Abstract
Aims
The onset of fulminant type 1 diabetes mellitus is sometimes accompanied by sudden death or cardiac arrest. The aim of this study was to determine the risk factors for the development of these conditions at the onset of fulminant type 1 diabetes mellitus.
Methods
We conducted a search of the literature on fulminant type 1 diabetes and sudden death or cardiac arrest published up to 2012 in PubMed and Ichushi (a Japanese article database), and a questionnaire survey was administered to the authors of the articles and to diabetes specialists affiliated to the Japan Diabetes Society. We analyzed the clinical data at disease onset of 17 patients with fulminant type 1 diabetes mellitus who experienced sudden death or cardiac arrest, and those of 257 patients who did not develop these conditions.
Results
Patients with sudden death or cardiac arrest were younger, had a higher rate of impaired consciousness, more severe acidosis, hyperglycemia, hyponatremia, hyperkalemia, and hypochloremia, a higher serum blood urea nitrogen level, a higher serum creatinine level, and a higher plasma osmolality level than the other patients. In multiple logistic regression analyses, plasma glucose level was positively associated with sudden death or cardiac arrest. Receiver operating characteristic curve analyses showed that patients with a plasma glucose level over 1000 mg/dl (55.5 mmol/l) were at a high risk of cardiac arrest.
Conclusions
Severe metabolic derangement, especially a high plasma glucose level, is associated with sudden death or cardiac arrest at the onset of fulminant type 1 diabetes mellitus.
Keywords: Fulminant type 1 diabetes mellitus, Sudden death, Cardiac arrest, Risk factors
Introduction
The two major subtypes of diabetes mellitus are type 1 and type 2 diabetes mellitus. Type 1 diabetes mellitus is characterized by the loss of more than 80 % of insulin-secreting beta cells [1]. In type 1A diabetes, which is a major form of type 1 diabetes mellitus, pancreatic beta cells are thought to be progressively destroyed over several months or even several years. Islet-related autoantibodies have been observed in patients’ sera before the onset of hyperglycemia. Although hyperglycemic symptoms accompanying diabetic ketosis or ketoacidosis appear more suddenly in type 1 diabetes than in type 2 diabetes, hyperglycemia itself progresses gradually. The Diabetes Prevention Trial of Type 1 (DPT-1) revealed that postprandial hyperglycemia appeared first, followed by fasting hyperglycemia, in autoantibody-positive first- or second-degree relatives of type 1A diabetes patients [2]. The elevated level of HbA1c at the onset of overt type 1A diabetes also suggests that hyperglycemia continues for a certain period before the development of hyperglycemic symptoms [3].
Fulminant type 1 diabetes mellitus (FT1DM) is a severe subtype of type 1 diabetes [3, 4]. It is commonly observed in East Asia, where it accounts for approximately 20 % of acute-onset type 1 diabetes cases in Japan [4] and 7.1 % of all type 1 diabetes cases in Korea [5]. The essential characteristic of FT1DM is severe insulin insufficiency based on almost complete destruction of pancreatic beta cells, even at clinical onset [6]. Based on this unique characteristic, FT1DM patients can easily undergo a severe clinical course, especially at onset. In fact, almost all patients with FT1DM also show ketoacidosis at disease onset [3]. At the onset of FT1DM, plasma glucose (PG) levels can be as high as 800 mg/dl, but HbA1c levels can be as low as 7.0 % on average, demonstrating the rapid progression and short duration of hyperglycemia. Sekine et al. reported that the PG level of a FT1DM patient suddenly increased from within the normal range to over 1000 mg/dl, and that the serum C peptide level decreased from approximately 1 ng/dl to an undetectable level in a day [7].
Moreover, there have been several reports of FT1DM patients with particularly severe clinical courses [7–25]. In such patients, many complications, such as cardiac disorders (including T-wave inversion, atrial fibrillation, acute myocarditis, cardiac arrest [8–14, 16–21]), acute renal failure [22], rhabdomyolysis [22], hypersensitivity syndrome [7], and even sudden death [15, 23–25], have been reported at the onset of FT1DM. It is important to predict and avoid such life-threatening situations. Therefore, we undertook this study to identify the risk factors for sudden death or cardiac arrest at the onset of FT1DM.
