Abstract
The safety and efficacy of sitagliptin as add-on therapy to glinides, rapid-acting insulin secretagogues, were evaluated for Japanese patients with type 2 diabetes mellitus. This 52-week study consisted of a 12-week double-blind period, followed by a 40-week open-label period. During the double-blind period, patients were randomized to sitagliptin 50 mg q.d. (S/S group) or placebo (P/S group) as add-on therapy to glinide monotherapy. During the open-label period, all patients in both groups were administered sitagliptin 50 mg q.d. (or 100 mg q.d. after up-titration). During the double-blind period, the overall occurrence of adverse experiences (AE) was similar in both treatment groups. The frequency of reported AE of hypoglycemia in both groups was low and not notably different. The nature of clinical AE during the open-label period for both groups was not notably different from that of clinical AE in the sitagliptin group during the double-blind period. The between-group difference in HbA1c least squares (LS) mean of change from baseline (95 % CI) at Week 12 was −1.1 % (−1.3, −0.8) in favor of sitagliptin (P < 0.001). LS mean of reductions from baseline of fasting plasma glucose and 2-h postmeal glucose were significantly greater in the sitagliptin group than in the placebo group: −23.1 mg/dL (−32.2, −13.9) and −51.2 mg/dL (−67.4, −35.0), respectively (both P < 0.001). The changes from baseline in glycemic data in the S/S group remained generally stable throughout the 52-week treatment period.
Electronic supplementary material
The online version of this article (doi:10.1007/s13340-015-0230-2) contains supplementary material, which is available to authorized users.
Keywords: Add-on, Sitagliptin, Incretins, Glinide, Type 2 diabetes mellitus
Introduction
Dipeptidyl peptidase-4 (DPP-4) inhibitors and rapid-acting insulin secretagogues (glinides) reduce plasma glucose levels by stimulating insulin secretion from pancreatic β-cells, although by different mechanisms of action [1, 2]. DPP-4 inhibitors, for example sitagliptin, inhibit degradation of the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). These incretins then bind to their respective receptor (GLP-1R or GIPR) on pancreatic β-cells, activating adenylate cyclase and elevating intracellular concentrations of 3′5′-cyclic adenosine monophosphate (cAMP), thereby stimulating insulin secretion in a manner that depends on the plasma glucose level, such that greater amounts of insulin are released at higher glucose concentrations. In addition, when plasma glucose is elevated, GLP-1 reduces glucagon secretion by pancreatic α-cells. In contrast, glinides bind to the sulfonylurea (SU) receptor on pancreatic β-cells and promote insulin secretion by inhibiting the adenosine triphosphate (ATP)-dependent K+ channel in a manner that is independent of plasma glucose level. Thus, when added to glinide for patients with type 2 diabetes mellitus (T2DM) whose plasma glucose levels are not adequately controlled, sitagliptin is expected to achieve complementary glucose reduction.
Japanese patients with T2DM may have lower capacity to secrete insulin than patients of European ancestry, and, characteristically, relatively low levels of insulin secretion are observed after meals [3]. Thus, these patients are believed to particularly benefit from agents that promote insulin secretion from pancreatic β-cells. Glinides are characterized by more rapid reduction of plasma glucose than SU agents, and are used for correcting postprandial hyperglycemia. Although glinides are widely prescribed in Japan for treatment of patients with T2DM, there has been no reported study of sitagliptin as add-on to glinides for Japanese patients with T2DM. Because, as described above, sitagliptin causes insulin secretion by a mechanism different from that of glinides, the effect of adding sitagliptin to glinides was examined for Japanese patients with T2DM.
Materials and methods
This multicenter, randomized clinical trial (registered at http://www.clinicaltrials.gov as NCT01517321) was conducted at 30 sites in Japan from October 2011 to April 2013. The study design included an initial 12-week placebo-controlled, parallel-group, double-blind treatment period with sitagliptin 50 mg q.d. or placebo added to ongoing glinide (180–360 mg/day nateglinide or 15–60 mg/day mitiglinide) with diet and exercise therapy. This double-blind placebo-controlled period was followed by an open-label uncontrolled treatment period of 40 weeks, during which all patients received sitagliptin at a dose of at least 50 mg q.d. added to their ongoing glinide therapy. Patients in the placebo group during the double-blind period were switched to sitagliptin 50 mg q.d. during the open-label period. Starting at Week 16, the sitagliptin dose could be up-titrated to 100 mg q.d. for both treatment groups if fasting plasma glucose (FPG) or HbA1c did not meet the prespecified criteria. This study was designed and conducted in accordance with the good clinical practice guidelines and ethical principles stated in the Declaration of Helsinki. The study protocol (ONO-5435-17, MK-0431 P282) was approved by the institutional review board at each study site, and all patients provided written informed consent.
This study used Japan Diabetes Society (JDS)-certified HbA1c values, the standard at the time the study was conducted. HbA1c values reported here have been converted to national glycohemoglobin standardization program (NGSP) values as follows: HbA1c [NGSP] [%] = 1.02 × HbA1c [JDS] [%] + 0.25% [4].
Patients
In this study, patients were screened on the basis of JDS-certified HbA1c values (HbA1c ≥7.0 to <10.0 %); ≥7.4 to <10.5 % after conversion to NGSP values. This study enrolled Japanese patients ≥20 years of age with T2DM (HbA1c ≥7.4 to <10.5 % and FPG ≥126 to ≤270 mg/dL) at Week-2; the patients had been on diet and exercise for ≥8 weeks and on glinide monotherapy for ≥12 weeks at Week 0. The main exclusion criteria were:
type 1 diabetes mellitus;
recent treatment with insulin, pioglitazone hydrochloride, GLP-1 analogues, DPP-4 inhibitors, SUs, or combination agents containing active ingredients listed above within 8 weeks before the start of the screening period;
the presence of progressive diabetes complications;
unstable cardiovascular disease or uncontrolled severe hypertension (systolic blood pressure >160 mmHg or diastolic blood pressure >100 mmHg);
increased serum creatinine (>1.5 mg/dL for men or >1.3 mg/dL for women) or increased alanine aminotransferase or aspartate aminotransferase >2-fold the upper limit of normal;
hemoglobin <11.0 g/dL for men or <10.0 g/dL for women; or
body mass index (BMI) <18 or >40 kg/m2.
