Abstract
Purpose
Several guidelines have recently recommended exercise for prevention and treatment of type 2 diabetes. However, determining the optimum exercise conditions, e.g., the intensity, amount, frequency, and type of exercise, is difficult, particularly by patients themselves. We have investigated the acute effect of fast walking on postprandial blood glucose levels among patients with type 2 diabetes.
Methods
Fourteen patients diagnosed with type 2 diabetes at least 1 year previously were eligible for inclusion in this study during educational hospitalization. Three walking programs, natural walking (walking at a natural speed), 10 % fast walking, and 20 % fast walking, were performed 1 h after lunch in a randomized sequence with a washout period of 1 day. Walking time was 30 min in all the programs. Primary outcome was determined by self-monitoring of blood glucose. Blood glucose levels were measured before walking, after walking for 15 min, and at the end of walking. Heart rate and systolic and diastolic pressure were also measured for safety reasons.
Results
All the participants completed the study with no adverse effects. Compared with natural walking, fast walking markedly improved postprandial glucose excursion in an intensity-dependent manner without any adverse effects.
Conclusion
Fast walking acutely reduced postprandial blood glucose levels among patients with type 2 diabetes. Our method has major implications for the practice of diabetes education in clinical rehabilitation.
Keywords: Fast walking, Type 2 diabetes, Postprandial glucose levels, Rehabilitation, Oxygen uptake
Introduction
Type 2 diabetes is a metabolic disorder characterized by deteriorating glycemic control and an associated risk of complications [1, 2]. Evidence from clinical trials suggests that improving glycemic control can substantially reduce the long-term microvascular complications associated with diabetes [3–6]. Several guidelines, including those of the American Diabetes Association and American College of Sports Medicine [7, 8], have recently recommended exercise for prevention and treatment of type 2 diabetes. However, determining the optimum exercise conditions, e.g., the intensity, amount, and type of exercise, is difficult, particularly by patients themselves. Although it is generally recommended that exercise intensity is determined by use of maximum oxygen uptake (VO2max) with cardiopulmonary exercise testing (CPX), patients are usually unable to examine their VO2max in daily life [7, 8]. We believe it is important for patients themselves to be able to determine the appropriate amount of exercise to obtain maximum benefit at lowest risk in their daily life.
In this study, we used fast walking as intervention, and the speed of fast walking was determined on the basis of natural walking (walking at a natural speed). Although the long-term effects of several walking exercises for controlling blood glucose levels and/or preventing adverse effects have been reported elsewhere [3, 9], the acute effects of fast walking have not been reported with definitive evidence [10]. Exercise intensity was set at a relatively low level considering that patients with type 2 diabetes were not active in their daily life [8, 11, 12]. Furthermore, exercise intensity was based on the natural walking speed that patients themselves could set. A randomized, open-label, crossover clinical trial was used to investigate the immediate effect of fast walking on postprandial blood glucose levels among patients with type 2 diabetes and to establish definitive evidence for its widespread use.
Materials and methods
Ethics statement
This study was approved by the local ethics committee and conducted at the Naruto Prefecture Hospital, Japan, in accordance with the guidelines of Good Clinical Practice and the principles of the Declaration of Helsinki. It was also registered with the UMIN Clinical Trials Registry (UMIN000013253). All participants provided written informed consent before participating in the study.
Participants
Twenty-two patients (14 males and 8 females) with type 2 diabetes, aged 30–70 years with a body mass index (BMI) <30 kg/m2 were eligible for this study during educational hospitalization for initiation of medication, exercise, and nutrition therapy. As pre-intervention screening, medical history, physical examination, including gait speed and CPX, and medical and laboratory data were evaluated. Gait speed was calculated as the natural walking speed of each patient. Participants were required to be free of clinically major medical and laboratory abnormalities, to have glycosylated hemoglobin (HbA1c) <8 % and fasting plasma glucose (FPG) <250 mg/dL, and to have type 2 diabetes that was well controlled by diet alone or with medication. Standard exclusion criteria included inability to perform exercise because of poor gait, renal disease, advanced retinopathy, coronary arterial disease, and heart failure. Total calorie consumption by the participants was 1471.4 ± 95.8 kcal (1400 kcal, n = 9; 1600 kcal, n = 5). The participants’ diets consisted of 50 % carbohydrate, 31 ± 2 % fat, and 19 ± 2 % protein; the carbohydrate was supplied from rice. The mean calorie count of breakfast and lunch was 426 ± 19 (400 kcal, n = 9; 440 kcal, n = 5) and 440 kcal (440 kcal, n = 9; 440 kcal, n = 5), respectively.