Methods
A literature search by the key words of “fulminant,” “diabetes,” “arrest,” and/or “death” was conducted in PubMed and Ichushi (a Japanese article database) to identify articles published up to 2012 that described patients who developed sudden death or cardiac arrest at the onset of FT1DM, and a questionnaire survey was administered to the authors of the articles. We excluded one autopsy case which was complicated by Reye syndrome [26], and one case of death after caesarean section [27], and analyzed 17 patients with FT1DM who had been described. Five of the patients had been found dead at home and 12 patients had experienced a cardiac arrest before or after they visited the hospital. Ten of these 12 patients went into cardiac arrest on the same day they visited the hospital. One patient went into cardiac arrest before visiting the hospital, one went into cardiac arrest in the ambulance, and the other eight patients went into cardiac arrest soon after arriving at the hospital. Regarding the other two patients, one visited a clinic for thirst and fatigue and was diagnosed with a cold. The next day, she had trouble breathing, called an ambulance, and went into cardiac arrest in the ambulance. The other patient visited a clinic for thirst, upper abdominal pain, and vomiting and was diagnosed with acute gastroenteritis. Two days later, he presented with restlessness and visited the emergency room, where he went into cardiac arrest. Among the 12 patients, five patients died despite cardiopulmonary resuscitation. An autopsy was performed in these ten dead patients.
To collect control cases, we administered a nationwide questionnaire survey to diabetes specialists affiliated to the Japan Diabetes Society, and asked them whether they had experienced FT1DM cases without sudden death or cardiac arrest until 2012. We recruited the resulting 257 patients as controls. The questionnaire included information on subjective symptoms, body size measurements, blood, and urine examinations of the FT1DM patients on the day of the hospital visit or on admission. Diagnosis of FT1DM was provided by the attending physician based on the following criteria, which are based on the Report of the Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research: (1) ketosis or ketoacidosis within 1 week after the onset of hyperglycemic symptoms; (2) urinary C-peptide below 10 μg/day or fasting serum C-peptide below 0.3 ng/ml and serum C-peptide below 0.5 ng/ml after glucagon injection or meal load soon after the onset of disease; and (3) PG level above 288 mg/dl and HbA1c below 8.7 % at the first visit [28]. The contents of the questionnaire used to collect the data on FT1DM patients with sudden death or cardiac arrest and the corresponding data on the control patients were same. All data on the clinical and biological characteristics of the subjects were collected at the initial visit, except in the cases of sudden death. The laboratory data on patients with sudden death were excluded because of the possibility of postmortem biological changes (except for HbA1c, which is reported to be stable in postmortem blood [24, 29]).
The value of HbA1c (%) was estimated as a National Glycohemoglobin Standardization Program (NGSP) equivalent value (%), which was calculated by the following formula: HbA1c (%) = 1.019 × HbA1c (JDS) (%) + 0.3 %, considering the relational expression of HbA1c (JDS) (%) measured by the previous Japanese standard substance and measurement methods and HbA1c (NGSP) [30]. The value of plasma osmolality (Posm) was calculated by the following formula: Posm = 2 × [sodium (Na, meq/l) + potassium (K, meq/l)] + PG (mg/dl)/18 + blood urea nitrogen (BUN, mg/dl)/2.8.
Statistical analyses were performed with the Windows-based SPSS software (version 22, IBM Co., Armonk, NY, USA). Values are expressed as the median and the interquartile range. The Mann–Whitney U test and Pearson’s chi square test were conducted to determine the difference between FT1DM patients who experienced sudden death/cardiac arrest and other FT1DM patients. Single and multiple logistic regression analyses were applied to examine the relationships between sudden death/cardiac arrest and other factors: age, drowsiness, PG, pH, Na, K, chloride (Cl), BUN, creatinine (Cr), and Posm levels. Receiver operating characteristic (ROC) analyses were conducted to identify the optimal PG cutoff value for predicting cardiac arrest using the Youden index. Statistical significance was determined at p < 0.05.
The Ethical Committees of the Japan Diabetes Society and Osaka University Hospital approved this study. All procedures followed were in accordance with the Helsinki Declaration of 1964 and later revisions.
Results
Clinical courses of FT1DM cases with sudden death or cardiac arrest
We identified 17 reported cases [8–12, 14–20, 23–25] of FT1DM associated with sudden death or cardiac arrest at the onset of the disease. Five patients were found dead and the pathological examination of their pancreata by autopsy revealed the complete loss of pancreatic beta cells, which was compatible with FT1DM. In the other 12 patients who suffered a cardiac arrest, cardiopulmonary resuscitation was performed, but five could not be rescued. Autopsies were performed in these ten dead patients, and we confirmed that seven of them did not show viral myocarditis, structural heart disease, or thrombosis. We also confirmed that four of them did not show encephalitis. There were no descriptions of heart or brain lesions in the autopsy reports for the other cases.
