Abstract
Objective
To identify the attenuating factor of glycated hemoglobin (HbA1c)-lowering effects of liraglutide in type 2 diabetes (T2D) patients over the long term.
Methods
Forty-six T2D patients received liraglutide-glimepiride combination therapy. Clinical characteristics were compared between the following two subgroups: the relapse group (≥0.4 % increase in HbA1c in 48 weeks compared to 12 weeks) and non-relapse group (remaining patients). A glucagon-loading test was performed to evaluate baseline endogenous insulin secretion.
Results
In the relapse group, significantly reduced HbA1c, as observed at 12 weeks, tended to increase at 24 and 48 weeks. In the non-relapse group, reduced HbA1c was maintained for 48 weeks. Body weight was decreased at 12 weeks and then recovered at 48 weeks in both groups. Baseline BMI was significantly higher in the relapse group than in the non-relapse group. Age, HbA1c, duration of diabetes, fasting C-peptide, daily glimepiride dose and the duration of glimepiride treatment were comparable between both groups. Multiple logistic regression analysis revealed that baseline BMI was independently associated with the relapse group.
Conclusion
A higher BMI is the leading factor for attenuating long-term glycemic control by liraglutide in T2D patients undergoing sulfonylurea-based therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s13340-016-0269-8) contains supplementary material, which is available to authorized users.
Keywords: BMI, GLP-1 receptor agonist, HbA1c-lowering effect, Liraglutide, Obesity
Introduction
The prevalence of type 2 diabetes (T2D) has been rapidly increasing worldwide, particularly in Asian countries [1]. Obesity is a major risk factor for insulin resistance, which plays a key pathogenic role in T2D [2]. Weight loss constitutes a therapeutic basis for obese T2D patients, yet controlling the appetite and body weight of these patients is often difficult. In addition, treatment with insulin and sulfonylurea often increases adiposity, causing weight gain that further hampers glycemic control [3]. Glucagon-like peptide-1 (GLP-1) is an incretin hormone that exerts a potent blood-glucose-lowering action by inducing insulin secretion in a glucose-dependent manner [4]. Beyond the glucose-lowering effect, GLP-1 also promotes weight loss by delaying gastric emptying and inducing satiety.
Liraglutide is a once-daily human GLP-1 analog that improves glycemic control in T2D patients, lowers the risk of hypoglycemia, and reduces body weight [5, 6].
The position statement of the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) recommends GLP-1 receptor agonists as reliable agents for maintaining good glycemic control and weight loss in T2D patients [7]. Several reports have demonstrated that a short duration of diabetes, a high 6-min value of C-peptide immunoreactivity (CPR) after glucagon stimulation, and a low BMI at the start of treatment may be predictive of significant liraglutide effects by reducing blood glucose in T2D patients [8–11].
However, no studies have elucidated whether the liraglutide effect on glycemic control can be maintained over longer periods in T2D patients. Recently, Inoue et al. reported that glycemic control was adequately maintained in T2D patients receiving long-term liraglutide treatment over 2 years [12]. Glycated hemoglobin (HbA1c) levels were significantly decreased at 3 months after commencement of liraglutide (7.4 ± 1.1 %); however, there was a tendency for levels to increase at 6, 12, and 24 months (7.8 %). The clinical parameters underlying the relapse of HbA1c after 3 months were not elucidated in that study. Furthermore, in the Liraglutide Effect and Action in Diabetes (LEAD)-2 core trial, a reduction in HbA1c after 2 years of liraglutide treatment was less pronounced than that after 26 weeks (−0.6 vs. −1.0 %, respectively) [13].
The current study aimed to clarify the attenuating factors of the HbA1c-lowering effects of liraglutide over the long term in T2D patients.
