoptotic cells from the portal circulation [53]. Kupffer cells consist of primary yolk sac-derived monocytes and fetal liver monocytes, and they develop independent from hematopoietic stem cells [3, 54, 55]. Recently, it has been reported that circulating monocytes can differentiate into Kupffer cells if the niche is available to them [56]. In hepatic steatosis, activated M1 Kupffer cells increase lipid storage in hepatocytes and trigger the recruitment of monocyte-derived macrophages into the liver [57], thereby inducing non-alcoholic steatohepatitis (NASH), a more severe type of non-alcoholic fatty liver disease (NAFLD). In general, alternatively activated M2 macrophages are involved in tissue remodeling through the production of TGF-β1, but in the liver, inflammatory monocyte-derived macrophages express a higher level of TGF-β1, which activates hepatic stellate cells and promotes fibrosis [58]. A protective role of alternatively activated M2 macrophages/Kupffer cells against alcoholic fatty liver and NAFLD, including NASH, by promoting caspase-3-dependent apoptosis of M1 macrophages/Kupffer cells has recently been reported [59]. Furthermore, alternatively activated M2 macrophages/Kupffer cells limit hepatocyte apoptosis and steatosis in alcohol-induced liver injury by secreting IL-6 [60].
Roles of M2 macrophages in muscle
Macrophages play an important role in regulating glucose metabolism. It has been well documented that M2 macrophages are pivotal in regulating normal insulin sensitivity in metabolic tissues including WAT, liver tissue, and skeletal muscle tissue, and depletion of M2 macrophages abrogate these effects [8, 14, 15, 35, 61, 62]. Various M2 macrophages are present in normal skeletal muscle, mainly outside the basal lamina [63]. Skeletal muscles are known to be highly adaptable to external stimuli or damage. Since exercise increases insulin sensitivity in skeletal muscle and upregulates M2 macrophages [62], M2 macrophages maintain the muscle environment in balance so that muscles are able to adapt to external stimuli. Both in vivo and in vitro studies have demonstrated that M1 macrophages are more abundant in skeletal muscle tissue in the early stage of an injury and that M2 macrophages are more abundant in the late stage [64]. It is noteworthy that M2 macrophages increase in skeletal muscle after exercise and muscle injury, after which they are thought to be involved in increasing insulin sensitivity and in resolution of inflammation or the healing process, respectively. There is evidence that muscle has strong regenerative capacity after acute injury and that it maintains its internal environment by recruiting various cytokines and macrophages to the site of injury, where they resolve the inflammation and complete the healing process. Several groups of researchers have reported observing increased numbers of M2 macrophages in muscle tissue 3–4 days after an injury [63, 65–67].
Diabetic mice showed impaired muscle regeneration with fatty accumulation in conjugation with M2 macrophage [68, 69]. Diabetes may cause sarcopenia by mediating fibro-/adipogenic progenitor (FAP) cell differentiation [66]. How M2 macrophages regulate FAP activity in injuries remains unknown. Fatty degeneration and toxin injury are both thought to increase M2 macrophages in muscle. M2 macrophages stimulate myogenesis and adipogenesis after a muscle injury [67, 69–71]. It has been hypothesized that these macrophages regulate or potentiate muscle resident cells/satellite cells or myoblasts to inhibit differentiation into myofibers. It is important to know that what kind of M2 macrophages help to maintain the muscle environment and promote the regeneration process.
Roles of M2 macrophages in the kidney
The same as in other organs, the phenotypic switch of macrophages in the kidney is determined by the microenvironment during the course of kidney injury, inflammation, fibrosis, and repair [72]. Pro-inflammatory M1 macrophages are dominant in the early phase of kidney injury [72, 73], and regulatory T cells are then recruited into the injured kidney [72]. Apoptotic cells and regulatory T cell-derived anti-inflammatory cytokines, including IL-10 and TGF-β, drive macrophage polarization toward M2 macrophages [72]. M2 macrophages suppress kidney inflammation by secreting IL-10 and TGF-β. TGF-β has profibrotic functions as well as anti-inflammatory functions. Recent studies have shown that M2 macrophage-derived TGF-β promotes epithelial-mesenchymal transition (EMT) and subsequent kidney fibrosis [74]. Moreover, M2 macrophages produce trophic factors such as Wnt7b, heme-oxygenase-1 (HO-1), and chitinase-like protein BRP-39, which promote kidney repair [70, 72, 75, 76]. However, since the M2 macrophages in kidney diseases exhibit phenotypic heterogeneity [77], the role of M2 macrophages in kidney diseases is still a matter of controversy.