Clinical and biological characteristics
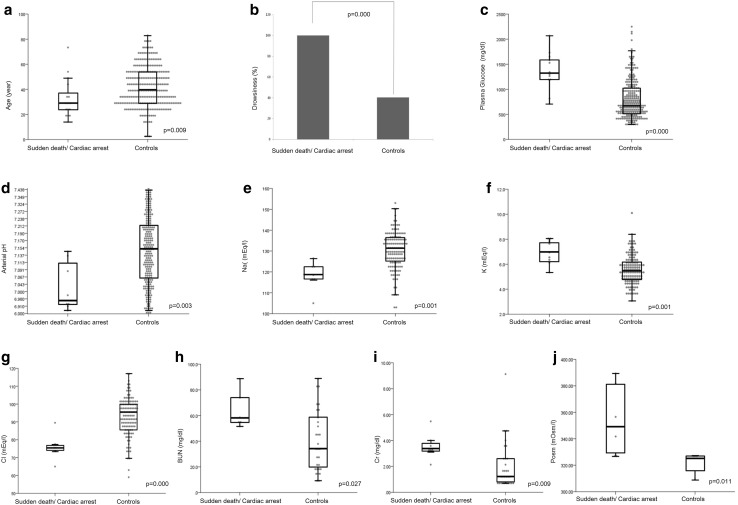
Clinical and biological characteristics of the subjects are shown in Table 1. There were no differences between the two groups regarding sex, BMI, duration, family history, pregnancy, thirst, flu-like symptoms, and abdominal symptoms. Patients who experienced sudden death or cardiac arrest were younger (aged 29 vs 40 years) and showed a higher rate of drowsiness (100 vs 40.3 %), a higher PG level [median, 1323 (interquartile range, 1198–1602) vs 710 (535–1035) mg/dl], a lower arterial pH [6.965 (6.920–7.111) vs 7.150 (7.060–7.213)], a lower Na concentration [119 (117–123) vs 131 (125–136) meq/l], a higher K concentration [7.0 (6.3–7.8) vs 5.6 (4.8–6.3) meq/l], a lower chloride concentration [75 (74–77) vs 94 (87–100) meq/l], a higher BUN level [57 (54–74) vs 34 (19–58) mg/dl], a higher Cr level [3.40 (3.20–3.80) vs 1.28 (0.80–2.60) mg/dl], and a higher Posm level [349 (330–382) vs 325 (316–327) mOsm/l] than other FT1DM patients. A bar graph and box plots of the data are shown in Fig. 1a–j. Levels of HbA1c, urinary C peptide reactivity (CPR), fasting serum CPR, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine phosphokinase (CPK), and anti-GAD antibody were not significantly different between the two groups. The electrocardiogram examined before cardiac arrest showed ventricular tachycardia in three patients, ST elevation in two patients, pulseless electrical activity in one patient, and ventricular flutter in one patient. Coronary angiography was performed for all patients with ST elevation, but no patients showed structural heart disease.
Table 1.
