Abstract
Objective
Efficacy and safety were compared between insulin degludec (IDeg) once daily (OD) in combination with mealtime insulin aspart (IAsp) and insulin detemir (IDet) OD or twice daily (BID) in combination with mealtime IAsp in Japanese subjects with type 1 diabetes mellitus (T1DM).
Materials and methods
This was a post hoc analysis of a multinational, controlled, open-label, parallel-group, treat-to-target trial that randomised adults [aged ≥18 years (≥20 years for Japan)] with T1DM for ≥12 months to basal IDeg OD (n = 124) or IDet (n = 62), both with mealtime bolus IAsp. The IDet dosing was adjusted to BID if required at ≥8 weeks.
Results
The estimated mean change in HbA1c from baseline to week 26 (the primary outcome measure) was −1.03 % in the IDeg + IAsp group and −0.94 % in the IDet + IAsp group (mean estimated treatment difference [ETD] −0.09; 95 % confidence interval [CI] −0.29, 0.10). Significantly greater reductions in fasting plasma glucose were observed in the IDeg + IAsp group (mean ETD −39.36 mg/dL; 95 % CI −56.04, −22.68). Both groups had similar rates of confirmed hypoglycaemia (59.9 and 59.2 per patient-year of exposure [PYE] with IDeg + IAsp and IDet + IAsp, respectively). Rates of nocturnal confirmed hypoglycaemia were significantly lower with IDeg + IAsp than with IDet + IAsp (5.2 vs 9.5 episodes per PYE; estimated ratio 0.48; 95 % CI 0.31, 0.75). Adverse event profiles were similar.
Conclusion
The findings were consistent with those of the global trial population. IDeg + IAsp may represent an improvement on current standard treatments for Japanese patients with T1DM.
Keywords: Type 1 diabetes mellitus, Insulin, Bolus treatment, Japanese
Introduction
The risk of developing type 1 diabetes mellitus (T1DM) varies significantly according to country of residence and race [1]. Although the incidence of T1DM is increasing in the Japanese population, it continues to have one of the lowest rates in the world [1, 2]. The reasons for this are poorly understood, but may be partly influenced by specific immunological, genetic or environmental interactions [1, 2].
Landmark trials have demonstrated the importance of maintaining tight glycaemic control to reduce the risk of long-term complications associated with T1DM [3–5]. As a result, the American Diabetes Association (ADA) now recommends target glycosylated haemoglobin (HbA1c) levels of <7.0 % in adults with T1DM [6]. The Japan Diabetes Society recommends the same target when aiming to prevent complications [7].
As in other countries, basal–bolus insulin regimens remain the cornerstone of T1DM treatment in Japan [7]. In these regimens, a short-acting insulin is injected at mealtimes to maintain prandial glucose control, and a long-acting insulin is injected once daily (OD) or twice daily (BID) to meet basal insulin requirements.
Despite advances in pharmacotherapy, glycaemic control is suboptimal for many people with T1DM in Japan and elsewhere [8, 9]. Hypoglycaemia remains an important barrier to achieving glycaemic control [10], and hence efforts are ongoing to develop treatments that reduce this risk.
Insulin degludec (IDeg) is a basal insulin that, upon injection, forms stable multi-hexamers in the subcutaneous tissue [11]. Monomers slowly separate from this depot and are absorbed into the circulation, resulting in a considerably longer half-life than insulin glargine (IGlar) [12] and a duration of action of >42 h [11], as well as reduced day-to-day variability in glucose-lowering effect [13]. In addition, in the event of a missed dose, the pharmacokinetic characteristics of IDeg mean that it can be taken as soon as practically possible (provided a minimum interval of 8 h is maintained between doses) [14].
A trial in patients with T1DM showed that IDeg OD, in conjunction with mealtime insulin aspart (IAsp), effectively reduces HbA1c and fasting plasma glucose (FPG), with a lower risk of nocturnal hypoglycaemia than with IGlar and mealtime IAsp [15].
More recently, the efficacy and safety of IDeg in T1DM, given OD as part of a basal–bolus regimen with mealtime IAsp, were demonstrated in the global phase III BEGIN Basal–bolus Type 1 study (Brazil, Finland, India, Italy, Japan, Republic of Macedonia, UK) [16]. In this trial, patients receiving IDeg achieved improved long-term glycaemic control, with a lower risk of nocturnal confirmed hypoglycaemia compared with IDet [16]. The current analysis was performed to assess whether the efficacy and safety of IDeg OD plus mealtime IAsp was maintained in a subpopulation of Japanese patients with T1DM from this trial.
