Abstract
A randomized, double-blind, placebo-controlled trial was performed to investigate the effect of cinnamon supplementation on glucose, triglyceride (TG), and high density lipoprotein-cholesterol (HDL-C) levels, TG/HDL-C ratio, blood pressure (BP), and estimated glomerular filtration rate (eGFR) for ninety-nine type 2 diabetes mellitus (T2DM) patients. Forty nine (49) patients with T2DM were in the cinnamon group whereas 50 were in the placebo group. All participants received either a cinnamon or placebo capsule daily for the 60-day study period. At the end of the study, median glucose, TG, TG/HDL-C ratio, and BP were significantly decreased (p < 0.005) whereas HDL-C and eGFR levels were significantly increased (p < 0.005) in the cinnamon supplementation group. Interestingly, the study also revealed reduction of HbA1c levels after the 60-day study period. In the placebo group, glucose, TG levels tended to increase and HDL-C levels tended to decrease whereas systolic and diastolic BP, HbA1c, and BUN CT were significantly increased and eGFR was significantly reduced (p < 0.005). Cinnamon supplementation reduced plasma glucose, HbA1c, triglyceride, TG/HDL-C ratio, and BP and increased HDL-C levels and eGFR in subjects with T2DM. Cinnamon supplementation may be beneficial for those with T2DM to prevent and control diabetic complications.
Keywords: Type 2 diabetes mellitus, Cinnamon, Glucose, TG/HDL-C ratio, Blood pressure, Estimated glomerular filtration rate
Introduction
Type 2 diabetes mellitus (T2DM), a metabolic disorder with multiple etiology, is one of the leading causes of morbidity and mortality worldwide [1, 2]. The prevalence of T2DM is increasing and is predicted to occur in approximately 5 % of the global population by 2025 [3]. The disorder is characterized by insulin resistance, hyperinsulinemia, β-cell dysfunction, and subsequent β-cell failure [4]; T2DM is associated with both microvascular (including retinopathy, nephropathy, and neuropathy) and macrovascular (cardiovascular diseases) complications [5–7]. The incidence of cardiovascular diseases is two to fourfold greater among people with T2DM [8]. Although the causes of T2DM and cardiovascular diseases are multifactorial, diet definitely affects the incidence and severity of these diseases.
Strategies for reducing blood glucose, lipid levels, and blood pressure (BP) include pharmacologic treatment, lifestyle modification, and dietary changes. However, an increasing number of literature reports focus on use of natural supplements for treatment of diabetes. Cinnamon (Cinnamomum cassia) has become a natural product of interest because it has been hypothesized to provide health benefits, for example the ability to reduce blood glucose and levels of serum lipids. It has been suggested that the effect of cinnamon on blood glucose can be attributed to its active component cinnamaldehyde [9]. Cinnamon has been of research interest for patients with diabetes since the 1990s, when peroxisome proliferator-activated receptors (PPARs) were recognized as a possible therapeutic target for diabetes and dyslipidemia [10]. Cinnamon extracts have been shown to increase in-vitro glucose uptake and glycogen synthesis and to increase phosphorylation of the insulin receptor. Cinnamon extracts are also likely to aid initiation of the insulin cascade system [11, 12]. Insulin is also crucially important in lipid metabolism [12]. We postulated that consumption of cinnamon might improve plasma glucose, blood lipid levels, estimated glomerular filtration rate (eGFR), and blood pressure for patients with T2DM.
Materials and methods
Subjects
The study was a randomized, double-blind, placebo-controlled trial. All patients were recruited from a volunteer list of patients who had previously attended the Diabetes Care Clinic activities of Ladyao Hospital, Nakonsawan Province, Thailand (December 2012–December 2013) and had been diagnosed with T2DM for over 5 years. All were ≥40 years old, not on insulin therapy at randomization, ambulatory outpatients with no acute cardiovascular or neurologic event 6 months before the study, no history of active tobacco smoking, and no oral intake of vitamin supplements in the last 60 days. Medical treatment for diabetes had to be stable for the last 3 months, with HbA1c ≤9 % (≤74.9 mmol/mol). All participants received at least two sessions of diet and lifestyle advice during the study period, as for standard care. Medications were not altered for either group during the study period. Exclusion criteria were cardiovascular, neuromuscular, arthritic, pulmonary, or other debilitating diseases, and those who currently smoked, had poor glycemic control, or were on insulin treatment. Patients were randomized, by use of a table of random numbers with blocks of four, to receive cinnamon or placebo. Treatment was concealed by placing the patients’ assignments in sequence in sealed opaque envelopes that were drawn in ascending consecutive order. Treatment was kept strictly confidential, so researchers and subjects were unaware, and revealed at the end of the study. Ninety-nine T2DM patients participated in the study. The duration of diabetes was similar for both groups: 9.04 ± 4.76 years for the cinnamon group and 8.14 ± 2.75 years for the placebo group.