Clinical and biological characteristics of subjects
| Sudden death/cardiac arrest | Other FT1DM | p-valuea | |
|---|---|---|---|
| N | 17 | 257 | |
| Age (years) | 29 (23, 36) (17) | 40 (29, 54) (257) | 0.009 |
| Sex (male/female) | 12/5 | 137/120 | 0.166 |
| BMI (kg/m2) | 22.7 (20.8, 24.8) (6) | 20.8 (18.8, 22.3) (231) | 0.127 |
| Duration (days) | 3 (1, 3) (6) | 4 (2, 7) (251) | 0.127 |
| Days before cardiac arrest (days) | 0 (0, 0) (13) | – | – |
| Family history of type 2 diabetes (−/+) | 3/3 (6) | 175/70 (245) | 0254 |
| Pregnancy (−/+) | 5/0 (5) | 93/21 (114) | 0.290 |
| Thirst (−/+) | 0/5 (5) | 28/210 (238) | 0.415 |
| Flu-like symptoms (−/+) | 0/11 (11) | 55/193 (248) | 0.207 |
| Abdominal symptoms (−/+) | 1/12 (13) | 59/189 (248) | 0.393 |
| Drowsiness (−/+) | 0/10 (10) | 139/94 (233) | 0.000 |
| PG (mg/dl) | 1323 (1198, 1602) (12) | 710 (535, 1035) (257) | 0.000 |
| HbA1c (%, NGSP) | 6.3 (6.1, 6.8) (15) | 6.6 (6.2, 7.2) (257) | 0.139 |
| Arterial pH | 6.965 (6.920, 7.111) (8) | 7.150 (7.060, 7.213) (219) | 0.003 |
| Urinary CPR (μg/day) | 5.10 (2.80, 7.20) (6) | 3.20 (1.30, 5.95) (211) | 0.323 |
| Fasting serum CPR (ng/ml) | 0.15 (0.10, 0.20) (2) | 0.20 (0.10, 0.30) (188) | 0.788 |
| Na (meq/l) | 119 (117, 123) (7) | 131 (125, 136) (128) | 0.001 |
| K (meq/l) | 7.0 (6.3, 7.8) (9) | 5.6 (4.8, 6.3) (129) | 0.001 |
| Cl (meq/l) | 75 (74, 77) (7) | 94 (87, 100) (123) | 0.000 |
| AST (IU/l) | 35 (24, 59) (7) | 23 (18, 43) (129) | 0.113 |
| ALT (IU/l) | 46 (27, 54) (7) | 29 (19, 46) (128) | 0.135 |
| BUN (mg/dl) | 57 (54, 74) (6) | 34 (19, 58) (30) | 0.027 |
| Cr (mg/dl) | 3.40 (3.20, 3.80) (7) | 1.28 (0.80, 2.60) (30) | 0.009 |
| CPK (IU/l) | 318 (208, 527) (5) | 270 (135, 600) (21) | 0.720 |
| Posm (mOsm/l) | 349 (330, 382) (6) | 325 (316, 327) (4) | 0.011 |
| Anti-GAD antibody (−/+) | 6/2 (8) | 117/25 (142) | 0.596 |
Values are expressed as median (interquartile range)
Italicized numbers in parentheses are the total number of cases with data available
Duration is the time between the onset of hyperglycemia symptoms and the first visit
Days before cardiac arrest is the time between the first visit and cardiac arrest
Pregnancy data refer to female patients 13–49 years old who had type 1 diabetes during or after pregnancy
aMann–Whitney U test and Pearson’s chi square test, with significance assumed when p < 0.05
Fig. 1.
Scatter and box plots showing age (a), plasma glucose levels (c), arterial pH (d), sodium (e), potassium (f), chloride (g), serum blood urea nitrogen (h), serum creatinine (i), and plasma osmolality (j) at the onset of fulminant type 1 diabetes mellitus with and without sudden death or cardiac arrest. The bottom and top of each box show the first and third quartiles, and the band inside the box is the median. The ends of the whiskers represent the lowest datum within the 1.5 interquartile range of the lower quartile and the highest datum within the 1.5 interquartile range of the upper quartile. The bar graph (b) shows the percentage of the patients who presented drowsiness
Single and multiple logistic regression analysis
The results of single logistic regression analysis are shown in Table 2. The levels of PG, K, and BUN were positively associated with sudden death or cardiac arrest. Patients with K ≥ 6.0 meq/l were at a higher risk of sudden death or cardiac arrest than those with lower levels of K. Age, pH, Na, and Cl were negatively associated with sudden death or cardiac arrest. In multiple logistic regression analysis, the PG level was positively associated with sudden death or cardiac arrest [odds ratio, 1.003 (95 % CI, 1.001–1.005); p = 0.004].
Table 2.