Materials and methods
Study design and procedures
The design, methodology and study procedures of the trial have been reported in full elsewhere [16]. In brief, the study was a 26-week, randomised, controlled, open-label, parallel-group, non-inferiority trial, with a treat-to-target design, in line with current Food and Drug Administration recommendations for the evaluation of novel insulin preparations [17]. Eligible participants were randomised 2:1 to either IDeg OD (Tresiba®, 100 U/mL) or IDet (Levemir®, 100 U/mL) as basal insulin, each in combination with mealtime IAsp (NovoRapid®, 100 U/mL) (all from Novo Nordisk, Bagsværd, Denmark). Participants transferred to their randomised treatment from their pre-trial insulin (basal insulin) on a unit-to-unit basis, as described in the full trial report [16].
Insulin products were injected subcutaneously using a 3 mL FlexPen® (Novo Nordisk). IDeg and IDet were dose-titrated individually to plasma glucose values of 70–89 mg/dL (3.9–4.9 mmol/L) converted from self-monitoring of blood glucose (SMBG) based on pre-breakfast or pre-main evening meal SMBG levels (mean values from three consecutive days). IDeg and IDet were injected subcutaneously OD in the evening (from start of main evening meal to bedtime). IAsp was injected subcutaneously as mealtime insulin. In the IDet group, a second dose of IDet could be added if there was inadequate glycaemic control [<0.5 % improvement in HbA1c if baseline HbA1c was ≥8.0 % (63.9 mmol/mol), or any deterioration in HbA1c if baseline HbA1c was <8.0 %, in conjunction with a mean pre-dinner plasma glucose level >6.0 mmol/L and no diagnosis of a treatable concurrent disease causing hyperglycaemia] after ≥8 weeks of IDet OD treatment.
The trial was registered with clinicaltrials.gov (NCT01074268).
Study population—Japanese subgroup
Results from the Japanese subgroup are described in this report. The trial included male or female Japanese patients aged ≥20 years (as compared with ≥18 years in other populations) with a clinical diagnosis of T1DM for ≥12 months. Subjects currently treated with any basal–bolus insulin regimen for ≥12 months, HbA1c ≤ 10.0 % (85.8 mmol/mol), and body mass index ≤35.0 kg/m2 at the time of screening were eligible for participation. Inclusion and exclusion criteria were reported previously for the full trial population [16].
Assessments
The primary assessment was the change in HbA1c after 26 weeks of treatment. Secondary efficacy assessments included laboratory-measured FPG, plasma glucose values obtained from 9-point SMBG profiles and automatically calibrated to plasma equivalent glucose values based on glucose meter measurements, and doses of basal and bolus insulin. The proportion of responders to HbA1c target levels of <7.0 % was also calculated, as was the proportion who reached these targets without severe hypoglycaemia (in the last 12 weeks of treatment).
Safety variables included the number of confirmed hypoglycaemic episodes, adverse events (AEs), body weight, standard clinical and laboratory assessments, electrocardiogram (data not shown), fundoscopy/fundus photography (data not shown) and injection-site reactions.
Severe hypoglycaemia (according to the ADA definition) was defined as an episode requiring the assistance of another person to actively administer carbohydrates, glucagon or other resuscitative actions. Confirmed hypoglycaemia was defined as plasma glucose <3.1 mmol/L (56 mg/dL), regardless of symptoms, or severe episodes (requiring assistance from another person). “Nocturnal” episodes were confirmed episodes with onsets occurring between 00.01 and 05.59. The daytime period was classified as the period between 06:00 and 00:00 (both included).
Statistical analyses
Analyses of the Japanese subpopulation were performed post hoc. Generally, the statistical approaches used were the same as in the pre-planned analyses of the full trial population [16].
Changes from baseline in HbA1c, FPG and weight after 26 weeks of treatment were analysed separately using an analysis of variance, with treatment, antidiabetic therapy at screening and sex as fixed factors, and age and the corresponding baseline value as covariates. HbA1c responder endpoints (HbA1c < 7.0 %), including whether targets were achieved without confirmed or severe hypoglycaemia, were analysed separately based on a logistic regression model. The model included treatment, antidiabetic therapy at screening and sex as fixed factors, and age and baseline HbA1c as covariates.
After 26 weeks of treatment, 9-point SMBG profile values were analysed jointly using a linear mixed model with an unstructured residual covariance structure and with treatment, time point and an interaction between treatment and time point, antidiabetic treatment at screening and sex as fixed factors and age and baseline response per time point as covariates. The numbers of overall confirmed, nocturnal confirmed and severe hypoglycaemic episodes were analysed using a negative binomial regression model which included treatment, antidiabetic therapy at screening and sex as fixed factors and age as the covariate.