Roles of M2 macrophages in tumor cell metabolism
The communication between tumor cells and M2 tumor-associated macrophages (TAMs) remains poorly understood. M2 TAMs express arginase-1, which catalyzes the conversion of l-arginine to l-ornithine. l-Ornithine is a precursor of polyamines, which promote M2 polarization, and is capable of upregulating tumor cell proliferation [78–80]. M2 macrophage-derived arginase-1 impairs the anti-tumor immunity of T cells through L-arginase depletion, which inhibits expression of the CD3ζ chain after internalization in response to antigen stimulation and TCR signaling [81–83]. In contrast, M1 TAMs express iNOS, which converts l-arginine to NO, which is cytotoxic to tumor cells [78, 79]. Colegio et al. demonstrated that tumor-cell-derived lactic acid induces the expression of vascular endothelial growth factor (VEGF), arginase-1, and other M2-associated genes in macrophages [84, 85]. These lactic acid-induced M2 TAMs, which are the source of arginase-1, play a pivotal role in tumor cell proliferation.
Conclusion
Recent data have suggested that the role of M2 ATMs in metabolism is not limited to an anti-inflammatory function during the development of obesity, and they seem to be involved in clearance of apoptotic cells, browning of subcutaneous white adipose tissue, and the prevention of the lipodystrophic phenotype. They are also involved in promoting fibrosis, which is related to insulin resistance. M2 macrophages express other various functional molecules, which may have many other unknown functions that maintain homeostasis in adipose tissue and the whole body.
Further research will clarify the novel roles of M2 macrophages and their molecular mechanisms.
Conflict of interest
The authors declare the following financial interests: AstraZeneca K.K., Merck & Co., Inc., Medical Review Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma, Novo Nordisk Pharma, Ltd., Kowa Pharmaceutical Co., Ltd., Astellas Pharma Inc., and Fuji Chemical Industries Co., Ltd.
Human rights statement and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Shiho Fujisaka, Phone: +81-76-434-7287, Email: shihof@med.u-toyama.ac.jp.
Isao Usui, Email: isaousui@med.u-toyama.ac.jp.
Kazuyuki Tobe, Email: tobe@med.u-toyama.ac.jp.
References
- 1.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209(6):1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi O, Akira S. Signaling pathways activated by microorganisms. Curr Opin Cell Biol. 2007;19(2):185–191. doi: 10.1016/j.ceb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 10.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262(1):134–152. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31(9):1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 14.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, Mahmood A, Goda N, Nagai Y, Takatsu K, et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1alpha-dependent and HIF-1alpha-independent manner in obese mice. Diabetologia. 2013;56(6):1403–1412. doi: 10.1007/s00125-013-2885-1. [DOI] [PubMed] [Google Scholar]
- 17.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281(36):26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 19.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25(10):2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 20.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8(4):301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185(1):605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 22.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15(6):813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, Macintyre AN, Goraksha-Hicks P, Rathmell JC, Makowski L. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289(11):7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galvan-Pena S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang SC, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O’Neill CM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15(9):846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer-Posovszky P, Wang QA, Asterholm IW, Rutkowski JM, Scherer PE. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology. 2011;152(8):3074–3081. doi: 10.1210/en.2011-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7(6):496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7(6):485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Chartouni C, Schwarzfischer L, Rehli M. Interleukin-4 induced interferon regulatory factor (Irf) 4 participates in the regulation of alternative macrophage priming. Immunobiology. 2010;215(9–10):821–825. doi: 10.1016/j.imbio.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 35.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 37.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013;18(3):355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabbiano S, Suarez-Zamorano N, Rigo D, Veyrat-Durebex C, Stevanovic Dokic A, Colin DJ, Trajkovski M. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab. 2016;24(3):434–46. doi: 10.1016/j.cmet.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, Feng T, Zhong C, Wang Y, Lam KS, et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 2015;22(2):279–290. doi: 10.1016/j.cmet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Lee YH, Kim SN, Kwon HJ, Maddipati KR, Granneman JG. Adipogenic role of alternatively activated macrophages in beta-adrenergic remodeling of white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2016;310(1):R55–R65. doi: 10.1152/ajpregu.00355.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YH, Kim SN, Kwon HJ, Maddipati KR, Granneman JG. Adipogenic role of alternatively activated macrophages in beta-adrenergic remodeling of white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2016;310(1):4. doi: 10.1152/ajpregu.00355.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210(3):535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160(1–2):74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 46.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 47.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519(7542):242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flach M, Diefenbach A. Adipose tissue: ILC2 crank up the heat. Cell Metab. 2015;21(2):152–153. doi: 10.1016/j.cmet.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Satoh T, Kidoya H, Naito H, Yamamoto M, Takemura N, Nakagawa K, Yoshioka Y, Morii E, Takakura N, Takeuchi O, et al. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495(7442):524–528. doi: 10.1038/nature11930. [DOI] [PubMed] [Google Scholar]