Intervention
During the screening period natural walking speed was measured, and the fast walking speed was calculated on the basis of the natural walking speed for each participant. After exclusion of eight participants (for poor blood glucose control, n = 4; heart failure, n = 2; poor gait ability, n = 2), the 14 remaining participants (9 males and 5 females) were randomly allocated to three walking programs. No changes to diet or medication were made for any patient during the intervention. Mean values at the time of assessment were: age, 55.5 ± 7.6 years; height, 167.0 ± 6.8 cm; weight, 67.2 ± 5.8 kg; BMI, 24.2 ± 0.9 kg/m2; HbA1c, 6.7 ± 0.6 %; IRI, 4.2 ± 1.7 μU/ml, HOMA-IR, 1.8 ± 0.7; duration of diabetes, 5.1 ± 4.0 years, and O2max, 24.2 ± 4.4 ml/kg/min. The characteristics of the participants are listed in Table 1. All patients participated in three walking programs, in a random order, by using a treadmill 1 h after lunch with a washout period of 1 day. The programs were: natural walking (walking at a natural speed), 10 % fast walking, and 20 % fast walking. All patients walked naturally for 3 min; the distance walked was measured and the natural walking speed was calculated. Walking time was 30 min in all the programs. The screening period was a week. Patients were randomly assigned to the walking programs.
Table 1.
Characteristics of participants
| Participant | Sex | Age | BMI | HbA1c (%) | IRI | HOMA-IR | Duration (year) | Medication |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 64 | 22.9 | 7.8 | 4.1 | 1.8 | 9 | Metformin |
| 2 | Female | 60 | 24.5 | 6.8 | 3.3 | 1.4 | 1 | None |
| 3 | Female | 56 | 22.9 | 7.4 | 8.3 | 3.6 | 1 | None |
| 4 | Male | 50 | 23.4 | 7.1 | 7.3 | 2.8 | 5 | Sulfonylurea |
| 5 | Male | 66 | 23.0 | 5.8 | 6.4 | 2.6 | 10 | Sulfonylurea |
| 6 | Male | 48 | 23.6 | 6.5 | 3.2 | 1.4 | 6 | Metformin |
| 7 | Female | 50 | 23.9 | 7.2 | 4.2 | 1.9 | 1 | None |
| 8 | Female | 53 | 24.7 | 6.2 | 6.9 | 2.6 | 2 | Metformin |
| 9 | Male | 55 | 24.6 | 6.4 | 4.4 | 1.5 | 8 | Sulfonylurea |
| 10 | Male | 64 | 24.0 | 5.9 | 5.3 | 1.8 | 11 | Sulfonylurea + voglibose |
| 11 | Male | 58 | 24.5 | 7.6 | 4.1 | 1.9 | 1 | None |
| 12 | Male | 38 | 26.6 | 6.4 | 2.8 | 1.0 | 1 | None |
| 13 | Female | 45 | 24.4 | 6.8 | 3.3 | 1.5 | 3 | Voglibose |
| 14 | 56 | 24.7 | 6.4 | 2.8 | 1.1 | 12 | Voglibose | |
| Mean ± SD | 54.5 ± 7.6 | 24.1 ± 0.9 | 6.7 ± 0.6 | 4.7 ± 1.7 | 1.9 ± 0.7 | 5.1 ± 4.0 |
Measurements
The primary outcome was determined on the basis of blood glucose levels, measured by self-monitoring of blood glucose (SMBG; Accu-Chek® Aviva; Roche Diagnostics, Mannheim, Germany). Blood glucose levels were measured before walking, after walking for 15 min, and at the end of walking. For safety reasons, heart rate and systolic and diastolic pressure were also measured as secondary outcomes.
Data analysis
Statistical analysis was performed by use of SPSS v. 21.0. Continuous variables were expressed as mean ± standard deviation. Statistical differences were denoted by p < 0.05. The effects of the three walking programs on blood glucose levels were analyzed by use of repeated-measures ANOVA and Bonferroni post-hoc group comparisons, with the three walking programs and time as the repeated factors. Changes (delta) in each measurement from before to after exercise were assessed by one-way ANOVA. A post-hoc test (Scheffe’s multiple comparison test) was then used to identify significant differences among the significant mean values. The homogeneity of variances was analyzed by use of the Shapiro–Wilk normality test.