Statistical analyses were performed on the full analysis set for efficacy endpoints, body weight and hypoglycaemia. Safety endpoints were summarised using the safety analysis set. Missing values were imputed using last observation carried forward.
Results
Patient characteristics
Among the 197 Japanese subjects initially screened, 11 failed the screening process (Fig. 1). A total of 186 subjects were therefore randomised 2:1 to receive either IDeg + IAsp (n = 124) or IDet + IAsp (n = 62), and 177 completed 26 weeks of study treatment (IDeg + IAsp, n = 121; IDet + IAsp, n = 56). The baseline characteristics of randomised Japanese subjects are shown in Table 1. There was a higher proportion of female patients, a higher mean age and a lower mean HbA1c in the IDeg arm. Otherwise, the baseline characteristics were generally comparable between the two treatment groups.
Fig. 1.
Trial flow diagram (Japanese patients). IAsp insulin aspart, IDeg insulin degludec, IDet insulin detemir
Table 1.
Baseline characteristics of randomised Japanese subjects (FAS)
| Characteristicsa | IDeg + IAsp (n = 124) | IDet + IAsp (n = 62) |
|---|---|---|
| Sex, male/female, % | 37.9/62.1 | 51.6/48.4 |
| Age, years | 48.6 (14.0) | 44.5 (14.4) |
| Weight, kg | 59.1 (10.2) | 60.8 (10.5) |
| BMI, kg/m2 | 22.7 (2.9) | 22.9 (3.3) |
| Duration of diabetes, years | 12.5 (9.6) | 12.9 (8.6) |
| HbA1c, % | 7.9 (0.9) | 8.2 (0.9) |
| FPG, mg/dL | 175.5 (65.9) | 171.3 (56.8) |
| mmol/L | 9.7 (3.7) | 9.5 (3.1) |
| Antidiabetic regimen at screening | ||
| Basal BID + bolus, n (%) | 37 (29.8) | 13 (21.0) |
| Basal OD + bolus, n (%) | 87 (70.2) | 49 (79.0) |
| Basal insulin type at screening | ||
| Insulin glargine, n (%) | 63 (50.8) | 37 (59.7) |
| Insulin detemir, n (%) | 51 (41.1) | 23 (37.1) |
| Insulin NPH, n (%) | 10 (8.1) | 2 (3.2) |
| Bolus insulin type at screening | ||
| Insulin aspart, n (%) | 83 (66.9) | 37 (59.7) |
| Insulin lispro, n (%) | 26 (21.0) | 15 (24.2) |
| Human insulin, n (%) | 9 (7.3) | 7 (11.3) |
| Human insulin + insulin aspart, n (%) | 4 (3.2) | 3 (4.8) |
| Human insulin + insulin lispro, n (%) | 2 (1.6) | – |
| Creatinine serum, µmol/L | 81 (16) | 83 (13) |
aMean (SD) values unless otherwise specified
BID twice daily, BMI body mass index, FAS full analysis set, FPG fasting plasma glucose, HbA 1c glycosylated haemoglobin, IAsp insulin aspart, IDeg insulin degludec, IDet insulin detemir, NPH neutral protamine hagedorn, OD once daily, SD standard deviation
Glycaemic control
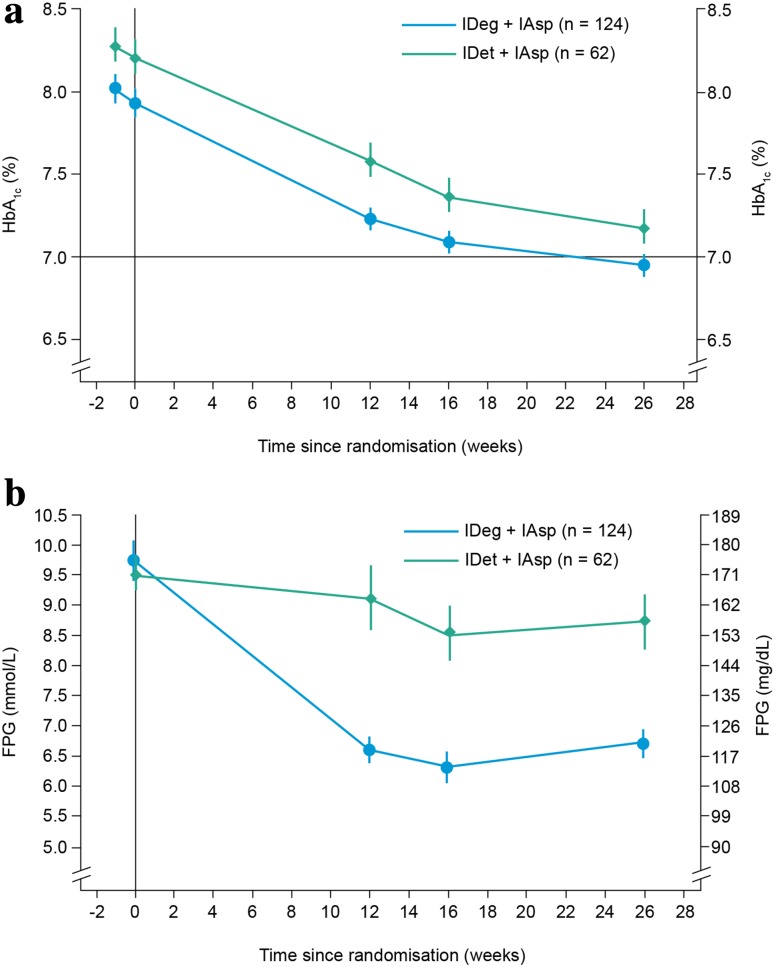
After 26 weeks, observed mean (standard deviation [SD]) HbA1c levels decreased from 7.9 (0.9) at baseline to 6.9 % (0.7) in the IDeg + IAsp group, and from 8.2 % (0.9) to 7.2 % (0.8) in the IDet + IAsp group (Fig. 2a).