Identical-looking capsules of cinnamon (500 mg) and placebo were purchased from the Government Pharmaceutical Organization, Thailand. After baseline assessment for eligibility, each subject was told to take one capsule immediately after meals three times a day (as suggested in a leaflet), for a total of 60 days. Each subject met a registered dietician at baseline and in the week before the end of the study to assess general daily dietary intake and cinnamon. Four 24 h recalls were conducted for each subject, especially elderly subjects. The study protocol was approved by the Ethics Committee of the Naresuan University (55-03-01-0020). All subjects provided written informed consent before participating and providing a blood sample for their health check in this study.
Anthropometric data and blood pressure measurement
Physical examination included anthropometry and blood pressure measurement. Height, weight, and blood pressure (BP) were measured and body mass index (BMI) was calculated. Waist circumference (WC) was measured at the midpoint between the rib cage and the top of lateral border of iliac crest during minimum respiration. BP was measured after the participants had been seated and rested for 5 min, as the mean value of at least two measurements for these participants on the same day with calibrated desktop sphygmomanometers. Hypertension was defined as an average BP ≥140/90 mmHg or if the participant was taking antihypertensive medications or had been diagnosed with HT.
Blood sample collection and biochemical determination
Venous blood samples were collected without stasis after a 12 h fast and 30 min rest in a supine position. Blood specimens were processed and assayed in the clinical laboratory of Ladyao Hospital, Nakornsawan, Thailand. Determination of fasting plasma glucose (Glu), total cholesterol (TC), triglycerides (TG), and high density lipoprotein cholesterol (HDL-C) was based on enzymatic techniques; determination of hemoglobin A1C (HbA1c) was based on the turbidimetric inhibition immunoassay (TINIA) on hemolyzed whole blood (standardized according to the International Federation of Clinical Chemistry) by use of an Hitachi 912 autoanalyzer (Roche Diagnostic, Switzerland) and low-density lipoprotein cholesterol (LDL-C) was calculated by use of Friedewald’s equation, which is valid for TG values less than or equal to 400 mg/dl.
eGFR was calculated by use of the Cockroft–Gault formula which incorporates age, body weight, and sex [13]. The formula is: eGFR = [(140 − age) × weight (kg) × constant]/[serum creatinine (µmol/L)] where 1.23 and 1.04 are constants for men and women, respectively. Serum creatinine was measured in µmol/L. eGFR was classified by stages, in accordance with the Kidney Disease Outcome Quality Initiative (KDOQI) [14] criteria, as follows: Stage I: normal eGFR (≥90 ml/min/1.73 m2); Stage II: mild eGFR (60–89 ml/min/1.73 m2); Stage III: moderate eGFR (30–59 ml/min/1.73 m2); Stage IV: severe eGFR (<30 ml/min/1.73 m2); and Stage V: end-stage renal disease: eGFR (<15 ml/min/1.73 m2). eGFR lower than 60 ml/min/1.73 m2 (moderate eGFR) was defined as chronic kidney disease (CKD).
Statistical analysis
Categorical data are reported as percentages. Continuous data are reported as mean ± standard deviation (SD), or median and interquartile range for non-normally distributed data, and tested by use of the Kolmogorov–Smirnov test. Differences between these clinical data for the same subjects were analyzed by use of the Friedman Test and were repeated with Wilcoxon signed-rank tests (2-tailed non-parametric tests); these tests were used to assess differences between baseline and after receiving supplementation in both groups (intragroup) for 60 days. The Mann–Whitney U-test was used to detect differences for the intergroup. p-values less than 0.05 were regarded as statistically significant. All analysis was performed by use of SPSS version 13.0 (SPSS, Chicago, IL, USA).
Results
No adverse effect was observed for forty-nine (90.7 %) of the group that took the cinnamon supplement continuously for the 60-day period. During the intervention period, three participants dropped out of the treatment group, and two (9.3 %) were later excluded from the study because they stopped taking their antidiabetic medication during the cinnamon intervention period. Fifty of the patients in the placebo group participated until the end of the study; three of the participants in the placebo group (5.7 %) dropped out of the study because they moved to work with their families in other provinces.