Relationships between sudden death/cardiac arrest and other variables
| Crude odds ratio | 95 % CI | p-valuea | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age (year) | 0.959 | 0.926 | 0.993 | 0.019 |
| Drowsiness (+) | Undetermined | – | – | – |
| PG (mg/dl) | 1.003 | 1.001 | 1.004 | 0.000 |
| pH | 0.030 | 0.001 | 0.704 | 0.029 |
| Na (meq/l) | 0.878 | 0.807 | 0.955 | 0.003 |
| K (meq/l) | 2.469 | 1.356 | 4.494 | 0.003 |
| K (≥5.5, meq/l) | 8.328 | 1.025 | 67.648 | 0.047 |
| K (≥6.0, meq/l) | 16.800 | 2.062 | 136.845 | 0.008 |
| Cl (meq/l) | 0.867 | 0.798 | 0.943 | 0.001 |
| BUN (mg/dl) | 1.048 | 1.002 | 1.096 | 0.042 |
| Cr (mg/dl) | 1.540 | 0.958 | 2.474 | 0.075 |
| Posm (mOsm/l) | Undetermined | – | – | – |
Objective variable: sudden death/cardiac arrest
aUnivariate logistic regression analysis, with significance assumed when p < 0.05
ROC analysis
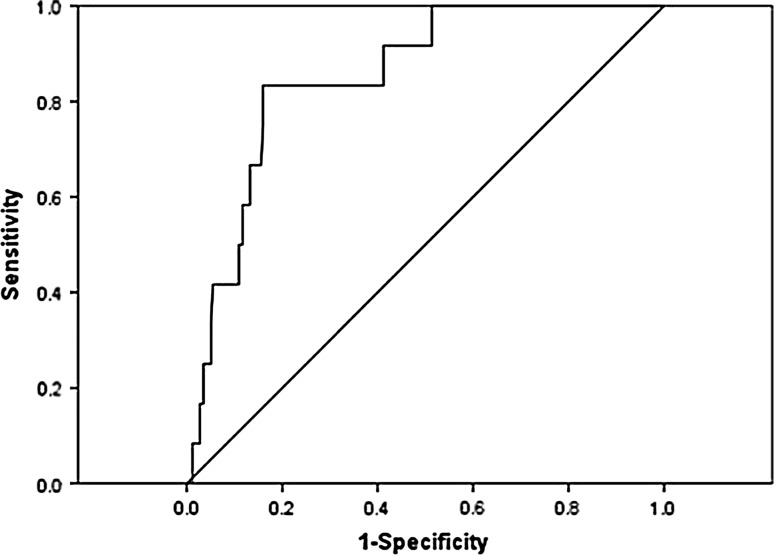
As PG level was measured in all cases at the onset of FT1DM, ROC analyses were conducted to identify the optimal PG cutoff value for predicting cardiac arrest. The ROC curve for PG level was created by plotting the range of sensitivity and specificity pairs for cardiac arrest (Fig. 2). The area under the curve was 0.852 (p = 0.000). According to the Youden index, the optimal PG cutoff value was 1197 mg/dl (sensitivity 0.833 and specificity 0.840). A level of PG over 1000 mg/dl (55.5 mmol/l) was found to have the same high sensitivity for cardiac arrest (sensitivity 0.833 and specificity 0.732). In this study, 80 FT1DM patients presented with a PG level of over 1000 mg/dl, and ten of them (12.5 %) went into cardiac arrest.
Fig. 2.
Receiver operating characteristic curve of the plasma glucose cutoff value for predicting cardiac arrest. The area under the curve is 0.852 (p = 0.000). The optimal plasma glucose cutoff value is 1197 mg/dl (sensitivity 0.833 and specificity 0.840)
Discussion
In this study, we analyzed and compared the patients who developed FT1DM with and without sudden death or cardiac arrest to identify the risk factors for these conditions at the onset of FT1DM. Patients with sudden death or cardiac arrest were younger than the others, had a higher rate of impaired consciousness, and had more severe acidosis, hyperglycemia, hyponatremia, hyperkalemia, and hypochloremia, a higher serum BUN level, a higher serum creatinine level, and a higher Posm level, indicating the existence of severe metabolic derangement. Based on these results, it was speculated that the severe metabolic disorder itself was the main cause of sudden death and cardiac arrest at the onset of FT1DM. In particular, the PG level, which was measured in all patients at the onset of FT1DM, was positively associated with the development of sudden death and cardiac arrest. The ROC curve showed that the optimal PG cutoff value for predicting cardiac arrest was 1197 mg/dl (sensitivity 0.833 and specificity 0.840). The easily comprehensible value of 1000 mg/dl (55.5 mmol/l) had the same sensitivity for cardiac arrest (sensitivity 0.833 and specificity 0.732). Therefore, the findings suggest that patients with a PG level of over 1000 mg/dl (55.5 mmol/l) are at high risk of cardiac arrest; indeed, 12.5 % of those patients went into cardiac arrest in this study.
According to the results of the autopsy and blood examinations of the patients who experienced sudden death or cardiac arrest, the only abnormal finding was the loss of pancreatic beta cells. The involvement of viral infections such as human herpesvirus 6 [31], herpes simplex virus [32], coxsackie B3 virus [33], influenza B virus [34], and cytomegalovirus [35] in the pathogenesis of FT1DM has been reported. Further, mice that develop encephalomyocarditis (EMC)-virus-induced diabetes have been used in a mouse model of human FT1DM [36]. However, none of the patients who experienced sudden death or cardiac arrest had viral myocarditis or encephalitis in this study, or in other reports [11, 14, 15, 19, 20, 23–25]. Furthermore, none of them showed any sign of structural heart disease that could be linked to sudden death or cardiac arrest. Therefore, severe metabolic derangement still seems to be the main cause of sudden death and cardiac arrest at the onset of FT1DM.