Fig. 2.
Changes in a mean HbA1c levels and b mean FPG levels over 26 weeks of treatment with IDeg + IAsp vs IDet + IAsp (FAS). FAS contains data imputed using LOCF, error bars show the ±standard error of the mean. FAS full analysis set, FPG fasting plasma glucose, HbA 1c glycosylated haemoglobin, IAsp insulin aspart, IDeg insulin degludec, IDet insulin detemir, LOCF last observation carried forward
The estimated mean change (standard error [SE]) from baseline to week 26 in HbA1c levels was −1.03 % (0.06) in the IDeg + IAsp group and −0.94 % (0.08) in the IDet + IAsp group. The mean estimated treatment difference (ETD; IDeg + IAsp minus IDet + IAsp) was −0.09 % [95 % confidence interval (CI) −0.29; 0.10].
The proportion of patients who achieved the HbA1c target of <7.0 % by week 26 was similar in both treatment groups: 53.2 and 46.8 % for IDeg + IAsp and IDet + IAsp, respectively (estimated odds ratio [OR; IDeg + IAsp/IDet + IAsp] 1.00; 95 % CI 0.48, 2.11). The proportion of patients who achieved the HbA1c target of <7.0 % without severe hypoglycaemia was also similar in both groups: 52.8 % with IDeg + IAsp and 46.7 % with IDet + IAsp (estimated OR 1.03; 95 % CI 0.49, 2.17).
Fasting plasma glucose
In the IDeg + IAsp group, the observed mean (SD) FPG level decreased from 175.5 (65.9) mg/dL (9.7 [3.7] mmol/L) at baseline to 120.9 (46.0) mg/dL (6.7 [2.6] mmol/L) at week 26 (Fig. 2b). In the IDet + IAsp group, it declined from 171.3 (56.8) mg/dL (9.5 [3.1] mmol/L) to 157.3 (68.9) mg/dL (8.7 [3.8] mmol/L).
The ETD between the IDeg + IAsp and IDet + IAsp groups was –39.36 mg/dL (95 % CI −56.04, –22.68) (−2.18 mmol/L; 95 % CI −3.11, −1.26).
Nine-point self-monitored plasma glucose
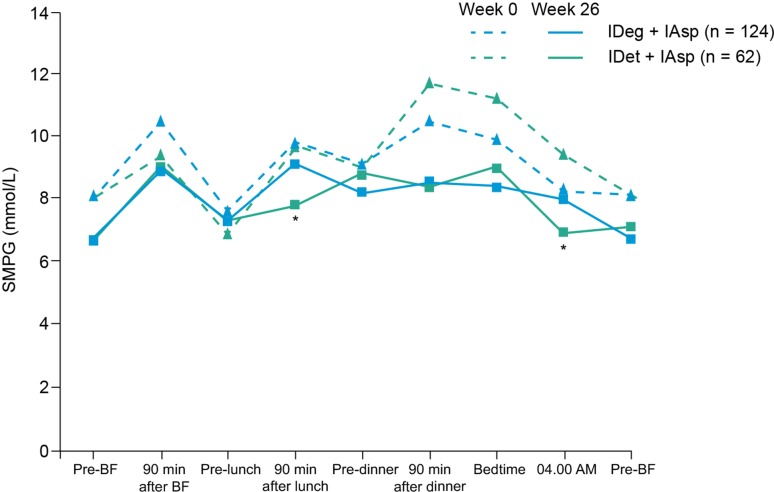
After 26 weeks, the 9-point SMBG profiles of the two groups were similar, except at 90 min after the start of lunch and at 4.00 am, when glucose concentrations were significantly lower with IDet + IAsp than IDeg + IAsp (ETD [IDeg + IAsp minus IDet + IAsp] 23.93 mg/dL, 95 % CI 2.60, 45.26; 1.33 mmol/L, 95 % CI 0.14, 2.51, P = 0.0281; and 23.87 mg/dL, 95 % CI 2.96, 44.78; 1.32 mmol/L, 95 % CI 0.16, 2.48, P = 0.0255, respectively) (Fig. 3).