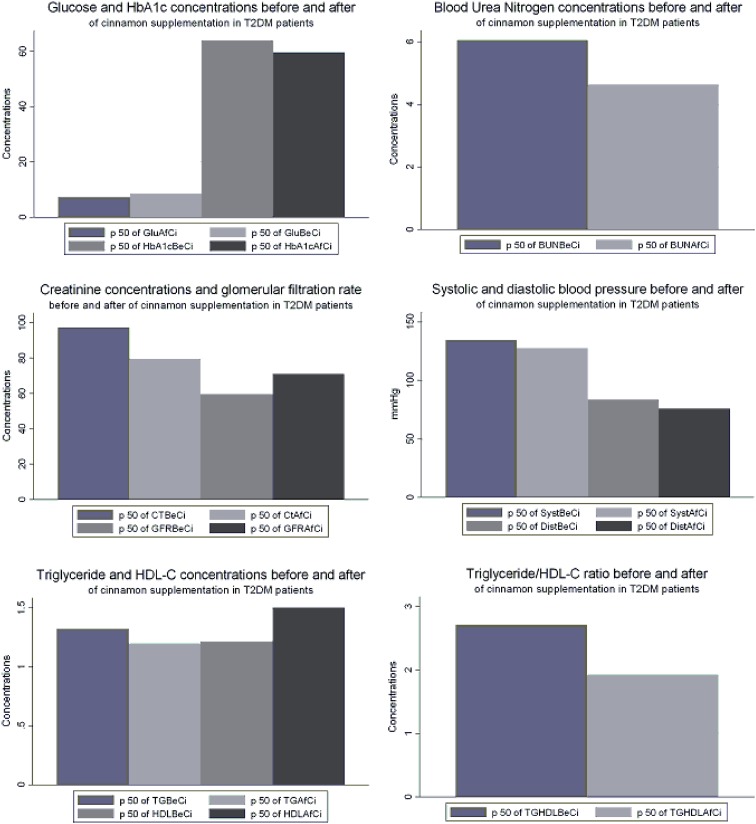
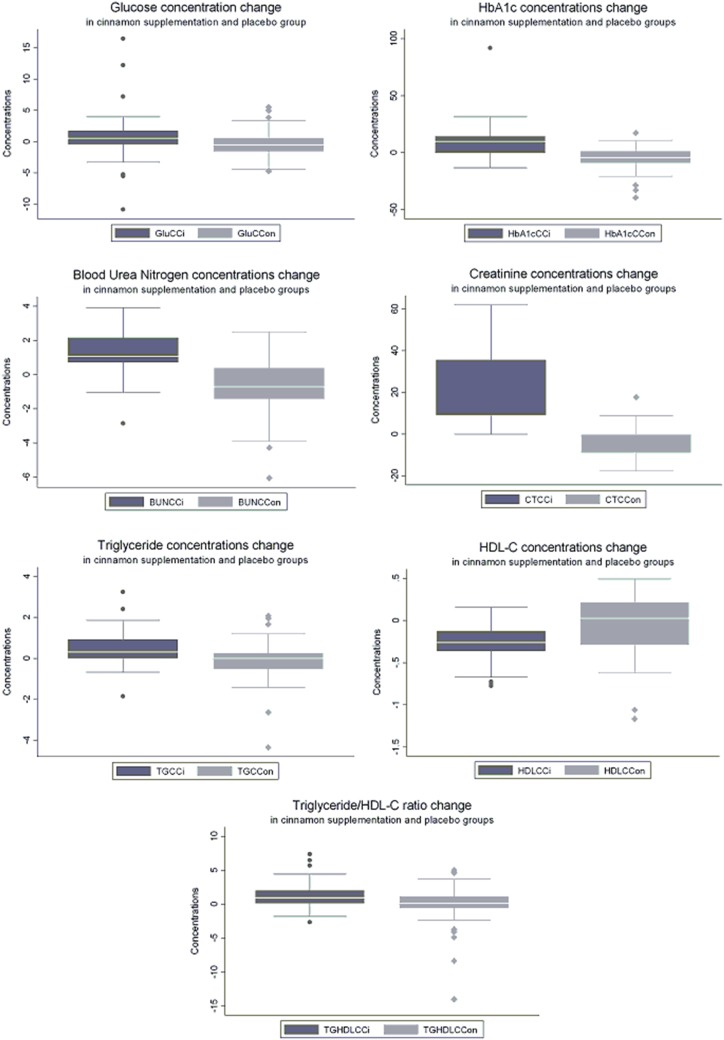
The data revealed significant differences between the groups (p < 0.05) by use of the Friedman Test. Further comparison was made between the baseline characteristics of the cinnamon and placebo groups; the results are shown in Table 1. At baseline both HDL-C and TG/HDL-C ratio were better in the placebo group than in the cinnamon supplementation group. At the end of the study, BMI and WC were not significantly different from the baseline. Supplementation of the daily diet with 1.5 g cinnamon led to statistically significant decreases in plasma glucose, TG, and TG/HDL-C ratio, and increases in serum HDL-C levels (p < 0.05) after 60 days (Wilcoxon signed-rank tests; Table 2; Fig. 1). Surprisingly, the study results also revealed a statistically significant decrease in HbA1c concentration, although our study period was only 60 days. This study also found that BP, both systolic and diastolic, was reduced and eGFR was increased in the cinnamon supplementation group, whereas BP had significantly increased and eGFR had significantly decreased in the placebo group at the end of the study. Values after 60 days had decreased significantly; increases ranged from 6.73 to 28.8 % in the cinnamon supplementation group. There were no significant changes in serum total cholesterol or LDL-C levels in the groups consuming cinnamon. It has been suggested that long-term consumption of cinnamon could be more beneficial than shorter use for reduction of triglyceride levels among people with type 2 diabetes [15]. Responses to different doses of cinnamon were not tested in this study. In the placebo group, glucose and TG/HDL-C ratio values were not significantly different, triglyceride levels tended to increase, HDL-C levels tend to decrease, and total cholesterol and HbA1c significantly increased at the end of the study, as shown in Table 3. All effects on clinical variables in the study are shown in Table 4 and Fig. 2.