- 50.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299(6):E1016–E1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bourlier V, Sengenes C, Zakaroff-Girard A, Decaunes P, Wdziekonski B, Galitzky J, Villageois P, Esteve D, Chiotasso P, Dani C, et al. TGFbeta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages. PLoS One. 2012;7(2):e31274. doi: 10.1371/journal.pone.0031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206(13):3143–3156. doi: 10.1084/jem.20091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59(5):2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 54.Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44(3):439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 55.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, Lippens S, Abels C, Schoonooghe S, Raes G, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem. 2012;287(48):40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109(46):E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59(1):130–142. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 60.Wan J, Benkdane M, Alons E, Lotersztajn S, Pavoine C. M2 kupffer cells promote hepatocyte senescence: an IL-6-dependent protective mechanism against alcoholic liver disease. Am J Pathol. 2014;184(6):1763–1772. doi: 10.1016/j.ajpath.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117(6):1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ikeda S, Tamura Y, Kakehi S, Takeno K, Kawaguchi M, Watanabe T, Sato F, Ogihara T, Kanazawa A, Fujitani Y, et al. Exercise-induced enhancement of insulin sensitivity is associated with accumulation of M2-polarized macrophages in mouse skeletal muscle. Biochem Biophys Res Commun. 2013;441(1):36–41. doi: 10.1016/j.bbrc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Saclier M, Cuvellier S, Magnan M, Mounier R, Chazaud B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 2013;280(17):4118–4130. doi: 10.1111/febs.12166. [DOI] [PubMed] [Google Scholar]
- 64.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93(6):875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 66.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mounier R, Theret M, Arnold L, Cuvellier S, Bultot L, Goransson O, Sanz N, Ferry A, Sakamoto K, Foretz M, et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18(2):251–264. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 68.Capote J, Kramerova I, Martinez L, Vetrone S, Barton ER, Sweeney HL, Miceli MC, Spencer MJ. Osteopontin ablation ameliorates muscular dystrophy by shifting macrophages to a pro-regenerative phenotype. J Cell Biol. 2016;213(2):275–288. doi: 10.1083/jcb.201510086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H, Melton DW, Porter L, Sarwar ZU, McManus LM, Shireman PK. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol. 2014;184(4):1167–1184. doi: 10.1016/j.ajpath.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferenbach DA, Nkejabega NC, McKay J, Choudhary AK, Vernon MA, Beesley MF, Clay S, Conway BC, Marson LP, Kluth DC, et al. The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int. 2011;79(9):966–976. doi: 10.1038/ki.2010.535. [DOI] [PubMed] [Google Scholar]
- 71.Villalta SA, Rinaldi C, Deng B, Liu G, Fedor B, Tidball JG. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet. 2011;20(4):790–805. doi: 10.1093/hmg/ddq523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao Q, Harris DC, Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 2015;30(3):183–194. doi: 10.1152/physiol.00046.2014. [DOI] [PubMed] [Google Scholar]
- 73.Tian S, Chen SY. Macrophage polarization in kidney diseases. Macrophage (Houst). 2015;2(1):e679. doi: 10.14800/macrophage.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen B, Liu X, Fan Y, Qiu J. Macrophages regulate renal fibrosis through modulating TGFbeta superfamily signaling. Inflammation. 2014;37(6):2076–2084. doi: 10.1007/s10753-014-9941-y. [DOI] [PubMed] [Google Scholar]
- 75.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA. 2010;107(9):4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt IM, Hall IE, Kale S, Lee S, He CH, Lee Y, Chupp GL, Moeckel GW, Lee CG, Elias JA, et al. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol. 2013;24(2):309–319. doi: 10.1681/ASN.2012060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80(9):915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 78.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511(7508):167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 79.Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32(6):463–488. doi: 10.1615/CritRevImmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 80.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. l-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171(3):1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 82.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64(16):5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 83.Popovic PJ, Zeh HJ, 3rd, Ochoa JB. Arginine and immunity. J Nutr. 2007;137(6 Suppl 2):1681S–1686S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 84.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bronte V. Tumor cells hijack macrophages via lactic acid. Immunol Cell Biol. 2014;92(8):647–649. doi: 10.1038/icb.2014.67. [DOI] [PubMed] [Google Scholar]