Results
Screening test
All participants completed our walking programs. The speeds of 10 % (4.5 ± 0.2 km/h) and 20 % (4.9 ± 0.3 km/h) fast walking were calculated on the basis of the speed of natural walking (4.1 ± 0.2 km/h). Maximum heart rate (HRmax) for natural walking, 10 % fast walking, and 20 % fast walking were 53.8 ± 3.7, 56.9 ± 4.0, and 60.7 ± 3.3 %, respectively. These HRmax values corresponded to 40–50 % VO2max.
Acute effect on blood glucose levels
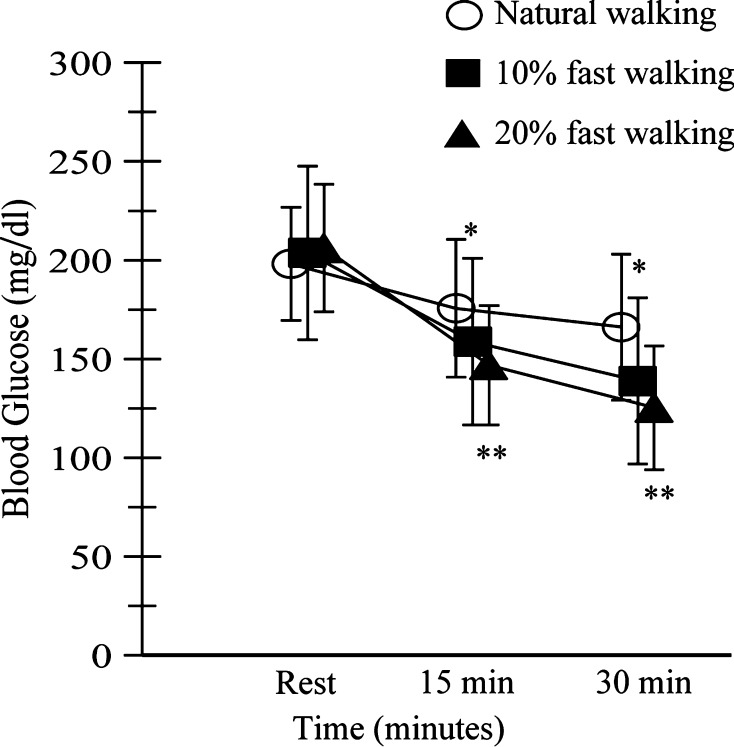
The primary outcome was a decrease in postprandial blood glucose levels with natural walking, 10 % fast walking, and 20 % fast walking (Fig. 1). Mean blood glucose values at baseline for natural walking, 10 % fast walking, and 20 % fast walking were 198.1 ± 28.6, 203.7 ± 43.9, and 206.1 ± 32.3 mg/dL, respectively. These values were comparable among the three walking programs. After 15 min, the mean blood glucose values for natural walking, 10 % fast walking, and 20 % fast walking were 175.6 ± 34.9, 158.8 ± 42.2, and 146.9 ± 30.2 mg/dL, respectively. A significant difference was observed between natural walking and 20 % fast walking. After 30 min, mean blood glucose values for natural walking, 10 % fast walking, and 20 % fast walking were 166.1 ± 34.9, 138.9 ± 42.1, and 125.3 ± 31.4 mg/dL, respectively. No significant changes were observed between 10 and 20 % fast walking, but changes were observed between natural walking and both 10 and 20 % fast walking. The changes in blood glucose after exercise were −16.9 ± 8.8, −32.7 ± 10.2, and −39.8 ± 8.4 % for natural walking, 10 % fast walking, and 20 % fast walking, respectively, with significant differences between 20 % fast walking and both natural walking and 10 % fast walking.
Fig. 1.