Fig. 3.
Mean 9-point self-measured blood glucose (SMBG) profiles at baseline (dashed lines) and week 26 (full lines). *p <0.05. BF breakfast, IDeg insulin degludec, IDet insulin detemir, IAsp insulin aspart
Insulin dose
The mean total (basal and bolus) daily insulin dose at baseline was similar in the IDeg + IAsp (0.69 U/kg) and IDet + IAsp (0.72 U/kg) groups. At week 26, mean total daily insulin doses were 0.72 U/kg and 0.89 U/kg with IDeg + IAsp and IDet + IAsp, respectively (insulin dose ratio [IDeg + IAsp/IDet + IAsp] 0.81).
The mean daily basal insulin dose at baseline was also similar in the two groups (0.25 U/kg in the IDeg + IAsp group; 0.26 U/kg in the IDet + IAsp group). At week 26, the mean daily basal insulin doses were 0.26 U/kg and 0.33 U/kg with IDeg and IDet, respectively (dose ratio 0.77), and a total of 39.3 % of subjects in the IDet group were receiving IDet BID.
Hypoglycaemic events
The rate of severe hypoglycaemic events was low in both groups (0.15 and 0 episodes per patient-year of exposure (PYE) with IDeg + IAsp and IDet + IAsp, respectively) (Table 2). The percentage of subjects with severe hypoglycaemia was 6.5 % for IDeg + IAsp and 0 % for IDet + IAsp. The rate of nocturnal severe hypoglycaemia was also low in both groups (0.02 and 0 episodes PYE with IDeg + IAsp and IDet + IAsp, respectively).
Table 2.
Episodes of hypoglycaemia occurring on or after the first day of exposure to treatment and no later than 7 days after the last day of treatment (SAS)
| IDeg + IAsp (n = 124) | IDet + IAsp (n = 61) | ETR (95 % CI)a | |||||
|---|---|---|---|---|---|---|---|
| n (%) | E | Rate/PYE | n (%) | E | Rate/PYE | ||
| Overall confirmed hypoglycaemia | 122 (98.4) | 3666 | 59.9 | 60 (98.4) | 1759 | 59.2 | 0.94 (0.70, 1.25) |
| Nocturnal confirmed hypoglycaemia | 74 (59.7) | 318 | 5.2 | 44 (72.1) | 281 | 9.5 | 0.48 (0.31, 0.75) |
| Severe hypoglycaemia | 8 (6.5) | 9 | 0.15 | 0 | 0 | 0 | b |
| Nocturnal severe hypoglycaemia | 1 (0.8) | 1 | 0.02 | 0 (0.0) | 0 | 0 | b |
| Daytime confirmed hypoglycaemia | 122 (98.4) | 3347 | 54.7 | 58 (95.1) | 1470 | 49.4 | 1.03 (0.77, 1.37) |
| Daytime severe hypoglycaemia | 6 (4.8) | 7 | 0.11 | 0 (0.0) | 0 | 0 | b |
Severe hypoglycaemia (according to the ADA definition): an episode requiring the assistance of another person to actively administer carbohydrates, glucagons or other resuscitative actions
Daytime period: the period between 06:00 and 00:00 (both included)
Nocturnal period: the period between 00:01 and 05:59 (both included)
ADA American Diabetes Association, CI confidence interval, E number of events, ETR estimated treatment ratio of IDeg + IAsp: IDet + IAsp, IAsp insulin aspart, IDeg insulin degludec, IDet insulin detemir, n number of subjects, PYE patient-years of exposure, SAS safety analysis set, % percentage of all randomised participants in treatment group
aFull analysis set
bStatistical analysis was not done due to there being only a few events in the IDeg + IAsp group and no events in the IDet + IAsp group
Confirmed hypoglycaemia was defined as plasma glucose <3.1 mmol/L (56 mg/dL), regardless of symptoms, or severe episodes (requiring assistance from another person)
Confirmed hypoglycaemia was reported in 98.4 % of patients in each of the two treatment groups. The rate of confirmed hypoglycaemia was similar in both groups (59.9 and 59.2 per PYE in the IDeg + IAsp and IDet + IAsp groups, respectively) (Table 2; Fig. 4a). The estimated ratio (IDeg + IAsp)/(IDet + IAsp) was 0.94 (95 % CI 0.70, 1.25).