Study design and procedures
The overall study design is shown in Fig. 1. Patients who met all the eligibility criteria entered a 2-week, single-blind, placebo run-in period. Otherwise, patients on combination therapy with a glinide and other oral hypoglycemic agents (OHA) (α-glucosidase inhibitor and/or biguanides), and who met all the other eligibility criteria, could enter the 2-week placebo run-in period after a 6-week wash-out period of non-glinide OHA. This design ensured that all patients received at least 8 weeks of diet and exercise therapy and at least 12 weeks of glinide therapy (and at least 8 weeks as monotherapy) at a stable dose before randomization. All patients were instructed to follow a stable program of diet and exercise throughout the study.
Fig. 1.
Study design
Patients were eligible for randomization if they had an HbA1c ≥7.4 and <10.5 % and an FPG ≥126 and ≤270 mg/dL immediately before initiation of the placebo run-in and ≥75 % treatment adherence (on the basis of pill counts) during the placebo run-in period. Eligible patients were randomized to either sitagliptin 50 mg q.d. or matching placebo (1:1) for 12 weeks in a double-blind fashion, by use of a computer-generated allocation schedule.
On completion of the double-blind period, patients entered a 40-week, open-label treatment period. Patients who received sitagliptin during the double-blind period continued to do so in the open-label period (S/S group). Patients who received placebo in the double-blind period started to receive sitagliptin 50 mg q.d. on entry to the open-label period (P/S group). The dose of sitagliptin in the open-label period was up-titrated from 50 to 100 mg for patients meeting the protocol-specified criteria FPG ≥140 mg/dL from Week 16 to 36 or HbA1c ≥7.4 % from Week 24 to 36. After Week 40, the sitagliptin dose remained stable until completion of the study. Investigators were allowed to reduce the sitagliptin dose back to 50 mg q.d. if treatment with 100 mg q.d. was not regarded as well tolerated. The starting glinide dose was maintained during the entire 52-week period.
Meal tolerance tests (MTT) were performed at Weeks 0, 12, and 52 or at the visit for discontinuation, starting 30 min after administration of the study drug (at Week 0, patients were administered a dose of matching placebo) and just after administration of glinide. The test meal contained approximately 500 kcal (60 % carbohydrate, 15 % protein, and 25 % fat) and was to be consumed within 15 min. Blood samples were drawn before beginning the test meal, and 0.5, 1, and 2 h after beginning the test meal.
Study endpoints
The primary endpoint of this study was assessment of the safety and tolerability of addition of sitagliptin for 12 weeks and for up to 52 weeks.
Adverse experiences (AE) were monitored throughout the study up to 2 weeks post-treatment and were rated by investigators on the basis of their intensity and relationship to the study drug. Hypoglycemia was predefined as an AE of interest and was diagnosed by the investigators on the basis of patients’ reports. Patients were instructed to measure self-monitored blood glucose (SMBG) values if they had clinical symptoms of hypoglycemia (e.g., sweating, anxiety, palpitations, headache, blurred vision, and loss of consciousness). Patients were instructed to notify the study site if the fasting SMBG values at any point were <60 or >270 mg/dL. During the study, safety and tolerability were also assessed by physical examination, monitoring of vital signs, electrocardiogram (ECG), and safety laboratory tests that included hematology, serum chemistry, and urinalysis. All laboratory assays were performed at one central laboratory (Mitsubishi Chemical Medience Corporation, Tokyo, Japan).
Changes from baseline in HbA1c, FPG, and 2-h postmeal glucose (2-h PMG) at Week 12 were secondary efficacy endpoints. In the open-label period, HbA1c, FPG, and 2-h PMG were assessed as exploratory endpoints. In addition, fasting 1,5-anhydroglucitol (1,5-AG), fasting insulin, insulinogenic index [(insulinogenic index = ΔIRI μU/mL (30′)/ΔPG mg/dL (30′); ΔIRI (30′) is difference between serum insulin values prior to meal tolerance test and 30 min after initiating test, ΔPG (30′) is difference between serum glucose values prior to meal tolerance test and 30 min after initiating test], homeostasis model assessment of β-cell function (HOMA-β = 20 × fasting serum insulin (μU/mL)/[FPG (mg/dL)/18–3.5]), homeostasis model assessment of insulin resistance (HOMA-IR = fasting serum insulin (μU/mL) × [FPG (mg/dL)/405]), 2-h postmeal insulin, postmeal glucose area under the concentration-versus-time curve (AUC), insulin AUC, and C-peptide AUC were assessed as exploratory endpoints at Weeks 12 and 52. In this study, the proportions of patients with HbA1c values meeting the therapeutic objectives of <7.0 and <6.5 % in HbA1c (JDS) (corresponding to <7.4 and <6.9 % in HbA1c (NGSP), respectively), were also assessed at Week 12. Although these criteria were the therapeutic objectives in Japan at the time of study design, a new therapeutic objective (<7.0 %) was applied in Japan from June, 2013. Therefore, in this paper, the proportions of patients with HbA1c values meeting the new HbA1c objective of <7.0 % at Week 12 were analyzed post hoc.
Statistical analysis
Safety and tolerability
Safety and tolerability analysis was performed for the all patients-as-treated (APaT) population. For the double-blind period, this consisted of randomized patients who received at least one dose of double-blind study drug. The AE of hypoglycemia during the double-blind period was prespecified as an event of interest. P values and 95 % confidence intervals (CI) for the between-group difference in the percentage of patients with events were calculated by use of the Miettinen and Nurminen (M&N) method.
For long-term safety assessment, the patient population included all patients who received at least one dose of sitagliptin in the open-label part of the study (i.e., from Week 12 to 52). Safety data were summarized by treatment group.
Efficacy
Efficacy analysis was performed for the full analysis set (FAS) population, which included all randomized patients who had taken at least one dose of study drug and had a baseline measurement or at least one measurement post-randomization. To evaluate continuous outcome change from baseline at Week 12, a constrained longitudinal data analysis (cLDA) method [5] included terms for treatment, previous OHA status (yes/no), type of glinide, time, and the interaction terms between time and the other main terms with the restriction of a common baseline mean across treatment groups. Missing values were handled by use of the cLDA model, without explicit imputation. The least squares (LS) difference and the corresponding 95 % CI were estimated from the cLDA model. A P value <0.05 (two-sided) was regarded as statistically significant. The proportions of patients with HbA1c values meeting the HbA1c objectives of <8.0 and <7.0 % at Week 12 were analyzed post hoc by use of the M&N method, stratified by previous OHA status and type of glinide. The last observation carried forward (LOCF) method was used to determine whether a value met the objective when the HbA1c measurement at Week 12 was not available. For HbA1c, FPG, and 2-h PMG, a subgroup analysis of glinide type was conducted in the context of the primary efficacy analysis model within each subgroup. The subgroup analyses for FPG and 2-h PMG were post hoc.