Table 1.
Baseline characteristics of treatment and placebo groups
| Treatment group (n = 49) | Placebo group (n = 50) | p value* | |
|---|---|---|---|
| Age (years) | 57.2 ± 1.1 | 56.9 ± 1.2 | 0.939 |
| Sex | |||
| Male | 16 (32.7 %) | 16 (32 %) | – |
| Female | 33 (67.3 %) | 34 (68 %) | – |
| BMI (kg/m2) | 24.7 (22.1–27.4) | 24.7 (22.5–27.4) | 0.086 |
| WC (cm) | 90.0 (84.5–96.5) | 89.0 (85.0–96.0) | 0.852 |
| Systolic BP (mmHg) | 134.5 (126.8–144.0) | 135.0 (127.0–142.0) | 0.162 |
| Diastolic BP (mmHg) | 83.5 (79.0–91.3) | 80.0 (73.5–90.0) | 0.131 |
| Baseline glucose (mmol/l) | 8.53 (7.26–10.56) | 7.59 (6.38–9.19) | 0.887 |
| Baseline HbA1c (mmol/mol) | 63.9 (57.4–80.9) | 60.7 (56.3–68.3) | 0.150 |
| BUN (mmol/l) | 6.06 (4.99–7.13) | 4.64 (3.57–6.07) | 0.572 |
| CT(µmol/l) | 97.24 (79.56–114.92) | 79.56 (70.72–88.40) | 0.128 |
| eGFR (ml/min/1.73 m2) | 59.61 (47.70–80.99) | 61.10 (58.57–74.12) | 0.783 |
| Baseline lipid profiles | |||
| Total cholesterol (mmol/l) | 4.26 (3.56–4.77) | 4.39 (3.79–5.02) | 0.207 |
| Triglycerides (mmol/l) | 1.32 (1.02–2.27) | 1.40 (1.01–2.28) | 0.934 |
| HDL-C (mmol/l) | 1.22 (1.01–1.44) | 1.42 (1.15–1.67) | 0.019 |
| LDL-C (mmol/l) | 2.19 (1.63–2.61) | 2.32 (1.86–2.83) | 0.512 |
| TG/HDL-C ratio | 2.71 (1.57–5.25) | 2.39 (1.45–4.42) | 0.031 |
| Patients who had diabetes medications stopped during the study period (n) | 3 | 2 | – |
| Number of capsules remaining (capsule count after 12 weeks) | – | 1 | – |
* Mann–Whitney U test
Table 2.