Methods
Subjects
The current study was an observational study of T2D patients who were started on liraglutide therapy at Showa University Hospital (Tokyo, Japan) between December 2010 and January 2012. They continued the liraglutide treatment for a period of 1 year. The commencement of liraglutide treatment required discontinuation of all other oral anti-diabetic agents except sulfonylureas. Patients were introduced to liraglutide once daily with a starting dose of 0.3 mg, and the dosage was gradually increased up to 0.9 mg, which is the maximum dose used in Japan. Each treatment was administered by an attending physician. When the current study was started, sulfonylurea was the only agent approved as a combination therapy with liraglutide in Japan. All patients on sulfonylurea were treated with glimepiride at baseline. The glimepiride dose was reduced by half (from 2.0 ± 1.7 mg to 0.5 ± 0.6 mg) to decrease the risk of hypoglycemia when the liraglutide therapy commenced. Patients with type 1 diabetes, gestational diabetes, overt nephropathy (a urine albumin-to-creatinine ratio >300 mg/g, creatinine or glomerular filtration rate <60 ml/min/1.73 m2), liver disease, insulin treatment, infections, or malignant tumors were excluded. Patients who were on medications known to affect glycemic control (e.g., corticosteroid) were also excluded. Diabetic retinopathy was examined by an ophthalmologist. The study was approved by the Institutional Review Board for Clinical Research at the Showa University of Medicine. All patients gave their informed consent to participate.
Study design
T2D patients exhibited insufficient glycemic control with sulfonylurea-based therapy and were administered liraglutide as a second line of therapy after discontinuing antidiabetic agents except glimepiride. As a rule, the glimepiride dosage was not changed during the study.
HbA1c levels were measured before and at 4, 12, 24, 36, and 48 weeks after the initial administration of liraglutide. Body weight was measured at 0, 12, and 48 weeks. Body mass index (BMI) was calculated as body weight (kg)/height2 (m2). The glucagon test to assess the residual β-cell function was carried out in the morning after a 10-h fast, just before the administration of liraglutide. The fasting CPR (FCPR) and 6-min CPR were measured. In addition, clinical characteristics were compared between the following two subgroups: the relapse group (HbA1c increased by 0.4 % or more at 48 weeks compared to 12 weeks) and non-relapse group comprising the remaining patients, referring to the previous report by Kubota et al. [14].
Laboratory measurements
Plasma glucose levels were measured using the glucose-oxidase method, and plasma CPR concentrations were measured using the immunoenzymometric assay (ST E test Tosoh II C-peptide; Tosoh, Tokyo, Japan). The HbA1c (%) values used in this study were estimated using National Glycohemoglobin Standardization Program (NGSP) equivalent values (%) and calculated using the following formula in consideration of the relational expression of HbA1c [Japan Diabetes Society (JDS)] (%) (measured using previous Japanese standard substance and measurement methods) and HbA1c (NGSP): HbA1c (%) = HbA1c (JDS) (%) + 0.4 % [15].
Statistical analysis
All statistical analyses were performed using JMP® 12 (SAS Institute Inc., Cary, NC, USA), and descriptive data were expressed as means ± standard deviations or as numbers. Continuous variables compared between two groups were analyzed using t tests. Logistic regression analysis was used to examine the relationships between variables. Receiver-operating characteristic (ROC) curves were constructed for BMI. Sensitivity, specificity, cutoff points, and areas under the curve (AUC) were calculated. p values <0.05 were considered statistically significant.
Results
Baseline clinical characteristics of subjects
Table 1 shows the baseline characteristics of patients in the study. Forty-six T2D patients received the liraglutide-glimepiride combination therapy. Mean age was 56.9 ± 12.3 years, mean BMI was 27.6 ± 4.0 kg/m2, and baseline HbA1c was 9.0 ± 2.0 %. The fasting CPR, 6-min CPR, and CPR index for beta cell function were preserved well. The daily glimepiride dose was 2.0 ± 1.7 mg, and the duration of glimepiride treatment was 4.7 ± 3.8 years. Retinopathy, albuminuria, and neuropathy were present in 47.8, 52.2, and 60.9 % patients, respectively. Previous antidiabetic drugs in combination with glimepiride were as follows: biguanides (56.5 %), thiazolidinediones (6.5 %), and α-glucosidase inhibitors (15.2 %).
Table 1.