For long-term efficacy assessment, summary statistics for efficacy endpoints were provided by treatment group (S/S or P/S) at each time point in which the endpoint was measured up to Week 52; missing values were not imputed. At Week 52, the within-group mean change from baseline (i.e., Week 0) for all efficacy endpoints were assessed by use of a paired-Student’s t test. For P/S, comparisons versus baseline (Week 0) were performed post hoc.
The effect of increasing the dose of sitagliptin to 100 mg q.d. was assessed post hoc. Among patients whose sitagliptin dose was increased and whose HbA1c value at the time of up-titration was ≥7.4 %, the proportion of patients with HbA1c values <7.0 % 12 weeks after up-titration was tabulated. In addition, for patients whose sitagliptin dose was increased and who completed the study, the proportion of patients with HbA1c values <7.0 % at Week 52 was also assessed. In these analyses, missing values were not imputed.
Results
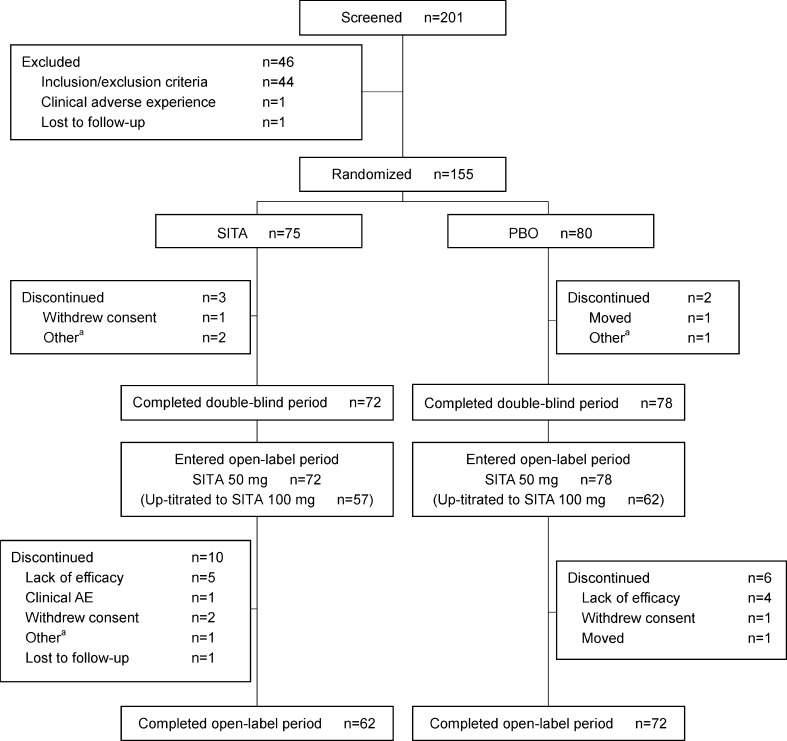
Two hundred and one patients were screened, of whom 155 were randomized to treatment (75 to sitagliptin and 80 to placebo) (Fig. 2). Demographic, anthropometric, and disease characteristics were usually balanced between the two treatment groups except for body weight. Mean body weight was slightly higher in the sitagliptin group than in the placebo group, but no statistically significant differences between the groups were found for demographic, anthropometric, or disease characteristics. (Table 1). Patients had mild to moderate hyperglycemia with a baseline mean HbA1c of 8.5 % and mean FPG of 171.6 mg/dL. The average duration of known T2DM was 8.6 years and the mean BMI was 25.2 kg/m2. For most of the patients, nateglinide dose was 270 mg/day and mitiglinide dose was 30 mg/day, which is consistent with the usual dose of these glinides approved in Japan.
Fig. 2.
Patient disposition. Patients in the P/S group received placebo during the double-blind period and switched to sitagliptin in the open-label period. Patients in the S/S group received sitagliptin in both periods
Table 1.
Baseline characteristics and demographics for randomized patients
| Characteristic | PBO (n = 80) | SITA 50 mg (n = 75) | All (n = 155) |
|---|---|---|---|
| Age (years) | 59.5 ± 10.3 | 60.2 ± 10.8 | 59.9 ± 10.5 |
| Gender, n (%) | |||
| Females | 21 (26.3) | 17 (22.7) | 38 (24.5) |
| Males | 59 (73.8) | 58 (77.3) | 117 (75.5) |
| Body weight, kg | 66.6 ± 12.8 | 69.1 ± 13.1 | 67.8 ± 13.0 |
| Body mass index, kg/m2 | 24.8 ± 3.5 | 25.6 ± 3.9 | 25.2 ± 3.7 |
| HbA1c (%) (range) | 8.4 ± 0.7 (7.3–10.2) | 8.6 ± 0.7 (7.2–10.5) | 8.5 ± 0.7 |
| Fasting plasma glucose, mg/dL | 167.1 ± 29.0 | 176.4 ± 39.1 | 171.6 ± 34.5 |
| 2-h postmeal glucose, mg/dL | 215.3 ± 54.7 | 223.3 ± 67.1 | 219.2 ± 61.0 |
| Duration of type 2 diabetes, years | 8.3 ± 4.9 | 9.0 ± 7.5 | 8.6 ± 6.3 |
| Type of glinide, n (%) | |||
| Nateglinide | 33 (41.3) | 28 (37.3) | 61 (39.4) |
| 180 mg/day | 1 (3.0) | 1 (3.6) | 2 (3.3) |
| 270 mg/day | 31 (93.9) | 25 (89.3) | 56 (91.8) |
| 360 mg/day | 1 (3.0) | 2 (7.1) | 3 (4.9) |
| Mitiglinide | 47 (58.8) | 47 (62.7) | 94 (60.6) |
| 15 mg/day | 4 (8.5) | 7 (14.9) | 11 (11.7) |
| 30 mg/day | 42 (89.4) | 39 (83.0) | 81 (86.2) |
| 45 mg/day | 1 (2.1) | 0 (0.0) | 1 (1.1) |
| 60 mg/day | 0 (0.0) | 1 (2.1) | 1 (1.1) |
Data are expressed as mean ± SD or n (%)
PBO placebo, SITA sitagliptin
One hundred fifty patients completed the double-blind period and entered the open-label period; of those, 134 patients subsequently completed the open-label period (Fig. 2).
Safety
Double-blind period (Weeks 0 through 12)
In the double-blind period (Weeks 0–12), the frequency of overall clinical AE reported was 26.7 % (20/75) in the sitagliptin group and 33.8 % (27/80) in the placebo group (Table 2). Drug-related clinical AE occurred for 5.3 % (4/75) in the sitagliptin group and 5.0 % (4/80) in the placebo group (Table 2). There were no notable differences between treatment groups in the frequency of overall clinical or drug-related clinical AE at Week 12.