Effect of cinnamon on glucose, triglycerides, HDL-C, and HbA1c for T2DM patients
| Baseline (n = 49) | Final treatment (n = 49) | Difference | p value* | |
|---|---|---|---|---|
| Systolic BP (mmHg) | 134.5 (126.8–144.0) | 127.5 (119.3–134.0) | 8.5 (5.0 to 13.3) | <0.001 |
| Diastolic BP (mmHg) | 83.5 (79.0–91.3) | 76.0 (70.0–81.3) | 8.0 (5.0 to 12.0) | <0.001 |
| Glucose (mmol/l) | 8.53 (7.26–10.56) | 7.32 (6.52–9.85) | 0.55 (0.47 to 1.82) | 0.026 |
| BUN (mmol/l) | 6.06 (4.99–7.13) | 4.64 (3.92–6.06) | 1.07 (0.53 to 2.14) | 0.020 |
| CT (µmol/l) | 97.24 (79.56–114.92) | 79.56 (70.72–88.4) | 8.84 (8.84 to 0.40) | 0.003 |
| eGFR (ml/min/1.73 m2) | 59.61 (49.70–80.99) | 70.96 (64.12–92.97) | −6.85 (−25.98 to 9.36) | <0.001 |
| Total cholesterol (mmol/l) | 4.26 (3.56–4.77) | 4.28 (3.73–5.03) | −0.15 (−0.89 to 0.31) | 0.120 |
| Triglyceride (mmol/l) | 1.32 (1.02–2.27) | 1.20 (0.79–1.70) | 0.32 (−0.05 to 0.95) | 0.001 |
| HDL-C (mmol/l) | 1.22 (1.01–1.44) | 1.50 (1.24–1.75) | −0.26 (−0.36 to −0.12) | <0.001 |
| LDL-C (mmol/l) | 2.19 (1.63–2.61) | 2.22(1.86–2.73) | −0.18 (−0.69 to 0.34) | 0.100 |
| TG/HDL-C ratio | 2.71 (1.57–5.25) | 1.93 (1.15–3.01) | 0.95 (0.18 to 2.02) | <0.001 |
| HbA1c (mmol/mol) | 63.9 (57.4–80.9) | 59.6 (50.8–71.0) | 0.9 (0.0 to 1.3) | <0.001 |
* Wilcoxon signed-rank tests
Fig. 1.
Median concentration of each variable before and after cinnamon supplementation for T2DM patients. P50 median, GluBeCi glucose concentration at baseline, GluAfCi glucose concentration after cinnamon supplementation, HbA1cBeCi HbA1c concentration at baseline, HbA1cAfCi concentration after cinnamon supplementation, BUNBeCi BUN concentration at baseline, BUNAfCi BUN concentration after cinnamon supplementation, GFRBeCi glomerular filtration rate at baseline, GFRAfCi glomerular filtration rate after cinnamon supplementation, SystBeCi systolic blood pressure at baseline, SystAfCi systolic blood pressure after cinnamon supplementation, DiastBeCi diastolic blood pressure at baseline, DiastAfCi diastolic blood pressure after cinnamon supplementation, TGBeCi triglyceride concentration at baseline, TGAfCi triglyceride concentration after cinnamon supplementation, HDLBeCi HDL-C concentration at baseline, HDLAfCi HDL-C concentration after cinnamon supplementation, TGHDLBeCi TG/HDL-C ratio at baseline, TGHDLAfCi TG/HDL-C ratio after cinnamon supplementation
Table 3.
Effect of placebo supplementation on biochemical variables for T2DM patients
| Baseline (n = 50) | Final treatment (n = 50) | Difference | p value* | |
|---|---|---|---|---|
| Systolic BP (mmHg) | 130.0 (121.0–140.0) | 136.5 (127.8–143.5) | −5.0 (−13.3 to 3.0) | 0.007 |
| Diastolic BP (mmHg) | 80.0 (73.5–90.0) | 83.0 (78.0–95.0) | −0.5 (−14.0 to 5.3) | 0.044 |
| Glucose (mmol/l) | 7.59 (6.38–9.19) | 8.03 (6.38–10.51) | −0.50 (−1.60 to 0.52) | 0.058 |
| BUN (mmol/l) | 4.64 (3.57–6.07) | 4.99 (4.28–6.42) | −0.71 (−2.14 to 1.25) | 0.001 |
| CT(µmol/l) | 79.56 (70.72–88.40) | 88.40 (79.56–106.068 | −8.84 (−26.52 to 8.84) | 0.001 |
| eGFR (ml/min/1.73 m2) | 61.10 (58.57–74.12) | 57.58 (52.88–64.12) | 3.47 (2.02 to 9.52) | <0.001 |
| Total cholesterol (mmol/l) | 4.39 (3.79–5.02) | 4.59 (4.05–5.30) | −0.28 (−0.68 to 0.26) | 0.012 |
| Triglyceride (mmol/l) | 1.40 (1.01–2.28) | 1.49 (1.05–1.99) | −0.011 (−0.50 to 0.25) | 0.937 |
| HDL-C (mmol/l) | 1.42 (1.15–1.67) | 1.40 (1.17–1.68) | 0.026 (−0.285 to 0.207) | 0.549 |
| LDL-C (mmol/l) | 2.32 (1.86–2.83) | 2.17 (1.84–2.97) | 0.05 (−0.44 to 0.40) | 0.940 |
| TG/HDL-C ratio | 2.39 (1.45–4.42) | 2.04 (1.32–3.11) | 0.14 (−0.62 to 1.21) | 0.363 |
| HbA1c (mmol/mol) | 60.7 (56.3–68.3) | 63.9 (59.6–73.8) | −0.14 (−0.62 to 1.21) | 0.002 |
* Wilcoxon signed-rank tests
Table 4.