Baseline characteristics of all patients
| N (male/female) | 46 (35/11) |
|---|---|
| Age (years) | 56.9 ± 12.3 |
| Height (cm) | 165.3 ± 9.9 |
| Body weight (kg) | 75.6 ± 12.9 |
| BMI (kg/m2) | 27.6 ± 4.0 |
| Waist circumstance (cm) | 96.2 ± 14.1 |
| FPG (mg/dl) | 163.6 ± 46 |
| Baseline HbA1c (%) | 9.0 ± 2.0 |
| Fasting CPR (ng/ml) | 2.3 ± 1.1 |
| 6 min-CPR (ng/ml) | 4.6 ± 1.8 |
| ΔCPR (ng/ml) | 2.3 ± 1.2 |
| CPR index | 1.5 ± 0.8 |
| Glimepiride dose (mg) | 2.0 ± 1.7 |
| Duration of glimepiride (years) | 4.7 ± 3.8 |
| Retinopathy | 22 (47.8 %) |
| Nephropathy (UACR ≥30 mg/gCr) | 24 (52.2 %) |
| Neuropathy | 28 (60.9 %) |
| Previous anti-diabetic agents BG/TZD/α-GI | 26 (56.5 %)/3 (6.5 %)/7 (15.2 %) |
Data are expressed as numbers or mean ± standard deviation. N, number
BMI body mass index, FPG fasting plasma glucose, CPR C-peptide immunoreactive protein, ΔCPR fasting CPR value subtracted from 6-min CPR, CPR index fasting CPR value divided by FPG, UACR urine albumin-to-creatinine ratio, BG biguanides, TZD thiazolidinediones, α-GI alfa-glucosidase inhibitors
Changes in HbA1c levels
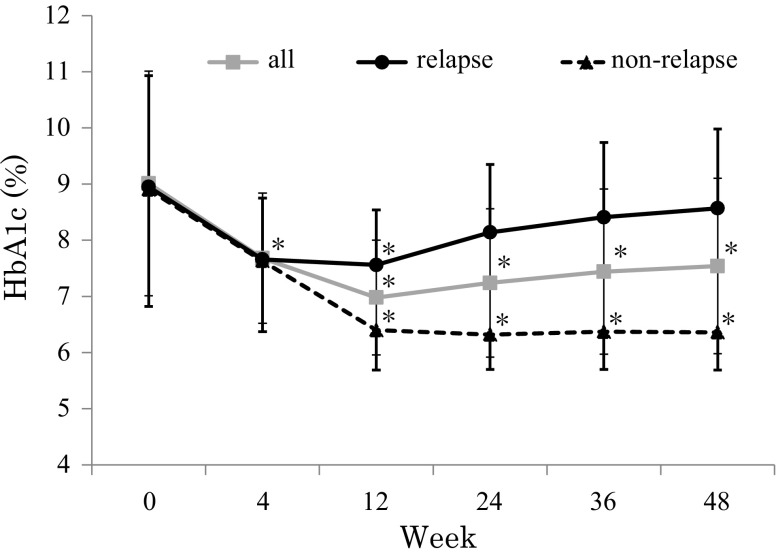
Figure 1 shows changes in HbA1c levels in all patients in the relapse and non-relapse group. In all patients, HbA1c was significantly decreased at 12 weeks (7.0 ± 1.1 %) with a tendency for levels to increase at 24, 36, and 48 weeks (7.2 ± 1.3, 7.4 ± 1.5, and 7.5 ± 1.6 %, respectively). In the relapse group, HbA1c was significantly decreased at 12 weeks (7.6 ± 1.0 %) and returned back to baseline levels at 36 and 48 weeks (8.4 ± 1.3 and 8.6 ± 1.4 %, respectively). In the non-relapse group, HbA1c was significantly decreased at 12 weeks (6.4 ± 0.7 %), and this was maintained up to 48 weeks. A significant decrease in HbA1c was observed at 12 weeks compared to baseline HbA1c in both groups, but the reduction in HbA1c at 12 weeks was significantly greater in the non-relapse group than in the relapse group (−2.6 ± 2.1 vs. −1.4 ± 1.9 %, p < 0.05, respectively).
Fig. 1.
Effect of liraglutide on glycated hemoglobin in all patients and the relapse and non-relapse group. Gray square with a gray line, all patients; black circle with a black line, relapse group (≥0.4 % increase in HbA1c in 48 weeks compared to 12 weeks); gray circle with a gray line, non-relapse group (<0.4 % increase in HbA1c in 48 weeks compared to 12 weeks). Each value represents mean ± standard deviation. *p < 0.05; in paired t test (vs. 0 weeks)
Changes in body weight
Figure 2 shows changes in body weight from baseline to 48 weeks. In all patients, body weight was significantly decreased at 12 weeks compared to baseline (−1.9 ± 3.2 kg) and subsequently returned to near baseline at 48 weeks. In both subgroups, body weight was also significantly decreased at 12 weeks compared to baseline (−2.0 ± 4.0 and −1.8 ± 2.1 kg, respectively) and subsequently increased back to baseline levels at 48 weeks. Therefore, we analyzed the correlation between ΔHbA1c and Δbody weight from 12 to 48 weeks to exclude any direct effects of increasing body weight on glycemic control in both subgroups. In our supplementary data (Fig. S1), individual changes in HbA1c and body weight from 12 to 48 weeks are shown as a scatter plot. Changes in body weight were not associated with changes in HbA1c from 12 to 48 weeks.