Table 2.
Safety and tolerability results
| Weeks 0–12 (double-blind period)a | Weeks 12–52 (open-label period) | |||
|---|---|---|---|---|
| PBO, n = 80, n (%) | SITA, n = 75, n (%) | P/Sb, n = 78, n (%) | S/Sb, n c = 72, n (%) | |
| Patients [n (%)] who had one or more | ||||
| Clinical AE | 27 (33.8) | 20 (26.7) | 53 (67.9) | 38 (52.8) |
| Drug-relatedc clinical AE | 4 (5.0) | 4 (5.3) | 8 (10.3) | 3 (4.2) |
| Serious clinical AE | 1 (1.3) | 2 (2.7) | 3 (3.8) | 6 (8.3) |
| Serious drug-relatedc clinical AE | 0 | 0 | 0 | 0 |
| Patients [n (%)] who | ||||
| Discontinued due to clinical AE | 0 | 0 | 0 | 1 (1.4) |
| Discontinued due to serious clinical AE | 0 | 0 | 0 | 0 |
| Discontinued due to drug-relatedc clinical AE | 0 | 0 | 0 | 0 |
| Discontinued due to serious drug-relatedc clinical AE | 0 | 0 | 0 | 0 |
| Died | 0 | 0 | 0 | 0 |
| Patients [n (%)] who had prespecified AE | ||||
| Hypoglycemia | 1 (1.3) | 3 (4.0) | 8 (10.3) | 1 (1.4) |
| Patients [n (%)] who had one or more | ||||
| Laboratory AE | 1 (1.3) | 1 (1.3) | 3 (3.8) | 4 (5.6) |
| Drug-relatedc laboratory AE | 1 (1.3) | 1 (1.3) | 1 (1.3) | 0 |
| Serious laboratory AE | 0 | 0 | 0 | 0 |
| Patients [n (%)] who | ||||
| Discontinued due to laboratory AE | 0 | 0 | 0 | 0 |
| Discontinued due to drug-relatedc laboratory AE | 0 | 0 | 0 | 0 |
| Patients [n (%)] who hadd | ||||
| Constipation | 1 (1.3) | 0 | 4 (5.1) | 3 (4.2) |
| Diarrhea | 0 | 0 | 2 (2.6) | 4 (5.6) |
| Cystitis | 1 (1.3) | 1 (1.3) | 5 (6.4) | 0 |
| Nasopharyngitis | 10 (12.5) | 4 (5.3) | 16 (20.5) | 17 (23.6) |
| Hypoglycemia | 1 (1.3) | 3 (4.0) | 8 (10.3) | 1 (1.4) |
AE adverse experience, PBO placebo, SITA sitagliptin
a P > 0.05 for hypoglycemia between groups from Week 0 to Week 12 (M&N method)
bP/S = PBO during double-blind period and SITA 50 mg (n = 16) or SITA 100 mg (n = 62) in the open-label period; S/S = SITA 50 mg during double-blind period and SITA 50 mg (n = 15) or SITA 100 mg (n = 57) in the open-label period
cDetermined by the investigator to be possibly, probably, or definitely related to the study drug
dAE of a ≥5 % frequency in either the sitagliptin or placebo group in the double-blind period (from Week 0 to 12), or in either the P/S or S/S group in the open-label period (from Week 12 to 52)
In the double-blind period, the frequency of serious clinical AE was low and usually similar in both treatment groups (2.7 % (2/75) in the sitagliptin group and 1.3 % (1/80) in the placebo group; Table 2). No serious drug-related clinical AE were reported for patients in either group (Table 2). No patients discontinued because of a clinical AE (Table 2).
The proportion of patients with hypoglycemia AE, which was low for both groups, was numerically higher for sitagliptin than for placebo (4.0 vs. 1.3 %; 95 % CI −3.2, 10.1 %) for the between-group difference. (Table 2). All episodes of hypoglycemia were mild in intensity and none led to discontinuation of therapy.
During the double-blind period, the frequencies of laboratory AE and drug-related laboratory AE were 1.3 % (1/75) and 1.3 % (1/80) in the sitagliptin and placebo groups, respectively (Table 2). No specific laboratory AE occurred for two or more patients in either group.
The change in body weight from baseline at Week 12 was −0.1 kg (95 % CI (−0.6, 0.3)) in the sitagliptin group and −0.4 kg (95 % CI (−0.7, −0.1)) in the placebo group. Whereas the change in body weight in the placebo group was statistically significant, no significant change was observed in the sitagliptin group. However, the reductions in weight in the two groups were small and not regarded as clinically meaningful.
Open-label period (Weeks 12 through 52)
Consistent with the longer period of observation for a patient population with T2DM, one or more clinical AE were reported for over half of the patients in both the S/S and P/S groups during the open-label period (Weeks 12 to 52; 52.8 % (38/72) of patients in the S/S group and 67.9 % (53/78) of patients in the P/S group; Table 2). Clinical AE reported with a frequency ≥5 % in either the S/S or P/S group included nasopharyngitis, hypoglycemia, cystitis, diarrhea, and constipation (Table 2). Drug-related clinical AE were reported by 4.2 % (3/72) and 10.3 % (8/78) of patients in the S/S and P/S groups, respectively (Table 2). The nature of clinical AE through Weeks 12–52 in both the S/S and P/S groups was not notably different from that during Weeks 0–12 in the sitagliptin group.
Serious clinical AE were reported for six patients in the S/S group and three patients in the P/S group. No serious clinical AE were believed by the investigator to be related to the study drug; one in the S/S group and none in the P/S group led to discontinuation because of clinical AE. No deaths were reported during the open-label period (Table 2).
In the open-label period, the frequency of hypoglycemia AE was 1.4 % (1/72) in the S/S group and 10.3 % (8/78) in the P/S group (Table 2). All episodes of hypoglycemia in the open-label period were regarded as either mild or moderate in intensity, none required medical attention, and no patient was discontinued from the study because of hypoglycemia AE.
In the open-label period, laboratory AE were reported by 5.6 % (4/72) patients in the S/S group and 3.8 % (3/78) patients in the P/S group, and drug-related laboratory AE were not reported in the S/S group and were reported by 1.3 % (1/78) patients in the P/S group; none was serious, and no patients discontinued because of a laboratory AE or a drug-related laboratory AE (Table 2).
Mean changes in body weight from Week 0 to Week 52 were 0.0 kg (−0.7, 0.7) and 0.1 kg (−0.5, 0.6) for patients in the S/S group and the P/S group, respectively.