Comparison of the changes in concentrations of each variable for the cinnamon supplementation and placebo T2DM groups at the end of the study
| Conc. change for cinnamon supplement (n = 49) | Conc. change for placebo group (n = 50) | p value* | |
|---|---|---|---|
| Systolic BP (mmHg) | 8.5 (5.0 to 13.3) | −5.0 (−13.3 to 3.0) | <0.001 |
| Diastolic BP (mmHg) | 8.0 (5.0 to 12.0) | −0.5 (−14.0 to 5.3) | 0.002 |
| Glucose (mmol/l) | 0.55 (0.47 to 1.82) | −0.50 (−1.60 to 0.52) | 0.001 |
| BUN (mmol/l) | 1.07 (0.53 to 2.14) | −0.71 (−2.14 to 1.25) | 0.006 |
| CT(µmol/l) | 8.84 (8.84 to 0.40) | −8.84 (−26.52 to 8.84) | <0.001 |
| eGFR (ml/min/1.73 m2) | −6.85 (−25.98 to 9.36) | 3.47 (2.02 to 9.52) | <0.001 |
| Total cholesterol (mmol/l) | −0.15 (−0.89 to 0.31) | −0.28 (−0.68 to 0.26) | <0.001 |
| Triglyceride (mmol/l) | 0.32 (−0.05 to 0.95) | −0.011 (−0.50 to 0.25) | <0.001 |
| HDL-C (mmol/l) | −0.26 (−0.36 to −0.12) | 0.026 (−0.285 to 0.207) | <0.001 |
| LDL-C (mmol/l) | −0.18 (−0.69 to 0.34) | 0.05 (−0.44 to 0.40) | 0.178 |
| TG/HDL-C ratio | 0.95 (0.18 to 2.02) | 0.14 (−0.62 to 1.21) | <0.001 |
| HbA1c (mmol/mol) | 0.9 (0.0 to 1.3) | −0.14 (−0.62 to 1.21) | <0.001 |
* Mann–Whitney U test
Fig. 2.
Concentration changes for each variable for the cinnamon supplementation group and placebo group. GluCCi glucose concentration change after cinnamon supplementation, GluCCon glucose concentration change in placebo group at the end of the study, HbA1CCCi HbA1c concentration change after cinnamon supplementation, HA1cCCCon HbA1c concentration change in placebo group at the end of the study, BUNCCi BUN concentration change after cinnamon supplementation, BUNCCon BUN concentration change in placebo group at the end of the study, CTCCi creatinine concentration change after cinnamon supplementation, CTCCon creatinine concentration change in placebo group at the end of the study, TGCCi triglyceride concentration change after cinnamon supplementation, TGCCon triglyceride concentration change in placebo group at the end of the study, HDLCCi HDL-C concentration change after cinnamon supplementation, HDLCCon HDL-C concentration change in placebo group at the end of the study, SystCCi systolic blood pressure change after cinnamon supplementation, concentration change after cinnamon supplementation, SystCCon systolic blood pressure change in placebo group at the end of the study, DiastCCi diastolic blood pressure change after cinnamon supplementation, DiastCCon diastolic blood pressure change in placebo group at the end of the study, TGHDLCCi TG/HDL-C ratio change after cinnamon supplementation, TGHDLCCon TG/HDL-C ratio change in placebo group at the end of the study
Discussion
Botanical products can improve glucose metabolism and the overall condition of individuals with diabetes not only by hypoglycemic effects but also by improving lipid metabolism, antioxidant status, and capillary function [15, 16]. This study revealed the effects of cinnamon (1.5 g per day) on reduction of glucose, triglyceride levels, TG/HDL-C ratio, and HbA1c and increasing of HDL-C levels for subjects with T2DM. The data were also reinforced by the observation that there were no significant changes for any of the patients in the placebo groups. No problems with compliance were noted; neither were there problems associated with consumption of 1.5 g cinnamon per day. Cinnamon has been shown to increase in-vitro glucose uptake and glycogen synthesis and to increase phosphorylation of the insulin receptor. In addition, cinnamon extracts are likely to aid in initiation of the insulin cascade system [11, 12] and insulin is also of crucial importance in lipid metabolism. Dyslipidemia in T2DM is characterized by elevated triglyceride, reduced HDL-C and small dense LDL-C particles, elevated triglyceride-rich remnant lipoprotein, and increased circulating insulin concentration [17]. This study also revealed a reduction in TG/HDL-C ratio among T2DM patients. Previous studies have demonstrated that lipoprotein ratio, TG/HDL-C, was associated with insulin resistance and increased risk of CVD [17]; cinnamon may have resulted in a decrease in insulin resistance and increased insulin sensitivity among the T2DM patients in this study. This study showed that consumption of cinnamon led to improved glucose and blood lipids levels in vivo. Anderson et al. [18] also demonstrated the beneficial effects of cinnamon on glucose control. Cinnamon extracts also increase glucose uptake and glycogen synthesis; an in-vivo study showed cinnamon possibly helped initiate the insulin cascade system [19]. In another study, cinnamon was claimed to be a natural insulin sensitizer [20]. The insulin-sensitizing effect of cinnamon has been established in in-vitro cell-line studies with adipocytes [12, 18, 20] and in in-vivo animal studies [19]. The bioactive compound methyl hydroxychalcone polymer (MHCP), isolated from cinnamon, acts as a mimetic of insulin [12].