Fig. 2.
Effect of liraglutide on body weight in all patients and the relapse and non-relapse group. Gray square with a gray line, all patients; black circle with a black line, relapse group (≥0.4 % increase in HbA1c in 48 weeks compared to 12 weeks); gray square with a gray line, non-relapse group (<0.4 % increase in HbA1c in 48 weeks compared with 12 weeks). Each value represents mean ± standard deviation. *p < 0.05; in paired t test (vs. 0 weeks), **p < 0.05; in paired t test (vs. 12 weeks)
Difference between non-relapse and relapse group
Table 2 shows a comparison of the baseline characteristics and Δbody weight of patients from 12 to 48 weeks in the non-relapse and relapse groups. Baseline BMI in the relapse group was significantly higher than in the non-relapse group (29.4 ± 3.9 vs. 25.7 ± 3.0 kg/m2, respectively, p < 0.05). There were no significant differences in sex, age, duration of diabetes, waist circumstance, fasting plasma glucose level, HbA1c, dose of glimepiride, or residual insulin secretion in either group. Three patients were pretreated with glimepiride and thiazolidinediones, and all these patients were classified into the relapse group (p < 0.05 vs. non-relapse group). The daily dose of biguanides and α-glucosidase inhibitors was not different between the relapse and non-relapse group (1250 ± 365 vs. 1200 ± 268 mg and 0.83 ± 0.15 vs. 0.70 ± 0.17 mg for metformin and voglibose, respectively).
Table 2.
Baseline characteristics of patients according to their glycemic control
| Non-relapse group (<0.4 % increase in HbA1c in 48 weeks compared with 12 weeks) | Relapse group (≥0.4 % increase in HbA1c in 48 weeks compared with 12 weeks) | |
|---|---|---|
| N (male/female) | 23 (15/8) | 23 (19/4) |
| Age (years) | 59.6 ± 10.2 | 55.3 ± 13.7 |
| Height (cm) | 166.8 ± 9.7 | 163.3 ± 9.7 |
| Weight (kg) | 71.9 ± 12.9 | 78.5 ± 12.0 |
| BMI (kg/m2) | 25.7 ± 3.0 | 29.4 ± 3.9* |
| Waist circumstance (cm) | 94.1 ± 17.2 | 97.4 ± 9.7 |
| FPG (mg/dl) | 165.2 ± 43.9 | 160.8 ± 47.9 |
| Baseline A1c (%) | 8.9 ± 2.1 | 8.9 ± 2.0 |
| Fasting-CPR (ng/ml) | 2.0 ± 0.8 | 2.5 ± 1.3 |
| 6 min-CPR (ng/ml) | 4.3 ± 1.6 | 4.8 ± 2.0 |
| ΔCPR (ng/ml) | 2.3 ± 1.2 | 2.3 ± 1.3 |
| CPR index | 1.3 ± 0.6 | 1.6 ± 0.8 |
| Glimepiride dose (mg) | 1.5 ± 1.4 | 2.4 ± 2.0 |
| Duration of Glimepiride use (years) | 3.5 ± 3.2 | 5.7 ± 4.3 |
| Retinopathy | 12 (52.2 %) | 10 (43.5 %) |
| Nephropathy (UACR ≥30 mg/gCr) | 9 (39.1 %) | 13 (56.5 %) |
| Neuropathy | 15 (65.2 %) | 13 (56.5 %) |
| Previous anti-diabetic drugs BG/TZD/α-GI | 10 (43.5 %)/0 (0.0 %)/3 (13.0 %) | 16 (69.6 %)/3 (13.0 %)*/4 (17.4 %) |
Non-relapse group: <0.4 % increase in HbA1c in 48 weeks compared with 12 weeks, relapse group: HbA1c ≥0.4 % increase in HbA1c in 48 weeks compared with 12 weeks. Data are expressed as numbers or mean ± standard deviation. p values were determined using the unpaired t test or χ 2 test for comparisons between the two groups
N number, BMI body mass index, FPG fasting plasma glucose, CPR C-peptide immunoreactive protein, ΔCPR fasting CPR value subtracted from 6-min CPR, UACR urine albumin to creatinine ratio, BG biguanides, TZD thiazolidinediones; α-GI, alfa-glucosidase inhibitors
* Significant difference from non-relapse group
Analysis of the predictive parameters of non-relapse
A multivariate logistic regression analysis was performed using the non-relapse group as the dependent variable and baseline BMI, fasting CPR, HbA1c, dosage of glimepiride, duration of glimepiride, and Δbody weight from 12 to 48 weeks as independent variables (Table 3). The only factor that significantly contributed to non-relapse was BMI (p < 0.01, χ 2 = 7.66).