Efficacy
Double-blind period (Weeks 0 through 12)
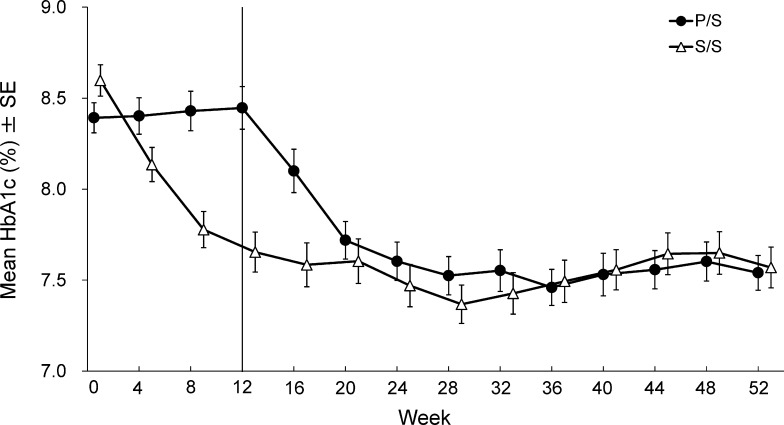
Table 3 shows fasting glycemic endpoints at Week 12 for patients treated with the addition of sitagliptin or placebo to ongoing glinide therapy. After 12 weeks, a significant reduction from baseline of HbA1c in the sitagliptin group compared with the placebo group was observed (LS mean difference (95 % CI): −1.1 % (−1.3, −0.8), P < 0.001, the first 12 weeks in Fig. 3). At Week 12, a greater proportion of patients in the sitagliptin group than in the placebo group had HbA1c values meeting the objectives (at the time of the study) of <7.4 % (50.7 vs. 10.0 %, P < 0.001), and <6.9 % (22.7 vs. 2.5 %, P < 0.001). Similarly, a greater proportion of patients in the sitagliptin group than in the placebo group had HbA1c values meeting the objectives (new objectives) of <8.0 % (76.0 vs. 43.8 %, P < 0.001), and <7.0 % (25.3 vs. 2.5 %, P < 0.001).
Table 3.
Fasting glycemic endpoints at Week 12 for Japanese patients with type 2 diabetes mellitus treated with sitagliptin or placebo added to glinide
| n | Week 0 mean (SD) | Week 12 mean (SD) | Change from Week 0 to Week 12 (LS mean (95 % CI)) | Between-group difference (LS mean (95 % CI)) | |
|---|---|---|---|---|---|
| HbA1c (%) | |||||
| Placebo | 78 | 8.4 (0.7) | 8.4 (1.0) | 0.4 (0.1, 0.6) | −1.1 (−1.3, −0.8)*** |
| Sitagliptin | 73 | 8.6 (0.8) | 7.7 (0.9) | −0.7 (−0.9, −0.5) | |
| Fasting plasma glucose (mg/dL) | |||||
| Placebo | 78 | 166.8 (29.3) | 167.9 (35.4) | 12.2 (1.6, 22.9) | −23.1 (−32.2, −13.9)*** |
| Sitagliptin | 73 | 177.4 (39.2) | 154.9 (38.6) | −10.8 (−20.7, −1.0) | |
| 1,5-anhydroglucitol (µg/mL) | |||||
| Placebo | 78 | 5.7 (4.5) | 5.7 (4.3) | −1.5 (−2.6, −0.3) | 5.4 (4.3, 6.4)*** |
| Sitagliptin | 73 | 5.7 (4.8) | 10.8 (7.0) | 3.9 (2.8, 5.0) | |
| Fasting insulin (µU/mL) | |||||
| Placebo | 78 | 8.2 (5.8) | 7.8 (5.9) | 0.1 (−1.1, 1.3) | 0.6 (−0.5, 1.7) |
| Sitagliptin | 73 | 8.1 (4.8) | 8.4 (5.2) | 0.7 (−0.4, 1.8) | |
| HOMA-IR | |||||
| Placebo | 78 | 3.4 (2.6) | 3.2 (2.5) | 0.1 (−0.7, 0.9) | −0.1 (−0.7, 0.6) |
| Sitagliptin | 73 | 3.5 (2.2) | 3.3 (2.9) | 0.0 (−0.7, 0.7) | |
| HOMA-β | |||||
| Placebo | 78 | 29.6 (21.3) | 28.9 (24.1) | 0.3 (−3.4, 4.1) | 7.9 (4.5, 11.3)*** |
| Sitagliptin | 73 | 28.1 (20.4) | 35.7 (23.1) | 8.3 (4.8, 11.7) | |
HOMA-IR homeostasis model assessment of insulin resistance, HOMA-β homeostasis model assessment of β-cell function, n number of patients who provided measurements at Weeks 0 and 12
*** P < 0.001
Fig. 3.
Time course of HbA1c for Japanese patients with type 2 diabetes mellitus treated with sitagliptin 50 mg q.d. or placebo added to glinide for the first 12 weeks and open-label sitagliptin 50 or 100 mg q.d. added to glinide for the subsequent 40 weeks. The data are presented as mean ± SE and results from the S/S and P/S treatment groups are indicated with triangles and circles, respectively
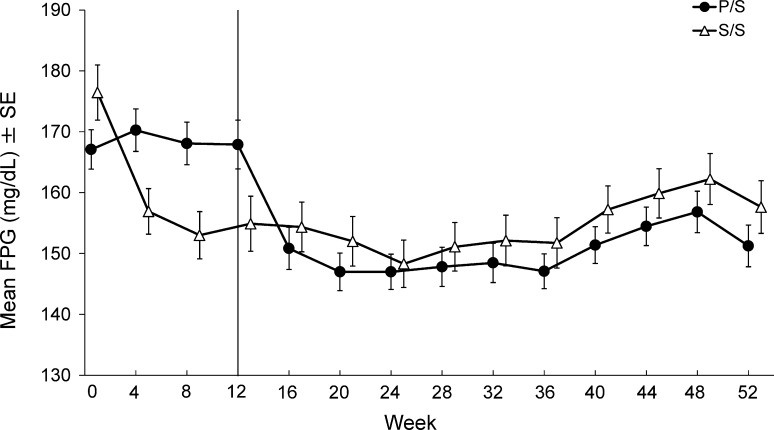
A significant reduction of FPG from baseline was observed in the sitagliptin group compared with the placebo group at Week 12 (LS mean difference (95 % CI): −23.1 mg/dL (−32.2, −13.9), P < 0.001, Table 3 and the first 12 weeks in Fig. 4).
Fig. 4.