In this study a decreased in HbA1c levels in diabetic patients was observed after only 60 days. The main mechanisms of the significant reduction of HbA1c could be the action of cinnamon in delaying gastric emptying [21], increasing glycogen synthesis by activating glycogen synthase and inhibiting glycogen synthase kinase 3β, and reducing glucose absorption in the small intestine by increasing glucosidase enzymes and inhibition of intestinal ATPase [18, 22]. Intake of cinnamon also improves insulin sensitivity and may lead to beneficial antioxidant effects [23]. A limitation of this study is that the amounts of polyphenolic polymers and flavonoids in the blood and the dose effect were not measured for the individual participants.
The relationship between BP and the kidney is complex; each may adversely affect the other. It has been hypothesized that slightly reduced GFR might be associated with increased BP or that increased GFR might be associated with reduced BP; this study demonstrated increased eGFR and reduced BP in T2DM patients with cinnamon supplementation. Cinnamon supports healthy circulation by bringing blood from the center of the body to the skin. Results obtained from use of animals as case studies are also indicative of the ability of cinnamon to regulate blood pressure via peripheral vasodilatation [24]. This mechanism may have caused increased eGFR in the cinnamon supplementation group and may be consistent with the hypothesis of the renal cause of essential hypertension, although it can also be explained by renal damage caused by elevated BP. Control of traditional risk factors (hypertension, diabetes, and dyslipidemia) has a substantial effect on CKD progression [25, 26]. Among the risk factors of CVD, hypertension had the greatest effect on CKD [27].
There are a variety of conflicting results from randomized controlled trials (RCTs) on the effects of cinnamon on glycemic and lipidemic reduction. Blevins et al. [28] demonstrated the reversed effect of increased plasma glucose levels and no significant change in HbA1c after 3 months of consuming 1 to 1.2 g/d of Cinnamon cassia versus placebo. However, this trial was published only as a research brief, so many of the study characteristics were unclear. Vanschoonbeek et al. [29] conducted RCTs on postmenopausal women and demonstrated that cinnamon did not improve insulin sensitivity, oral glucose tolerance, lipid profile and hemoglobin A1c levels after 30 days supplementation. Altschuler et al. [30] also demonstrated no beneficial effects of cinnamon on adolescents with type 1 diabetes.
Conclusion
Cinnamon supplementation reduced plasma glucose, HbA1c, triglyceride, TG/HDL-C ratio, and BP, and increased HDL-C levels and eGFR among people with type 2 diabetes. Cinnamon may be beneficial for those with T2DM to prevent and control diabetic complications.
Acknowledgments
We sincerely thank Naresuan University for financial support. We also want to specially thank those who participated and donated blood samples for this study. Finally, we sincerely thank Assistant Professor Dr Ronald A. Markwardt, Faculty of Public Health, Burapha University, for his critical reading and correction of the manuscript.
Abbreviation
- T2DM
Type 2 diabetes mellitus
- Glu
Glucose
- BUN
Blood urea nitrogen
- CT
Creatinine
- HbA1c
Hemoglobin A1c
- TC
Total cholesterol
- TG
Triglycerides
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- TG/HDL-C ratio
Triglycerides/high-density lipoprotein cholesterol ratio
- eGFR
Estimated glomerular filtration rate
- BP
Blood pressure
Conflict of interest
The authors declare that they have no competing interests.
Ethics policy
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients before inclusion in the study.