Table 3.
Independent predictors of non-relapse (<0.4 % increase in HbA1c in 48 weeks compared with 12 weeks) by multiple stepwise logistic regression analysis
| Estimated value | Standard error | χ 2 | p value | |
|---|---|---|---|---|
| Intercept | −12.77 | 4.50 | 8.03 | 0.005 |
| Baseline HbA1c | −0.20 | 0.28 | 0.54 | 0.464 |
| BMI | 0.45 | 0.16 | 7.66 | 0.006 |
| Fasting CPR | 0.03 | 0.57 | 0.00 | 0.960 |
| Duration of glimepiride | 0.24 | 0.19 | 1.64 | 0.201 |
| Glimepiride dose | 0.37 | 0.37 | 1.02 | 0.314 |
| Body weight changes 12–48 weeks |
0.33 | 0.19 | 2.79 | 0.095 |
The values show independent contributions to r 2 for each predictor in the model. The dependent variable was non-relapse (<0.4 % increase in HbA1c in 48 weeks compared with 12 weeks). The independent variables were baseline HbA1c, BMI, fasting CPR, duration of SU administration, glimepiride dose, and body weight changes from 12 weeks to 48 weeks
BMI body mass index, CPR C-peptide radio-immunoprotein
ROC analyses were carried out to determine the BMI cutoff value. AUC was 0.78 (p < 0.05), with an estimated cutoff point of 27.9 kg/m2, sensitivity of 69.6 %, and specificity of 82.6 % (Fig. 3).
Fig. 3.
Receiver-operating characteristic curve analysis of BMI for predicting non-relapse (<0.4 % increase in HbA1c in 48 weeks compared with 12 weeks). The AUC was 0.7769 with a sensitivity of 69.6 %, specificity was 82.6 %, and cutoff BMI value of 27.94 kg/m2
Discussion
In this study, we demonstrated for the first time that a higher BMI (average BMI: 29.4 kg/m2) attenuates the long-term HbA1c-lowering effects of liraglutide in T2D patients treated with sulfonylurea-based therapy.
Previous reports have suggested the usefulness of the 6-min CPR response to glucagon stimulation or CPR index as predictors of the glucose-lowering effect for 12 weeks after liraglutide initiation [9, 16]. These indices for endogenous insulin secretion were similar and indicated a relatively well-preserved β-cell function between the relapse and non-relapse groups. As expected, HbA1c decreased significantly from baseline to 12 weeks, but increased to baseline HbA1c levels at 48 weeks in the relapse group. In other words, endogenous insulin secretion was essential for the improvement of glycemic control at 12 weeks, but the baseline 6-min CPR response to glucagon stimulation and the CPR index could not be predictors of long-term HbA1c-lowering effects of liraglutide in the relapse group.
Several studies have reported that liraglutide leads to weight loss by delaying gastric emptying and inducing satiety [17]. It is well known that body weight reduction after liraglutide initiation is dependent on the baseline body weight of T2D patients [18]. Recently, Inoue et al. reported that a 0.3–0.9-mg dose of liraglutide resulted in a marked weight loss (−3.4 kg from baseline) over 2 years in obese T2D patients, although more than half of those patients were on insulin therapy [12]. Liraglutide treatment might be more effective at reducing body weight in obese T2D patients who gain weight after the start of insulin therapy. However, apparent weight loss was not observed in the present study. While the cause of the weight gain from week 12 to week 48 is still incomprehensible, it appears that 0.9 mg of liraglutide (the maximal daily dose permitted in Japan) may be insufficient to maintain weight loss over the long term. Liraglutide improves β-cell function as measured by a significant improvement in both first- and second-phase glucose-induced insulin secretion [19] and induces a robust enhancement of the post-challenge insulin compared with the placebo [20]. In addition, a significant increase in postprandial insulin secretion was observed in sulfonylurea and liraglutide combination therapy compared with sulfonylurea monotherapy [21]. Because hyperinsulinemia and obesity are so closely linked, a lasting insulin secretion by sulfonylurea and liraglutide combination therapy might cancel any body weight reduction after 12 weeks in a relapse group based on high BMI-caused insulin resistance.