Fig. 4.
Cumulative incidence rates of a overall confirmed hypoglycaemia and b nocturnal confirmed hypoglycaemia over 26 weeks of treatment with IDeg + IAsp vs IDet + IAsp (SAS). IAsp insulin aspart, IDeg insulin degludec, IDet insulin detemir, SAS safety analysis set
The rate of nocturnal confirmed hypoglycaemia was significantly lower with IDeg + IAsp than with IDet + IAsp (5.2 vs 9.5 episodes per PYE, respectively; estimated ratio 0.48; 95 % CI 0.31, 0.75) (Table 2; Fig. 4b).
There were no significant differences in daytime confirmed hypoglycaemia (54.7 and 49.4 episodes per PYE with IDeg + IAsp and IDet + IAsp, respectively; estimated ratio 1.03, 95 % CI 0.77, 1.37) and daytime severe hypoglycaemia (0.11 and 0 episodes per PYE with IDeg + IAsp and IDet + IAsp, respectively) between the two groups.
Body weight
Mean body weight increased from baseline to week 26 by 1.2 kg in the IDeg + IAsp group and by 0.2 kg in the IDet + IAsp group. The mean ETD (IDeg + IAsp minus IDet + IAsp) was 0.98 kg (95 % CI 0.26, 1.69).
Adverse events
The incidence and rate of AEs were similar in both the IDeg + IAsp and IDet + IAsp treatment groups (83 vs 87 %; 5.38 vs 3.90 per PYE, respectively). The majority of the AEs were mild to moderate in severity. In both treatment groups, the most frequent AE was nasopharyngitis (Table 3).
Table 3.
Common AEs reported in ≥5 % of Japanese subjects (SAS)
| Event rate per PYE | ||
|---|---|---|
| IDeg + IAsp (n = 124) | IDet + IAsp (n = 61) | |
| Bronchitis | 0.07 | 0.17 |
| Nasopharyngitis | 0.83 | 1.01 |
| Upper respiratory tract infection | 0.18 | 0.07 |
| Abdominal pain upper | 0.10 | 0.17 |
| Diarrhoea | 0.11 | 0 |
| Headache | 0.21 | 0.07 |
| Diabetic retinopathy | 0.10 | 0.13 |
| Back pain | 0.13 | 0.03 |
| Weight increased | 0.11 | 0 |
AE adverse event, IAsp insulin aspart, IDeg insulin degludec, IDet insulin detemir, PYE patient-years of exposure, SAS safety analysis set
The rate of AEs judged by investigators as probably related to the investigational basal insulin was low in both groups (0.16 and 0.03 per PYE with IDeg + IAsp and IDet + IAsp, respectively).
Rates of serious AEs were 0.26 and 0.07 per PYE in the IDeg + IAsp and IDet + IAsp groups, respectively. Rates of serious AEs judged as probably related to the investigational basal insulin were low in both groups (0.03 and 0.00 per PYE, respectively). Two serious AEs—hypoglycaemia and hypoglycaemic coma (both in the IDeg + IAsp group)—were considered to be probably related to the study insulin treatment.
The proportion of participants reporting injection-site reactions was similar in both the IDeg + IAsp (3.2 %) and the IDet + IAsp (1.6 %) groups. None of these reactions was classified as serious.
There were no other relevant clinical or laboratory safety findings.
Discussion
In this post hoc analysis of a multinational, randomised, controlled, open-label, parallel-group, treat-to-target trial, the safety and efficacy of IDeg were investigated in Japanese patients with T1DM, and the findings were consistent with those observed in the global trial population [16]. IDeg + IAsp improved glycaemic control over the 26-week treatment period, leading to reductions in HbA1c that were comparable to those observed with IDet + IAsp, as would be expected in a trial with a treat-to-target design. Estimated mean changes from baseline in HbA1c over the treatment period were similar for the two groups, suggesting similar levels of glycaemic control. The levels of overall HbA1c control obtained in the two arms were comparable to those achieved in previous treat-to-target trials with IDet using similar regimens [15, 18].