This is the first study to focus on and clarify the risk factors for sudden death and cardiac arrest at the onset of FT1DM. Although the precise mechanism of sudden death and cardiac arrest due to severe metabolic derangement is still unknown, to comprehend the severity of metabolic derangement on seeing patients who develop FT1DM will bring more information to solve this problem. A limitation of this study was that the number of the patients who experienced cardiac arrest or sudden death was small; hence, the data might be insufficient for multiple analyses. To confirm our results, we recommend that more patients are accumulated and assessed in the future.
In conclusion, we found that metabolic derangements were associated with sudden death and cardiac arrest at the onset of FT1DM. When clinicians are faced with patients who develop FT1DM, it is important to focus on metabolic markers such as PG, pH, Na, K, Cl, BUN, Cr, and Posm. In particular, patients with an extraordinary high PG level [over 1000 mg/dl (55.5 mmol/l)] may need to be more careful and seek treatment to avoid sudden death or cardiac arrest.
Acknowledgments
The other member of the Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research is Seiho Nagafuchi (Department of Medical Science and Technology, Graduate School of Medical Sciences, Kyushu University). The authors express their sincere gratitude to Dr. Kazuro Kaise, Dr. Satsuki Niitsuma, Dr. Ayako Ro, Dr. Kazuaki Shinohara, and all diabetes specialists affiliated to the Japan Diabetes Society for answering the questionnaire.
Conflict of interest statement
Toshiaki Hanafusa received honoraria for lectures from Ono Pharmaceutical, Mitsubishi Tanabe Pharma, and Sumitomo Dainippon Pharma. Hiroshi Ikegami received honoraria for lectures from Novo Nordisk Pharmaceuticals Industries, Inc., Sumitomo Dainippon Pharma, and Kowa Company, and scholarship grants from Ono Pharmaceutical and Novo Nordisk Pharmaceuticals Industries, Inc. Yumiko Kawabata received scholarship grants from Ono Pharmaceutical and Novo Nordisk Pharmaceuticals Industries, Inc. Other authors declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients before they were included in the study.
Footnotes
For the consultation of the Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research.
Contributor Information
Megu Yamaguchi Baden, Phone: +81 6 6879 3736, Email: mbaden@endmet.med.osaka-u.ac.jp.
Akihisa Imagawa, Phone: +81 6 6879 3736, Email: aimagawa@endmet.med.osaka-u.ac.jp.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenbaum CJ, Cuthbertson D, Krischer JP. Type I diabetes manifested solely by 2-h oral glucose tolerance test criteria. Diabetes. 2001;50:470–476. doi: 10.2337/diabetes.50.2.470. [DOI] [PubMed] [Google Scholar]
- 3.Hanafusa T, Imagawa A. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab. 2007;3:36–45. doi: 10.1038/ncpendmet0351. [DOI] [PubMed] [Google Scholar]
- 4.Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Toyoda T, Maruyama T, Makino H. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care. 2003;26:2345–2352. doi: 10.2337/diacare.26.8.2345. [DOI] [PubMed] [Google Scholar]
- 5.Cho YM, Kim JT, Ko KS, Koo BK, Yang SW, Park MH, Lee HK, Park KS. Fulminant type 1 diabetes in Korea: high prevalence among patients with adult-onset type 1 diabetes. Diabetologia. 2007;50:2276–2279. doi: 10.1007/s00125-007-0812-z. [DOI] [PubMed] [Google Scholar]
- 6.Sayama K, Imagawa A, Okita K, Uno S, Moriwaki M, Kozawa J, Iwahashi H, Yamagata K, Tamura S, Matsuzawa Y, Hanafusa T, Miyagawa J, Shimomura I. Pancreatic beta and alpha cells are both decreased in patients with fulminant type 1 diabetes: a morphometrical assessment. Diabetologia. 2005;48:1560–1564. doi: 10.1007/s00125-005-1829-9. [DOI] [PubMed] [Google Scholar]
- 7.Sekine N, Motokura T, Oki T, Umeda Y, Sasaki N, Hayashi M, Sato H, Fujita T, Kaneko T, Asano Y, Kikuchi K. Rapid loss of insulin secretion in a patient with fulminant type 1 diabetes mellitus and carbamazepine hypersensitivity syndrome. JAMA. 2001;285:1153–1154. doi: 10.1001/jama.285.9.1153. [DOI] [PubMed] [Google Scholar]
- 8.Uto Y, Uto K, Teno S, Isono K, Omori Y. A case of fulminant type 1 diabetes mellitus detected after cardiac arrest due to diabetic ketoacidosis. J Japan Diab Soc. 2002;45:689–693. [Google Scholar]
- 9.Shigeta M, Obana M, Iwamoto K, Ichikawa A, Nakata K, Hoshi D, Koshikawa Y, Nozaki H, Suzuki T, Osone Y, Akizuki T, Matsuoka Y, Shibayama K. Two cases of fulminant type 1 diabetes mellitus. Kawasakishi Ishikai Igakkaishi. 2003;20:118–24 (in Japanese).