To clarify whether relapsing HbA1c after 12 weeks is shown only in a liraglutide–glimepiride combination therapy, we analyzed the change in HbA1c in T2D patients treated with liraglutide monotherapy (n = 26, data not shown). In the liraglutide monotherapy group, baseline HbA1c (10.1 ± 2.1 %) was significantly decreased at 12 weeks (6.1 ± 0.7 %) and persisted over the 48-week treatment period. Although endogenous insulin secretion (fasting CPR: 2.2 ± 0.8 ng/ml) and excessive body weight (BMI: 27.1 ± 4.7 kg/m2) in the liraglutide monotherapy group were similar to those in the relapse group of the present study, the difference of change in HbA1c throughout the 48 weeks between both groups is of great interest. Liraglutide suppresses the fasting and postprandial glucagon levels in T2D patients [22]; however, the prandial glucagon level is increased by glimepiride [23]. The opposite effect on glucagon levels between liraglutide and glimepiride may have been attenuated in improved long-term glycemic control in the relapse group despite similar excessive body weight compared with those with liraglutide monotherapy.
Several limitations involved in the present study should be considered. First, there were no data available on endogenous insulin secretion after liraglutide administration; therefore, endogenous hyperinsulinemia could not be proved, leading to the attenuation effect of body weight reduction. Second, the lifestyle changes such as eating behavior and exercise during the study period were not assessed. An increase in HbA1c after 12 weeks in the relapse group might simply be due to an inappropriate lifestyle followed by increasing body weight after 12 weeks. Nonetheless, our conclusions would not be largely changed for the following reason: Baseline BMI was more closely associated with relapse for HbA1c than body weight changes after 12 weeks (Table 3) and body weight changes were not associated with HbA1c changes (supplementary data, Fig. S1). A recent report by Tajiri et al. found an association between a lower baseline score on a lifestyle assessment and relapsing HbA1c levels after sitagliptin treatment [24]. These results suggest that diet and exercise awareness should be persistently reinforced in patients receiving incretin-based therapy. Third, the present study was retrospective and conducted under an open-label design with no control arm, and the number of patients was relatively small. Finally, the HbA1c-lowering effect of liraglutide might be affected by seasonal variation of HbA1c because liraglutide was not administered at the same period.
In summary, we conclude that a higher BMI is associated with inadequate glycemic control in T2D patients treated using sulfonylurea-based therapy. We recommend that baseline BMI as well as endogenous insulin secretion should be considered before liraglutide initiation, particularly in T2D patients undergoing sulfonylurea-based therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Ethics policy
All procedures followed were in accordance with the Institutional Review Board for Clinical Research at the Showa University of Medicine and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients for being included in the study.
Conflict of interest
Takeshi Yamamoto, Tomoyasu Fukui, Akiko Higuchi, Makoto Ohara, and Toshiyuki Hayashi declare that they have no conflicts of interest. Tsutomu Hirano received a research grant from Eli Lilly Japan K.K. and Takeda Pharmaceutical Co., Ltd., and received lecture fees from Astra Zeneca, Mitsubishi Tanabe Pharma Corp., and MSD K.K.
Contributor Information
Takeshi Yamamoto, Phone: +81-3-3784-8947, Email: tak3105@aol.com.
Tomoyasu Fukui, Email: showauft@med.showa-u.ac.jp.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas Sixth Edition Poster Update 2014 (online), available from http://www.idf.org/diabetesatlas/update-2014 (accessed 2015-04-20).