Time course of fasting plasma glucose (FPG) results for Japanese patients with type 2 diabetes mellitus treated with sitagliptin 50 mg q.d. or placebo added to glinide for the first 12 weeks and open-label sitagliptin 50 or 100 mg q.d. added to glinide for the subsequent 40 weeks. The data are presented as mean ± SE. Results from the S/S and P/S treatment groups are indicated with triangles and circles, respectively
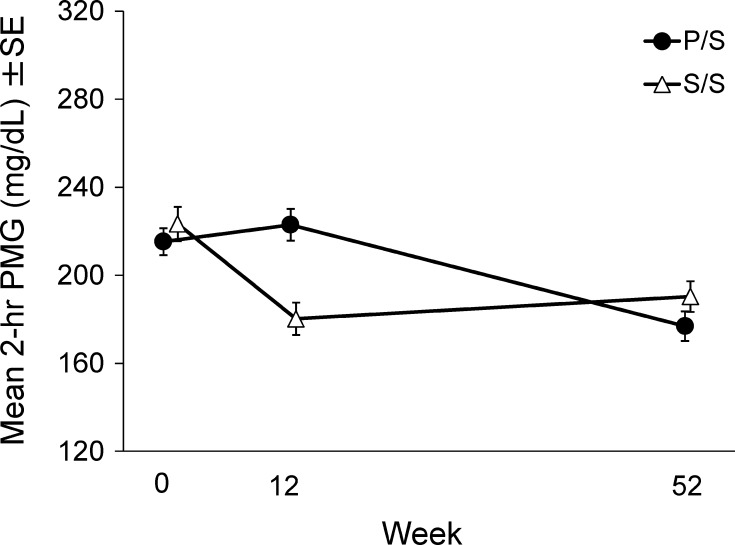
A significant mean reduction of 2-h PMG from baseline was also observed in the sitagliptin group compared with the placebo group (LS mean difference (95 % CI): −51.2 mg/dL (−67.4, −35.0), P < 0.001, Table 4; Fig. 5). Other exploratory efficacy results in the fasting state and results from MTT at Week 12 are shown in Tables 3 and 4, respectively. 1,5-AG in the sitagliptin group improved significantly compared with the placebo group. The treatment difference in HOMA-β was also statistically significant (Table 3). The reduction in total glucose AUC from baseline in the sitagliptin group was significantly greater than in the placebo group. The total C-peptide AUC decreased significantly from baseline in the placebo group whereas that in the sitagliptin group was maintained at the baseline level (Table 4).
Table 4.
Glycemic endpoints measured during meal-tolerance tests at Week 12 for Japanese patients with type 2 diabetes mellitus treated with sitagliptin or placebo added to glinide
| n | Week 0 mean (SD) | Week 12 mean (SD) | Change from Week 0 to Week 12 (LS mean (95 % CI)) | Between-group difference (LS mean (95 % CI)) | |
|---|---|---|---|---|---|
| 2-h postmeal glucose (mg/dL) | |||||
| Placebo | 78 | 214.8 (54.9) | 223.0 (63.6) | 18.7 (−0.4, 37.7) | −51.2 (−67.4, −35.0)*** |
| Sitagliptin | 73 | 224.3 (67.5) | 180.2 (63.0) | −32.5 (−50.1, −14.9) | |
| 2-h postmeal insulin (µU/mL) | |||||
| Placebo | 78 | 51.1 (34.2) | 46.3 (24.3) | −8.6 (−17.7, 0.4) | 2.1 (−4.5, 8.7) |
| Sitagliptin | 73 | 52.0 (34.2) | 48.6 (26.7) | −6.6 (−15.1, 2.0) | |
| Total glucose AUC (mg h/dL) | |||||
| Placebo | 78 | 432.8 (88.9) | 447.2 (100.2) | 42.0 (13.6, 70.4) | −80.6 (−105.2,-56.1)*** |
| Sitagliptin | 73 | 440.6 (100.7) | 376.9 (101.5) | −38.6 (−64.8, −12.5) | |
| Total insulin AUC (µU h/mL) | |||||
| Placebo | 78 | 98.1 (58.0) | 90.5 (47.7) | −10.6 (−20.3, −0.9) | 3.5 (−4.2, 11.2) |
| Sitagliptin | 73 | 96.4 (54.0) | 92.8 (45.3) | −7.1 (−16.2, 1.9) | |
| Total C-peptide AUC (ng h/mL) | |||||
| Placebo | 78 | 7.8 (2.6) | 7.3 (2.3) | −0.8 (−1.3, −0.3) | 0.6 (0.2, 1.0)** |
| Sitagliptin | 73 | 7.8 (2.8) | 7.8 (2.5) | −0.2 (−0.7,0.2) | |
| Insulinogenic index | |||||
| Placebo | 78 | 0.4 (3.5) | 0.7 (0.8) | 0.1 (−1.7, 1.9) | 0.3 (−1.3, 1.8) |
| Sitagliptin | 72 | 1.3 (1.9) | 1.2 (6.7) | 0.4 (−1.3, 2.1) | |
AUC total area under the concentration-versus-time curve, n number of patients who provided measurements at Weeks 0 and 12
*** P < 0.001, ** P < 0.01
Fig. 5.
Time course of meal tolerance results for Japanese patients with type 2 diabetes mellitus treated with sitagliptin 50 mg q.d. or placebo added to glinide for the first 12 weeks and open-label sitagliptin 50 or 100 mg q.d. added to glinide for the subsequent 40 weeks. The data are presented as mean ± SE
Consistent results were obtained in analyses of changes from baseline of other measures of efficacy between the treatment groups at Week 12 that were supportive of the secondary findings.
The changes from baseline and between-group differences for HbA1c, FPG, and 2-h PMG at Week 12 in the subgroups of patients on the basis of glinide type (Table S1) were consistent with those for the entire cohort (Tables 3, 4).
Open-label period (Weeks 12 through 52)
Significant reductions in HbA1c from baseline were observed during the open-label period in the S/S and P/S groups (P < 0.001 for all time points; Table S2 and Fig. 3). The proportion of patients with HbA1c values at Week 52 meeting the therapeutic objectives of <8.0 and <7.0 % was 71.0 and 35.5 %, respectively, in the S/S group, and 69.4 and 33.3 %, respectively, in the P/S group. The FPG and 2-h PMG were also significantly reduced from baseline (Week 0) at all time points in both the S/S and P/S groups (P < 0.01 for all time points, Fig. 4 and after 52 weeks; Fig. 5).