As was the case in the full trial population [16], IDeg + IAsp improved FPG control to a greater extent than IDet + IAsp. Consistent with results from a previous trial [19], a lower mean FPG with IDeg versus IGlar in subjects with T1DM was observed in the current Japanese subanalysis. However, there was no statistically significant difference between the 9-point SMBG profiles of the two groups, except at 90 min after the start of lunch and at 4.00 am, when glucose concentrations were lower with IDet + IAsp than IDeg + IAsp. This finding is similar to the results observed in the global trial population, where plasma glucose (PG) profiles after 26 weeks were similar between treatment groups, except at 4.00 am, where PG concentration was significantly lower with IDet than IDeg [16]. It could be speculated that the lower PG concentration with IDet may be a counterbalance against higher FPG concentrations in the IDet group in the morning, and may be reflective of the higher rate of nocturnal hypoglycaemia seen in this group.
Rates of overall confirmed hypoglycaemia among Japanese subjects in the present analysis were similar in both treatment groups. However, treatment with IDeg + IAsp was associated with a lower rate of nocturnal hypoglycaemia than treatment with IDet + IAsp. Both of these findings are in line with those from the full study population [16]. Although overall confirmed hypoglycaemia is driven by both the bolus and basal insulin, nocturnal hypoglycaemia is primarily driven by the basal component. Hence, rates of nocturnal hypoglycaemia may provide the most relevant comparison for the two basal insulin analogues [20].
The results from this study are also supported by data from a recent phase II study conducted in Japan, in which IDeg was associated with a 69 % lower rate of nocturnal confirmed hypoglycaemia than IDet in subjects with T1DM [21].
The findings from the present study are particularly notable given that the significant decreases in nocturnal hypoglycaemia with IDeg versus IDet were achieved in parallel with greater control of FPG, a finding that has been observed in previous trials in T1DM of IDeg OD versus IGlar [20, 22].
The overall safety profile of IDeg in Japanese patients was consistent with that reported in previous phase III trials in subjects with T1DM [15, 19, 22–24].
There were no notable disparities between the Japanese and full trial populations with regard to treatment differences between IDeg and IDet in glycaemic control, other key efficacy endpoints, insulin dose or AEs.
Extrapolation of the efficacy and safety results of the entire study population to the Japanese subgroup is therefore meaningful. As part of basal–bolus therapy, IDeg OD improves long-term glycaemic control in patients with T1DM, and is associated with a significantly lower rate of nocturnal confirmed hypoglycaemia and a significantly larger reduction in mean FPG compared with basal–bolus therapy with IDet (for which 39.3 % of subjects were receiving IDet BID rather than OD).
The data presented here demonstrate that IDeg + IAsp improved glycaemic control in comparison with IDet + IAsp, with no substantial differences in safety parameters. We conclude that IDeg may represent an improvement in current treatment standards for Japanese patients with T1DM.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients before they were included in the study.
Acknowledgments
Medical writing assistance was provided by apothecom scopemedical, UK, and funded by Novo Nordisk.
Compliance with ethical standards
Conflict of interest
Y. Ono has received grants from the Advanced Clinical Research Organization of Japan. T. Nishida is an employee of Novo Nordisk. J. Hyllested-Winge is an employee and shareholder in Novo Nordisk. H. Seino has received honoraria from Astellas, Eli Lilly, Novartis, Sanofi, Shionogi, Boehringer Ingelheim and Mitsubishi Tanabe. He has also received research grants from GSK, Sanofi, Novo Nordisk and Mitsubishi Tanabe Pharma. T. Sasaki has no conflicts to declare.
References
- 1.Kawasaki E, Matsuura N, Eguchi K. Type 1 diabetes in Japan. Diabetologia. 2006;49:828–836. doi: 10.1007/s00125-006-0213-8. [DOI] [PubMed] [Google Scholar]
- 2.Park Y. Why is type 1 diabetes uncommon in Asia? Ann NY Acad Sci. 2006;1079:31–40. doi: 10.1196/annals.1375.005. [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM. DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 years: overview. Diabetes Care. 2014;37:9–16. [DOI] [PMC free article] [PubMed]
- 4.Lachin JM, Orchard TJ, Nathan DM. DCCT/EDIC Research Group. Update on cardiovascular outcomes at 30 years of The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care. 2014;37:39–43. [DOI] [PMC free article] [PubMed]
- 5.Gubitosi-Klug RA. DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 years: summary and future directions. Diabetes Care. 2014;37:44–9. [DOI] [PMC free article] [PubMed]
- 6.Chiang JL, Kirkman MS, Laffel LMB, Peters AL. Type 1 diabetes sourcebook authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37:2034–2054. doi: 10.2337/dc14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Japan Diabetes Society. Treatment guide for diabetes (online). 2013. http://www.jds.or.jp/common/fckeditor/editor/filemanager/connectors/php/transfer.php?file=/uid000025_54726561746D656E745F47756964655F666F725F44696162657465735F323031322D323031332E706466. Accessed May 2015.