- 2.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34. Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 5.Harder H, Nielsen L, Tu DT, et al. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care. 2004;27:1915–1921. doi: 10.2337/diacare.27.8.1915. [DOI] [PubMed] [Google Scholar]
- 6.Madsbad S, Schmitz O, Ranstam J, et al. Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004;27:1335–1342. doi: 10.2337/diacare.27.6.1335. [DOI] [PubMed] [Google Scholar]
- 7.Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Management of Hyperglycemia in Type2 Diabetes A patient- Centered Approach. Diabetes Care. 2015;2015(38):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 8.Takabe M, Matsuda T, Hirota Y, et al. C-peptide response to glucagon challenge is correlated with improvement of early insulin secretion by liraglutide treatment. Diabetes Res Clin Pract. 2012;98:e32–e35. doi: 10.1016/j.diabres.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 9.Usui R, Yabe D, Kuwata H, Fujiwara S, et al. Retrospective analysis of safety and efficacy of insulin-to-liraglutide switch in Japanese type 2 diabetes: A caution against inappropriate use in patients with reduced β-cell function. J Diabetes Investig. 2013;4:585–594. doi: 10.1111/jdi.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toyoda M, Yokoyama H, Abe K, et al. Predictors of response to liraglutide in Japanese type 2 diabetes. Diabetes Res Clin Pract. 2014;106:451–457. doi: 10.1016/j.diabres.2014.09.052. [DOI] [PubMed] [Google Scholar]
- 11.Imai K, Tsujimoto T, Goto A, et al. Prediction of response to GLP-1 receptor agonist therapy in Japanese patients with type 2 diabetes. Diabetol Metab Syndr. 2014;6:110. doi: 10.1186/1758-5996-6-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue K, Maeda N, Fujishima Y, et al. Long-term impact of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight and glycemic control in Japanese type 2 diabetes: an observational study. Diabetol Metab Syndr. 2014;6:95. doi: 10.1186/1758-5996-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nauck M, Frid A, Hermansen K, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab. 2013;15:204–212. doi: 10.1111/dom.12012. [DOI] [PubMed] [Google Scholar]
- 14.Kubota A, Yabe D, Kanamori A, et al. Factors influencing the durability of the glucose-lowering effect of sitagliptin combined with a sulfonylurea. J Diabetes Investig. 2014;5:445–448. doi: 10.1111/jdi.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diabetes Soc 2010;53:450–467.
- 16.Kozawa J, Inoue K, Iwamoto R, et al. Liraglutide is effective in type 2 diabetic patients with sustained endogenous insulin-secreting capacity. J Diabetes Investig. 2012;3:294–297. doi: 10.1111/j.2040-1124.2011.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujishima Y, Maeda N, Inoue K, et al. Efficacy of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight, eating behavior, and glycemic control, in Japanese obese type 2 diabetes. Cardiovasc Diabetol. 2012;14(11):107. doi: 10.1186/1475-2840-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lapolla A, Frison V, Bettio M, et al. Correlation between baseline characteristics and clinical outcomes in a large population of diabetes patients treated with liraglutide in a real-world setting in Italy. Clin Ther. 2015;37:574–584. doi: 10.1016/j.clinthera.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Degn KB, Juhl CB, Sturis J, et al. One week’s treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187–1194. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 20.Kramer CK, Zinman B, Choi H, et al. The impact of chronic liraglutide therapy on glucagon secretion in type 2 diabetes: insight from the LIBRA trial. J Clin Endocrinol Metab. 2015;100:3702–3709. doi: 10.1210/jc.2015-2725. [DOI] [PubMed] [Google Scholar]
- 21.Seino Y, Rasmussen MF, Clauson P, et al. The once-daily human glucagon-like peptide-1 analog, liraglutide, improves β-cell function in Japanese patients with type 2 diabetes. J Diabetes Investig. 2012;3:388–395. doi: 10.1111/j.2040-1124.2012.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermansen K, Bækdal TA, Düring M, et al. Liraglutide suppresses postprandial triglyceride and apolipoprotein B48 elevations after a fat-rich meal in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, cross-over trial. Diabetes Obes Metab. 2013;15:1040–1048. doi: 10.1111/dom.12133. [DOI] [PubMed] [Google Scholar]
- 23.Ahrén B, Foley JE, Ferrannini E, et al. Changes in prandial glucagon levels after a 2-year treatment with vildagliptin or glimepiride in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2010;33:730–732. doi: 10.2337/dc09-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tajiri Y, Tsuruta M, Ohki T, et al. Long-term efficacy of sitagliptin for the treatment of type 2 diabetic patients in Japan. Endocr J. 2012;59:197–204. doi: 10.1507/endocrj.EJ11-0248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.