- 10.Nakagawa M, Abe S, Tsutsumi R, Goto D, Tsutsu N, Umeno J, Inou T. A case of fulminant type 1 diabetes mellitus suffered cardiopulmonary arrest. J Japan Diab Soc. 2004;47:671 (in Japanese).
- 11.Goto H, Abiru H, Iino M, Tamaki K. An autopsy case of fulminant type 1 diabetes mellitus. Nihon Hoigaku Zassi. 2005;59:201 (in Japanese).
- 12.Fukushima T, Abiru N, Moriuchi A, Fukushima K, Kuwahara H, Uotani S, Yamazaki H, Eguchi K, Kobayashi M, Kita A, Kawasaki E. Fulminant type 1 diabetes mellitus with cardiopulmonary arrest. J Japan Diab Soc. 2005;48:371 (in Japanese).
- 13.Kobayashi T, Isomine S, Goto M, Sato M, Kanazawa T, Sakaida K, Iwaoka H. A case of fulminant type 1 diabetes with ECG changes in the first trimester of pregnancy. J Jpn Soc Intensive Care Med. 2005;12:25–30 (in Japanese).
- 14.Tanaka S, Nishida Y, Aida K, Maruyama T, Shimada A, Suzuki M, Shimura H, Takizawa S, Takahashi M, Akiyama D, Arai-Yamashita S, Furuya F, Kawaguchi A, Kaneshige M, Katoh R, Endo T, Kobayashi T. Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated beta-cell failure in fulminant type 1 diabetes. Diabetes. 2009;58:2285–2291. doi: 10.2337/db09-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibasaki S, Imagawa A, Tauriainen S, Iino M, Oikarinen M, Abiru H, Tamaki K, Seino H, Nishi K, Takase I, Okada Y, Uno S, Murase-Mishiba Y, Terasaki J, Makino H, Shimomura I, Hyoty H, Hanafusa T. Expression of Toll-like receptors in the pancreas of recent-onset fulminant type 1 diabetes. Endocr J. 2010;57:211–9. [DOI] [PubMed]
- 16.Sato S, Honkura K, Yamashita R, Takahashi M, Seino J, Kaise K, Tanigawa T. Two cases of fulminant type 1 diabetes mellitus suffered cardiac complication within early days of onset. J Japan Diab Soc. 2010;53:S207 (in Japanese).
- 17.Kunieda T, Murayama M, Koga M, Hanatate F, Hayashi M, Yamakita N, Yasuda K. A case of ischemic stricture of the small intestine due to cardiac arrest caused by diabetic ketoacidosis in fulminant type 1 diabetes. J Japan Diab Soc. 2011;54:356–60 (in Japanese).
- 18.Maekawa Y, Yara T, Nashiro K, Masuda F, Kowatari M, China Y, Yamashiro A, Kinjyo M, Shiroma I. A survived case of fulminant type 1 diabetes after cardiac arrest with hyperkalemia and ST elevation. J Japan Diab Soc. 2011;54:359 (in Japanese).