- 8.Kobayashi M, Yamazaki K, Hirao K, Oishi M, Kanatsuka A, Yamauchi M, et al. The status of diabetes control and antidiabetic drug therapy in Japan—a cross-sectional survey of 17,000 patients with diabetes mellitus (JDDM 1). Diabetes Res Clin Pract. 2006;73:198–204. [DOI] [PubMed]
- 9.Kanatsuka A, Kawai K, Hirao K, Oishi M, Takagi H, Kobayashi M, et al. Actual usage and clinical effectiveness of insulin preparations in patients with type 1 diabetes mellitus in Japan: CoDiC-based analysis of clinical data obtained at multiple institutions (JDDM 3). Diabetes Res Clin Pract. 2006;72:277–83. [DOI] [PubMed]
- 10.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger P-M. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med J Br Diabet Assoc. 2012;29:682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtzhals P, Heise T, Strauss H, Bottcher SG, Granhall C, Haahr H, et al. Multi-hexamer formation is the underlying basis for the ultra-long glucose-lowering effect of insulin degludec. Diabetologia. 2011;54:S426. [Google Scholar]
- 12.Heise T, Hovelmann U, Nosek L, Bottcher S, Granhall C, Haahr H. Insulin degludec has a two-fold longer half-life and a more consistent pharmacokinetic profile compared with insulin glargine. 2012. http://www.endocrine-abstracts.org/ea/0028/ea0028p188.htm. Accessed May 2015.
- 13.Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–864. doi: 10.1111/j.1463-1326.2012.01627.x. [DOI] [PubMed] [Google Scholar]
- 14.Novo Nordisk. Tresiba®: product information. 2012. http://www.pmda.go.jp/english/service/pdf/drugs/tresiba_sep2012_e.pdf. Accessed May 2015.
- 15.Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489–1497. doi: 10.1016/S0140-6736(12)60204-9. [DOI] [PubMed] [Google Scholar]
- 16.Davies MJ, Gross JL, Ono Y, Sasaki T, Bantwal G, Gall MA, et al. Efficacy and safety of insulin degludec given as part of basal-bolus treatment with mealtime insulin aspart in type 1 diabetes: a 26-week randomized, open-label, treat-to-target non-inferiority trial. Diabetes Obes Metab. 2014;16:922–930. doi: 10.1111/dom.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. Diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention (draft guidance). 2008. http://www.fda.gov/downloads/Drugs/Guidances/ucm071624.pdf. Accessed May 2015.
- 18.Bartley PC, Bogoev M, Larsen J, Philotheou A. Long-term efficacy and safety of insulin detemir compared to Neutral Protamine Hagedorn insulin in patients with type 1 diabetes using a treat-to-target basal-bolus regimen with insulin aspart at meals: a 2-year, randomized, controlled trial. Diabet Med J Br Diabet Assoc. 2008;25:442–9. [DOI] [PMC free article] [PubMed]
- 19.Mathieu C, Hollander P, Miranda-Palma B, Cooper J, Franek E, Russell-Jones D, et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013;98:1154–1162. doi: 10.1210/jc.2012-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratner RE, Gough SCL, Mathieu C, Del Prato S, Bode B, Mersebach H, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175–184. doi: 10.1111/dom.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwamoto Y, Clauson P, Nishida T, Kaku K. Insulin degludec in Japanese patients with type 1 diabetes mellitus: a randomized controlled trial. J Diabetes Investig. 2013;4:62–68. doi: 10.1111/j.2040-1124.2012.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birkeland KI, Home PD, Wendisch U, Ratner RE, Johansen T, Endahl LA, et al. Insulin degludec in type 1 diabetes: a randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care. 2011;3(34):661–5. [DOI] [PMC free article] [PubMed]
- 23.Hirsch IB, Bode B, Courreges J-P, Dykiel P, Franek E, Hermansen K, et al. Insulin degludec/insulin aspart administered once daily at any meal, with insulin aspart at other meals versus a standard basal-bolus regimen in patients with type 1 diabetes: a 26-week, phase 3, randomized, open-label, treat-to-target trial. Diabetes Care. 2012;35:2174–2181. doi: 10.2337/dc11-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bode BW, Buse JB, Fisher M, Garg SK, Marre M, Merker L, et al. Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN(®) Basal-Bolus Type 1): 2-year results of a randomized clinical trial. Diabet Med J Br Diabet Assoc. 2013;30:1293–7. [DOI] [PMC free article] [PubMed]