Results from measurement of other exploratory fasting and postmeal efficacy data at Week 52 are shown in Table S2 and Table S3, respectively. With regard to the major data, 1,5-AG was significantly higher than baseline at Week 52 in both the S/S and P/S groups (all P < 0.001). The total glucose AUC in MTT was significantly reduced from baseline at Week 52 in both the S/S and P/S groups (all P < 0.001). The decrease in total insulin AUC from baseline at Week 52 was statistically significant in the S/S group (P = 0.018) but not significant in the P/S group (P = 0.980).
To provide additional glycemic efficacy data, dose titration of sitagliptin to 100 mg was allowed after Week 16 for patients meeting the predefined criteria of glycemic data. The sitagliptin dose was up-titrated for 57 out of the 72 patients in the S/S group and 62 out of the 78 patients in the P/S group. At 12 weeks post-escalation of HbA1c values were obtained from a total of 115 patients (S/S group, 54 patients; P/S group, 61 patients). Overall, 32.4 % (35/108) patients with a HbA1c ≥7.4 % before up-titration achieved HbA1c <7.0 % (treatment target achievement rate) at 12 weeks after up-titration. Additionally, among all patients whose dose was up-titrated, 72 patients completed the study; 22.2 % (16/72) of these patients had an HbA1c <7.0 % at Week 52.
Discussion
Addition of sitagliptin to glinide monotherapy was generally well tolerated by Japanese patients with T2DM receiving glinide monotherapy during both the double-blind (12-week) and open-label (52-week) periods. There were no notable differences in the nature of clinical AE or drug-related clinical AE during long-term administration of sitagliptin (Week 52) compared with short-term use of the drug (Week 12). The frequency of reported hypoglycemia AE in the sitagliptin group (4.0 %) relative to the placebo group (1.3 %) was low and was not notably different at Week 12. In the sitagliptin and placebo groups, all reported hypoglycemia AE were mild in intensity and none led to discontinuation of therapy, which is consistent with the glucose-dependent increase in insulin secretion and the suppression of glucagon release with incretin-based therapy [6]. An undesired side effect of some OHA (e.g., peroxisome proliferator-activated receptor (PPAR)γ agonists and SUs) is increased body weight [7]. In this study, no clinically meaningful increases in weight were noted for sitagliptin-treated patients.
Addition of sitagliptin for 12 weeks resulted in significant reductions of HbA1c, FPG, and 2-h PMG compared with placebo for Japanese patients with T2DM whose glycemic control was not adequate with glinide monotherapy. The LS mean difference of change of HbA1c from baseline was −1.1 %, and the proportion of patients achieving the objectives of HbA1c <8.0 and <7.0 % after sitagliptin treatment was greater than for placebo. Over the 52-week study period, which included the option for up-titration of sitagliptin, the efficacy data (HbA1c, FPG, and 2-h PMG) remained stable, although limited to the conditions within the study protocol. In addition, the observed reductions of HbA1c from baseline of 0.8 and 1.0 % are clinically meaningful. Of the 108 patients who did not achieve the objective of HbA1c ≥7.4 % with sitagliptin 50 mg, 35 patients (32.4 %) achieved the objective of HbA1c ≥7.0 % after up-titration to sitagliptin 100 mg. This result is consistent with results from previous studies of sitagliptin as monotherapy [8] and as add-on to metformin [9], pioglitazone [10], and glimepiride [11], and α-glucosidase inhibitor [12] among Japanese patients.
Furthermore, an improvement in HOMA-β was observed in the sitagliptin group compared with the placebo group, suggesting that addition of sitagliptin to glinide therapy may improve the function of pancreatic β-cells among Japanese patients. This result was consistent with those from previous multinational studies showing that treatment with sitagliptin as monotherapy or as add-on therapy improved HOMA-β [13, 14]. In addition, improvement in 1,5-anhydroglucitol in the sitagliptin group compared with the placebo group was demonstrated. Because 1,5-anhydroglucitol is a more sensitive indicator of changes in glucose levels than HbA1c and is useful in conjunction with HbA1c to assess glycemic control, sitagliptin add-on to glinide monotherapy may improve postprandial hyperglycemia, by reducing fluctuation of glucose levels, which is a risk factor for macrocardiovascular disease among T2DM patients [15–17].
Glinides, widely prescribed in Japan for treatment of patients with T2DM, stimulate insulin secretion by binding to SU receptors on pancreatic β-cells, exerting antihyperglycemic effects within a short time after dosing, and improving postprandial hyperglycemia. Japanese patients with T2DM may have a lower capacity to secrete insulin than patients of European ancestry with obesity, and characteristically tend to have postprandial hyperglycemia. Thus, antihyperglycemic agents that target postprandial hyperglycemia, for example glinides or α-glucosidase inhibitors (α-GIs) are preferable for Japanese patients. However, insulin secretagogues (glinides or SUs) are known to be associated with a risk of hypoglycemia. Considering the clinical use of anti-diabetic drugs, the efficacy and safety results obtained in this study suggest that adding sitagliptin to glinide might be more appropriate for the patients with inadequate glycemic control on glinide monotherapy than increasing the dose of glinide or switching to SU because of a lower risk of hypoglycemia and efficacy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank M. Kawashima, D. Yanagida, M. Ohkubo, H. Shuno, M. Kondo, H. Suna, H. Maekawa, T. Yoshida, T. Kuramoto, S. Shiono, Y. Hatori, K. Nagasawa, Y. Kamehara, N. Inaba, and M. Odani (ONO Pharmaceutical Co., Ltd., Japan) for their assistance in writing and preparing this paper for submission and publication.
Appendix 1
MK-0431 P282/ONO-5435-17 Primary Investigator list: Fuminobu Okuguchi (Okuguchi Clinic of Internal Medicine), Naoki Itabashi (Itabashi Clinic), Tomoyuki Arisaka (Arisaka Clinic), Takashi Ishii (Ishii Hospital), Yuichiro Makita (Koshigaya Municipal Hospital), Yoshihiko Suzuki (HDC Atlas Clinic), Yukiko Onishi (The Institute for Adult Diseases, Asahi Life Foundation), Masaharu Morohoshi (Sanraku Hospital, Lifestyle Diseases Clinic), Arihiro Kiyosue (Tokyo-Eki Center-Building Clinic), Masayo Yamada (Yokohama Sakae Kyosai Hospital), Taro Asakura (Tsuruma Kaneshiro Diabetes Clinic), Shin-ichiro Matsumura (Yokohama Cardiopulmonary Clinic), Ichitaro Takada (Takada Naika Clinic), Toshio Kiguchi (Kiguchi Clinic), Masaaki Ohashi (Saku Central Hospital), Akira Yamauchi (Suruga Clinic), Hideki Okamoto (Meitetsu Hospital), Genichi Watanabe (Watanabe Clinic), Mikihiro Nakayama (Nakayama Clinic), Masako Deguchi (Japanese Red Cross Kyoto Daini Hospital), Yasuro Kumeda (Minamiosaka Hospital), Soichi Kurioka (Komatsu Hospital), Takashi Ikawa (Osaka Rehabilitation Hospital), Kazushige Ejiri (Joyo Ejiri Hospital), Hidenori Ohno (Oofuji Clinic), Atsuyoshi Yuhara (Yuhara Clinic), Akira Matsutani (Shunan City Shin-nanyo Hospital), Makoto Kunisaki (Kunisaki Makoto Clinic), Shoichi Akazawa (Shinkoga Clinic), and Noriko Nakamura (Primula Clinic).