References
- 1.Ashcroft FM, Rorsman P. Diabetes mellitus and the beta cell: the last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal BR, Mehta AA. Diabetic cardiomyopathy: pathophysiological mechanisms and cardiac dysfunction. Hum Exp Toxicol. 2013;32:571–590. doi: 10.1177/0960327112450885. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 5.Arora MK, Singh UK. Molecular mechanisms in the pathogenesis of diabetic nephropathy: an update. Vascul Pharmacol. 2013;58:259–271. doi: 10.1016/j.vph.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen DV, Shaw LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol. 2012;3:170. doi: 10.3389/fendo.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman S, Rahman T, Ismail AA, Rashid AR. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab. 2007;9:767–780. doi: 10.1111/j.1463-1326.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 8.Raza A, Movahed A. Current concepts of cardiovascular diseases in diabetes mellitus. Int J Cardiol. 2003;89:123–134. doi: 10.1016/S0167-5273(02)00510-7. [DOI] [PubMed] [Google Scholar]
- 9.Ulbricht C, Seamon E, Windsor RC, Armbruester N, Bryan JK, Costa D, Giese N, Gruenwald J, Iovin R, Isaac R, Serrano JM, Tanguay-Colucci S, Weissner W, Yoon H, Zhang J. An evidence-based systematic review of cinnamon (Cinnamomum spp.) by the NaturalStandard Research Collaboration. J Diet Suppl. 2011;8:378–454. [DOI] [PubMed]
- 10.Sheng X, Zhang Y, Gong Z, Huang C, Zang Y. Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res. 2008;2008:1–9. doi: 10.1155/2008/581348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imparl-Radosevich J, Deas S, Polansky MM, Baedke DA, Ingebrutsen TS, Anderson RA, Graves DJ. Regulation of phosphorylase phosphatase (PTP-1) and insulin receptor kinase by fractions from cinnamon: implications for cinnamon regulation of insulin signaling. Horm Res. 1998;50:177–182. doi: 10.1159/000023270. [DOI] [PubMed] [Google Scholar]
- 12.Jarvill-Taylor KJ, Anderson RA, Graves DJ. A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3–L1 adipocytes. J Am Coll Nutr. 2001;20:327–336. doi: 10.1080/07315724.2001.10719053. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation, Kidney Disease Outcome Quality Initiative Advisory Board. Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis 2002;39:S1–246. [PubMed]
- 15.Alamkhan MS, Safdar M, Alikhan MM, Nawazkhattak K, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 16.Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12:553–564. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 17.Tangvarasittichai S, Poonsub P, Tangvarasittichai O. Association of serum lipoprotein ratios with insulin resistance in type 2 diabetes mellitus. Indian J Med Res. 2010;131:641–648. [PubMed] [Google Scholar]
- 18.Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP, Schoene NW, Graves DJ. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004;52:65–70. doi: 10.1021/jf034916b. [DOI] [PubMed] [Google Scholar]
- 19.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract. 2003;62:139–148. doi: 10.1016/S0168-8227(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 20.Broadhurst CL, Polansky MM, Anderson RA. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem. 2000;48:849–852. doi: 10.1021/jf9904517. [DOI] [PubMed] [Google Scholar]
- 21.Hlebowicz J, Darwiche G, Bjorgell O, Almer LO. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am J ClinNutr. 2007;85:1552–1556. doi: 10.1093/ajcn/85.6.1552. [DOI] [PubMed] [Google Scholar]
- 22.Solomon TP, Blannin AK. Effects of short-term cinnamon ingestion on in vivo glucose tolerance. Diabetes Obes Metab. 2007;9:895–901. doi: 10.1111/j.1463-1326.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 23.Gruenwald J, Freder J, Armbruester N. Cinnamon and health. Crit Rev Food Sci Nutr. 2010;50:822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 24.Preuss HG, Echard B, Polansky MM, Anderson R. Whole cinnamon and aqueous extracts ameliorate sucrose-induced blood pressure elevations in spontaneously hypertensive rats. J Am Coll Nutr. 2006;25:144–150. doi: 10.1080/07315724.2006.10719525. [DOI] [PubMed] [Google Scholar]
- 25.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 26.Cottone S, Lorito MC, Riccobene R, Nardi E, Mule G, Buscemi S, Geraci C, Guarneri M, Arsena R, Cerasola G. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J Nephrol. 2008;21:175–179. [PubMed] [Google Scholar]
- 27.Wang F, Ye P, Luo L, Xiao W, Wu H. Association of risk factors for cardiovascular disease and glomerular filtration rate: a community-based study of 4925 adults in Beijing. Nephrol Dial Transplant. 2010;25:3924–3931. doi: 10.1093/ndt/gfq327. [DOI] [PubMed] [Google Scholar]
- 28.Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabetes Care. 2007;30:2236–2237. doi: 10.2337/dc07-0098. [DOI] [PubMed] [Google Scholar]
- 29.Vanschoonbeek K, Thomassen BJ, Senden JM, Wodzig WK, van Loon LJ. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr. 2006;136:977–980. doi: 10.1093/jn/136.4.977. [DOI] [PubMed] [Google Scholar]
- 30.Altschuler JA, Casella SJ, MacKenzie TA, Curtis KM. The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care. 2007;30:813–816. doi: 10.2337/dc06-1871. [DOI] [PubMed] [Google Scholar]