- 19.Aida K, Nishida Y, Tanaka S, Maruyama T, Shimada A, Awata T, Suzuki M, Shimura H, Takizawa S, Ichijo M, Akiyama D, Furuya F, Kawaguchi A, Kaneshige M, Itakura J, Fujii H, Endo T, Kobayashi T. RIG-I- and MDA5-initiated innate immunity linked with adaptive immunity accelerates beta-cell death in fulminant type 1 diabetes. Diabetes. 2011;60:884–889. doi: 10.2337/db10-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inokuchi R, Matsumoto A, Odajima H, Shinohara K. Fulminant type 1 diabetes mellitus. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-006560. [DOI] [PMC free article] [PubMed]
- 21.Hiramatsu S, Komori K, Mori E, Ogo A, Maruyama S, Kato S. A case of fulminant type 1 diabetes mellitus accompanied by myocarditis. Endocr J. 2011;58:553–557. doi: 10.1507/endocrj.K11E-007. [DOI] [PubMed] [Google Scholar]
- 22.Iwaoka T. A case of fulminant type 1 diabetes with transiently positive anti-GAD antibodies. Endocr J. 2003;50:225–231. doi: 10.1507/endocrj.50.225. [DOI] [PubMed] [Google Scholar]
- 23.Mizutani T, Yoshimoto T, Kaneko R, Ishii A. Diagnosis of fulminant type 1 diabetes mellitus in an autopsy case with postmortem changes. Leg Med. 2011;13:250–253. doi: 10.1016/j.legalmed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Ro A, Hisashi Y, Kageyama N, Tanifuji T, Hayashi K, Fukunaga T, Fujita MQ. Two autopsy cases of sudden death in adolescent by fulminant type 1 diabetes mellitus. Houibyouri. 2006;12:22–6 (in Japanese).
- 25.Bird S. Failure to diagnose: diabetic ketoacidosis. Aust Fam Physician. 2010;39:867–868. [PubMed] [Google Scholar]
- 26.Ohashi R, Hasegawa G, Naito M. An autopsy case of fulminant type 1 diabetes complicated by Reye syndrome. Proc Jpn Soc Pathol. 2005;94:342 (in Japanese).
- 27.Tamaki E, Doi M, Masuda R, Kato Y. A case of fulminant type 1 diabetes occurred in late pregnancy. Kanto J Obst Gynec. 2007;44:187 (in Japanese).
- 28.Imagawa A, Hanafusa T, Awata T, Ikegami H, Uchigata Y, Osawa H, Kawasaki E, Kawabata Y, Kobayashi T, Shimada A, Shimizu I, Takahashi K, Nagata M, Makino H, Maruyama T. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus. J Diabetes Investig. 2012;3:536–539. doi: 10.1111/jdi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hindle EJ, Rostron GM, Gatt JA. The diagnostic value of glycated haemoglobin levels in post-mortem blood. Ann Clin Biochem. 1985;22:144–147. doi: 10.1177/000456328502200206. [DOI] [PubMed] [Google Scholar]
- 30.Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40. [DOI] [PMC free article] [PubMed]
- 31.Chiou CC, Chung WH, Hung SI, Yang LC, Hong HS. Fulminant type 1 diabetes mellitus caused by drug hypersensitivity syndrome with human herpesvirus 6 infection. J Am Acad Dermatol. 2006;54:S14–S17. doi: 10.1016/j.jaad.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 32.Nagaoka T, Terada M, Miyakoshi H. Insulin-dependent diabetes mellitus following acute pancreatitis caused by herpes simplex virus; a case report. J Japan Diab Soc. 2001;44:335–40 (in Japanese).
- 33.Nishida W, Hasebe S, Kawamura R, Hashiramoto M, Onuma H, Osawa H, Makino H. A case of fulminant type 1 diabetes associated with high titer of coxsackie B3 virus antibody. J Japan Diab Soc. 2005;48(suppl 1):A23–7 (in Japanese).
- 34.Sano H, Terasaki J, Tsutsumi C, Imagawa A, Hanafusa T. A case of fulminant type 1 diabetes mellitus after influenza B infection. Diabetes Res Clin Pract. 2008;79:e8–e9. doi: 10.1016/j.diabres.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Onuma H, Tohyama M, Imagawa A, Hanafusa T, Kobayashi T, Kano Y, Ohashi J, Hashimoto K, Osawa H, Makino H. High frequency of HLA B62 in fulminant type 1 diabetes with the drug-induced hypersensitivity syndrome. J Clin Endocrinol Metab. 2012;97:e2277–e2281. doi: 10.1210/jc.2012-2054. [DOI] [PubMed] [Google Scholar]
- 36.Shimada A, Maruyama T. Encephalomyocarditis-virus-induced diabetes model resembles “fulminant” type 1 diabetes in humans. Diabetologia. 2004;47:1854–1855. doi: 10.1007/s00125-004-1538-9. [DOI] [PubMed] [Google Scholar]