Conflict of interest
Authors Tajima and Kadowaki were coordinating investigators for this study. Author Odawara was the medical advisor for this study. Author Tajima has acted as advisory panel for Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd. and Sanofi K.K.; as consultant for MSD K.K. and Ono Pharmaceutical Co., Ltd.; as a speaker for Astellas Pharma Inc., Daiichi-Sankyo, Co., Ltd., Eli Lilly Japan K.K., Kissei Pharmaceutical Co., Ltd., MSD K.K., Boehringer Ingelheim Japan Inc., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd. and Dainippon Sumitomo Pharma Co., Ltd. Author Kadowaki has acted as advisory panel for AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., Sanofi K.K., and Takeda Pharmaceutical Co., Ltd.; as consultant for MSD K.K., and Ono Pharmaceutical Co., Ltd.; as a speaker for Astellas Pharma, Inc., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly Japan K.K., Kowa Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Mitsubishi Tanabe Pharma Corp., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Sanofi K. K., Sanwa Kagaku Kenkyusho Co., Ltd., Taisho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; obtained research support from Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., MSD K.K., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., and Takeda Pharmaceutical Co., Ltd.; and has been involved in research units endowed by MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co., Ltd., and Terumo Corp. Author Odawara has acted as consultant for MSD K.K. and Ono Pharmaceutical Co., Ltd.; as a speaker for MSD K.K. and Ono Pharmaceutical Co., Ltd.. Authors Minamide, Seki, and Oki are full-time employees of ONO Pharmaceutical Co., Ltd., Japan and authors Nagayasu is a full-time employee of MSD K.K.and Arjona Ferreira was a full-time employee of Merck Sharp & Dohme Corp. at the time of the study, and might potentially own stock and/or hold stock options in the company. No other potential conflicts of interest relevant to this article were reported. This study was sponsored by MSD K.K., Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, the manufacturer of sitagliptin, and by Ono Pharmaceutical Co. Ltd., Japan.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revision. Informed consent or substitute for it was obtained from all patients included in the study.
Footnotes
This study is registered with ClinicalTrials.gov: NCT01517321, “MK-0431/ONO-5435 Phase III Clinical Trial-Rapid-acting Insulin Secretagogue Add-on Study in Patients With Type 2 Diabetes”, http://www.clinicaltrials.gov/ct2/show/NCT01517321?term=NCT01517321&rank=1.
References
- 1.Idris I, Donnelly R. Dipeptidyl peptidase-IV inhibitors: a major new class of oral antidiabetic drug. Diabetes Obes Metab. 2007;9:153–165. doi: 10.1111/j.1463-1326.2007.00705.x. [DOI] [PubMed] [Google Scholar]
- 2.Prato SD, Pulizzi N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metab Clin Exp. 2006;55(Suppl 1):S20–S27. doi: 10.1016/j.metabol.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004;66S:S37–S43. doi: 10.1016/j.diabres.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H, Committee on the standardization of diabetes mellitus related laboratory testing of Japan Diabetes Society International clinical harmonization of glycated hemoglobin in Japan: from Japan diabetes society to national glycohemoglobin standardization program values. J Diabetes Investig. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang KY, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhya Indian J Stat. 2000;62:134–148. [Google Scholar]
- 6.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 7.Pi-Sunyer FX. The effects of pharmacologic agents for type 2 diabetes mellitus on body weight. Postgrad Med. 2008;120:5–17. doi: 10.3810/pgm.2008.07.1785. [DOI] [PubMed] [Google Scholar]
- 8.Nonaka K, Kakikawa T, Sato A, Okuyama K, Fujimoto G, Kato N, Suzuki H, Hirayama Y, Ahmed T, Davies MJ, Peter PS. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79:291–298. doi: 10.1016/j.diabres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki T, Tajima N, Odawara M, Nishii M, Taniguchi T, Arjona Ferreira JC. Addition of sitagliptin to ongoing metformin monotherapy improves glycemic control in Japanese patients with type 2 diabetes over 52 weeks. J Diabetes Investig. 2013;4:174–181. doi: 10.1111/jdi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashiwagi A, Kadowaki T, Tajima N, Nonaka K, Taniguchi T, Nishii M, Arjona Ferreira JC, Amatruda JM. Sitagliptin added to treatment with ongoing pioglitazone for up to 52 weeks improves glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig. 2011;2:381–390. doi: 10.1111/j.2040-1124.2011.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tajima N, Kadowaki T, Odawara M, Nishii M, Taniguchi T, Arjona Ferreira JC. Addition of sitagliptin to ongoing glimepiride therapy in Japanese patients with type 2 diabetes over 52 weeks leads to improved glycemic control. Diabetol Int. 2011;2:32–44. doi: 10.1007/s13340-011-0022-2. [DOI] [Google Scholar]
- 12.Tajima N, Kadowaki T, Okamoto T, Sato A, Okuyama K, Minamide T, Arjona Ferreira JC. Sitagliptin added to voglibose monotherapy improves glycemic control in patients with type 2 diabetes. J Diabetes Investig. 2013;4:595–604. doi: 10.1111/jdi.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, For the Sitagliptin Study 035 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–745. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 14.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, For the Sitagliptin Study 021 Group Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 15.The DECODE Study Group. On behalf of the European Diabetes Epidemiology Group Glucose tolerance and cardiovascular mortality. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 16.Nakagami T, Qiao Q, Tuomilehto J, Balkau B, Tajima N, Hu G, Borch-Johnsen K. Screen-detected diabetes, hypertension and hypercholesterolemia as predictors of cardiovascular mortality in five populations of Asian origin: the DECODA study. Eur J Cardiovasc Prev Rehabil. 2005;13:555–561. doi: 10.1097/01.hjr.0000183916.28354.69. [DOI] [PubMed] [Google Scholar]
- 17.Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S. 1,5-Anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderatory controlled patients with diabetes. Diabetes Care. 2006;29:1214–1219. doi: 10.2337/dc06-1910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.