Self-monitoring of blood glucose levels. Blood glucose levels were measured before walking, after walking for 15 min, and after walking for 30 min. *Significant (p < 0.05) difference between natural walking and fast walking. **Significant (p < 0.01) difference between natural walking and fast walking
Acute effects on heart rate and systolic and diastolic blood pressure
Secondary outcomes were changes in heart rate and systolic and diastolic blood pressure (Table 2). No significant changes from baseline were observed for heart rate or systolic and diastolic blood pressure among the three walking programs. After 10 and 20 % fast walking for 15 min, heart rate and systolic and diastolic blood pressure were significantly higher than after natural walking for 15 min (Table 2). After walking for 30 min, heart rate and systolic and diastolic blood pressure for both 10 and 20 % fast walking were significantly different from that after natural walking (Table 2). No significant increase in either heart rate or blood pressure was observed for 20 % fast walking compared with 10 % fast walking (Table 2). The intervention was safely completed without cardiovascular problems; ranges for heart rate, systolic blood pressure, and diastolic blood pressure were 72–108 bpm, 126–156, and 64–96 mmHg, respectively.
Table 2.
Acute effects on heart rate and systolic and diastolic blood pressure
| (a) | |||
|---|---|---|---|
| Walking speed | Heart rate (beats/min) | ||
| 0 min | 15 min | 30 min | |
| Natural | 80.8 ± 4.4 | 88.3 ± 3.5 | 89.4 ± 3.2 |
| 10 % fast | 80.0 ± 4.6 | 93.0 ± 4.2a | 95.0 ± 4.5a |
| 20 % fast | 81.2 ± 3.9 | 97.8 ± 3.0a | 102.6 ± 3.7a |
| (b) | |||
|---|---|---|---|
| Walking speed | Systolic pressure | ||
| 0 min | 15 min | 30 min | |
| Natural | 135.4 ± 5.2 | 143.1 ± 4.2 | 145.7 ± 4.1 |
| 10 % fast | 134.4 ± 3.7 | 147.9 ± 4.0a | 150.0 ± 5.3a |
| 20 % fast | 133.0 ± 3.3 | 148.5 ± 3.6a | 151.9 ± 3.8a |
| (c) | |||
|---|---|---|---|
| Walking speed | Diastolic pressure | ||
| 0 min | 15 min | 30 min | |
| Natural | 77.0 ± 6.2 | 79.9 ± 5.6 | 81.6 ± 6.0 |
| 10 % fast | 76.4 ± 5.4 | 84.0 ± 4.3a | 85.7 ± 4.5a |
| 20 % fast | 76.1 ± 4.5 | 87.7 ± 3.8a | 89.6 ± 3.5a |
aStatistically significant difference compared with natural walking
Discussion
This randomized, open-label, crossover study illustrated that fast walking reduced postprandial blood glucose levels in an intensity-dependent manner for patients with type 2 diabetes. Postprandial blood glucose levels decreased acutely during 15 and 30 min of 10 and 20 % fast walking. The difference between the acute effects on blood glucose levels of 10 and 20 % fast walking for 30 min was not significant. Therefore, patients with type 2 diabetes can experience the same immediate effect on postprandial blood glucose levels as a result of 20 % fast walking for 30 min as they can as a result of 10 % fast walking for 30 min. Furthermore, 10 and 20 % fast walking did not adversely affect the cardiovascular system, at least not within 30 min.
Previous studies have recommended fast walking as a method of reducing postprandial blood glucose levels because of its beneficial effects on the cardiovascular system in long-term follow-up [2, 6, 10, 13]. Our results are in agreement with these results and extend the findings to the acute effects of exercise on blood glucose levels as measured by SMBG [14, 15]. Although the participants in this study walked at relatively slow speeds, their relative exertion was 50–65 % of HRmax, which corresponded to 40–50 % of O2max [16]. According to previous reports, health benefits may not be complete for patients with type 2 diabetes at their natural walking speed, although participants achieve approximate popular volume recommendations [17]. On the basis of the results of this study, 10 and 20 % fast walking may be sufficient to achieve current recommendations for physical activity to enable type 2 diabetes patients experience health benefits. Furthermore, 10 % fast walking may also reduce postprandial blood glucose levels, so these findings could help to encourage patients with diabetes to start walking with a low intensity and build exercise habits.
Our results also indicate that fast walking was completed without adverse effects on the cardiovascular system. The changes in heart rate and systolic and diastolic blood pressure were within safe ranges during this study. Most previous reports revealed that exaggerated systolic and diastolic blood pressure in response to exercise was significantly associated with future hypertension [18]. Monitoring heart-rate responses during exercise is commonly believed to be important in assessment of exercise intensity because it linearly responds to workload and is closely associated with oxygen uptake [18, 19]. We showed that 10 and 20 % fast walking for 30 min were as safe as natural walking.
This open-label trial has several limitations. First, the small number of participants, the accuracy of SMBG, and limited sampling times for SMBG may have affected the study. Second, awareness of the programs conducted may have affected reporting of adverse effects, although it is unlikely to have affected the more objective endpoint of blood glucose levels. Third, such factors as dietary composition and physical activity during this trial may have affected the results. However, the amount of carbohydrates and total calories included in the breakfast and lunch were almost identical for each participant. Furthermore, a randomized, open-label, crossover clinical trial was used to reduce the effect of the other factors. Fourth, participants had well-controlled diabetes; effects for patients with uncontrolled diabetes were not studied. Furthermore, the long-term effect and associated patient adherence of fast walking remain unclear. Further studies are needed to investigate these problems.
Conclusion
Up to 20 % fast walking reduced postprandial blood glucose levels among patients with type 2 diabetes in an intensity-dependent manner without any cardiovascular adverse effects. Our method has significant implications for the practice of diabetes education. Patients themselves can easily use our method to determine their exercise intensity, making it possible to start exercise and continue walking in their daily life.
Acknowledgments
This work was partly supported with by Grant-in-Aid for Medical and Dental Research from General Incorporated Associations Kojinkai.
Conflict of interest
Authors KD, TE, NS, HM, YF, MM, and SK declare that they have no conflict of interest.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent was obtained from all patients before inclusion in the study.
Footnotes
K. Deguchi and T. Enishi contributed equally.
References
- 1.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno G, Mangione CM. Management of cardiovascular disease risk factors in older adults with type 2 diabetes mellitus: 2002–2012 literature review. J Am Geriatr Soc. 2013;61(11):2027–2037. doi: 10.1111/jgs.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner RC, Holman RR, Cull CA, Stratton IM, Matthews DR, Frighi V, et al. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 5.Stearne MR, Palmer SL, Hammersley MS, Franklin SL, Spivey RS, Levy JC, et al. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Brit Med J. 1998;317(7160):703–713. doi: 10.1136/bmj.317.7160.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA, J Am Med Assoc. 2002;288(13):1622–1631. doi: 10.1001/jama.288.13.1622. [DOI] [PubMed] [Google Scholar]
- 7.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes A Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sone H, Tanaka S, Iimuro S, Tanaka S, Oida K, Yamasaki Y, et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study) Diabetologia. 2010;53(3):419–428. doi: 10.1007/s00125-009-1622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes. Diabetes Care. 2007;30(3):744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 11.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in US adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30(2):203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 12.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus—a meta-analysis of controlled clinical trials. Jama J Am Med Assoc. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 13.Johnson ST, McCargar LJ, Bell GJ, Tudor-Locke C, Harber VJ, Bell RC. Walking faster: distilling a complex prescription for type 2 diabetes management through pedometry. Diabetes Care. 2006;29(7):1654–1655. doi: 10.2337/dc06-0761. [DOI] [PubMed] [Google Scholar]
- 14.Oberlin DJ, Mikus CR, Kearney ML, Hinton PS, Manrique C, Leidy HJ, et al. One bout of exercise alters free-living postprandial glycemia in type 2 diabetes. Med Sci Sports Exerc. 2014;46(2):232–238. doi: 10.1249/MSS.0b013e3182a54d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Heijden MM, van Dooren FE, Pop VJ, Pouwer F. Effects of exercise training on quality of life, symptoms of depression, symptoms of anxiety and emotional well-being in type 2 diabetes mellitus: a systematic review. Diabetologia. 2013;56(6):1210–1225. doi: 10.1007/s00125-013-2871-7. [DOI] [PubMed] [Google Scholar]
- 16.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 17.Johnson ST, Tudor-Locke C, McCargar LJ, Bell RC. Measuring habitual walking speed of people with type 2 diabetes: are they meeting recommendations? Diabetes Care. 2005;28(6):1503–1504. doi: 10.2337/diacare.28.6.1503. [DOI] [PubMed] [Google Scholar]
- 18.Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I. Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension. 2002;39(3):761–766. doi: 10.1161/hy0302.105777. [DOI] [PubMed] [Google Scholar]
- 19.Benhalima K, Mathieu C. The role of blood glucose monitoring in non-insulin treated type 2 diabetes: what is the evidence? Prim Care Diabetes. 2012;6(3):179–185. doi: 10.1016/j.pcd.2012.05.001. [DOI] [PubMed] [Google Scholar]