Abstract
Transplantation-associated thrombotic microangiopathy (TA-TMA) is an uncommon but severe complication in patients undergoing allogeneic stem cell transplantation (allo-SCT). However, the mechanism is unclear. From 2011 to 2014, 20 patients with TA-TMA, 20 patients without, and 54 patients with various other complications, including veno occlusive disease (VOD), graft-versus-host disease (GVHD), and infection, were recruited in the study. Plasma vWF antigen (vWFAg), vWF activity (vWFAc), and ADAMTS13 activity were determined in these patients by ELISAs and FRETS-vWF73 assay, respectively. Plasma C3b, sC5b-9, and CH50 were also determined by ELISAs. Plasma levels of C3b were significantly increased in patients with either TA-TMA (p < 0.0001) or GVHD (p < 0.01). Plasma sC5b-9 and CH50 levels in patients with TA-TMA were also significantly increased (p < 0.001). Plasma ADAMTS13 activity was lower in patients with VOD, but normal with other complications. Both plasma vWFAg and vWFAc levels were not elevated in patients with TA-TMA or VOD compared with those of other groups. Complement activation likely via an alternative pathway (increased C3b, sC5b-9, and CH50) may play a role in the pathogenesis of TA-TMA. ADAMTS13 activity is reduced in VOD, but the ADAMTS13/vWF axis appears to be unaffected in patients with TA-TMA.
Keywords: Stem cell transplantation, Transplantation-associated thrombotic microangiopathy, Complement activation, C3b, sC5b-9
Introduction
Transplantation-associated thrombotic microangiopathy (TA-TMA) is a potentially fatal complication following allogeneic stem cell transplantation (allo-SCT) [1, 2]. While it was recognized as a complication after allo-SCT in the early 1980s, its pathogenesis remains unclear [3–5]. Studies suggest that multiple factors resulting in endothelial cell injury, specifically, the abnormity of the complement system contribute to the pathogenesis [6, 7]. Complement can be activated via a classical, a lectin, and an alternative pathway, leading to the deposition of complement component C5b-9 complexes on endothelial cells and resulting in cell injury [8–10]. Jodele et al. observed complement regulatory defects in six patients developed TA-TMA after SCT (including 3 auto-SCT and 3 allo-SCT) [2]. These patients exhibited a high prevalence of a heterozygous CFHR3-CFHR1 deletion (83%) compared to that in the donor population (33%) [11]. Three of the six patients with TA-TMA after allo-SCT also demonstrated the presence of factor H autoantibodies [2]. Another study also showed that allo-SCT recipients with multiple complement gene variants (≥ 3) were at high risk for development of TA-TMA [12]. These results implied that increased numbers of complement gene variants predispose to TA-TMA, which contributes to the transplant-related mortality [12]. Arteriolar C4d deposition was found in the kidney disease following SCT [13]. Recently, an anti-C5 monoclonal antibody, eculizumab, has been used to treat TA-TMA with success [14–16]. Six children with severe TA-TMA were treated with eculizumab and four achieved remission over time [15]. Another study showed that eculizumab treatment in 12 TA-TMA patients led to hematological response and increased overall survival from 33 to 50% [14]. Thus, activation of the complement system may play a critical role in pathogenesis of TA-TMA.
However, TA-TMA has a similar clinical manifestation to autoimmune thrombotic thrombocytopenic purpura (TTP) with severe ADAMTS13 deficiency [1, 2]. Because of the difficulty in making diagnosis, the incidence of TA-TMA was reported to be from 0.5 to 76% and the mortality of 60–90% [2, 6, 17]. Although some TA-TMA patients respond to instant plasma exchange treatment, most survivals have suffered from long-term complications, including hypertension, chronic kidney disease, gastrointestinal or central nervous system disease, and pulmonary hypertension [6, 18], which dramatically impacts the quality of life. Moreover, TA-TMA is often undistinguished from other complications associated with allo-SCT, including GVHD, severe infection, and veno occlusive disease (VOD) [19]. While severe deficiency of ADAMTS13 activity is diagnostic for autoimmune TTP [20], TA-TMA patients usually have normal to modestly reduced plasma ADAMTS13 activity [21]. Therefore, assays for plasma ADAMTS13 help differential diagnosis and guides for therapy, but we still could not ignore those TA-TMA patients with anti-ADAMTS13 antibody.
In the present study, we determined the plasma levels of complement activation markers including C3b, sC5b-9, and CH50 and the levels of von Willebrand factor (vWF) antigen, collagen-binding activity, and ADAMTS13 activity in 20 patients with TA-TMA following allo-SCT and compared the results to those of 74 other patients without and with various other complications following allo-SCT. These include 20 patients receiving allo-SCT but no complications, 14 with VOD, 20 with GVHD, and 20 with severe infection. Statistical analysis was performed to assess the contribution of various biomarkers in pathogenesis of TA-TMA.
Materials and methods
Patients
Twenty patients diagnosed as TA-TMA who received allo-SCT at the first affiliated hospital of Soochow University from June 2011 through June 2014 were enrolled in this study. TA-TMA was diagnosed according to the criteria proposed by Cho et al. [22]. The criteria include the following: schistocytes ≥ 2 per high-power field in peripheral blood, increased lactate dehydrogenase (LDH), thrombocytopenia < 50 × 109/L or a 50% decrease in platelet count, decreased hemoglobin, negative of Coombs test, decreased haptoglobin, and no coagulopathy.
Seventy-four other patients following allo-SCT were randomly selected as control subjects including 14 cases ofVOD, 20 cases of severe infections, 20 cases of 3–4 grade GVHD, and 20 cases with no complications. All the patients provided written informed consent for the protocol, which was approved by our hospital’s Ethics Committee. Patients in the control group were matched with TA-TMA group for gender, age, and disease characteristic. Peripheral blood samples were collected and anti-coagulated with EDTA. Samples were then centrifuged at 800g for 10 min at room temperature to collect the supernatants that were stored at – 80 °C for further assessments.
Conditioning regimen and GVHD prophylaxis
Both myeloablative [23] and reduced-intensity conditioning (RIC) [24] were applied for allo-SCT in our center. Two major myeloablative regimens included: (1) modified Bu/Cy regimen: Me-CCNU, cytarabine, busulfan, and cyclophosphamide, (2) modified TBI/Cy regimen: Me-CCNU, fractional total body irradiation (TBI), and cytarabine. The RIC regimen was composed mainly of fludarabine, cytarabine, and busulfan. Seventy-one percent of the patients received the modified Bu/Cy regimen. The modified TBI/Cy regimen was employed for patients with lymphoid malignancies and extramedullary diseases, while the RIC regimen for patients of advanced age or poor performance.
GVHD prophylaxis consisted of cyclosporine A (CsA) and short-term methotrexate (MTX), along with mycophenolate mofetil as well as antithymocyte globulin for unrelated and haploidentical donor SCT.
Selection criteria for comparison analysis
In order to determine the value of parameters in the panel, we set four control groups from the whole cohort for comparison analysis: VOD group: patients diagnosed as VOD according to the Baltimore Criteria [25]; GVHD group: patients with grades 3–4 GVHD as confirmed pathologically and graded using the International Bone Marrow Transplant Research criteria [26]; infection group: Infectious complications were defined by clinical and laboratory parameters [27]; and non-complications group: patients without clinical and laboratory indications of post-SCT complications including relapse, TA-TMA, VOD, GVHD, infections, and rheumatic diseases. We also remove cases with the overlap between those complications above.
Assays for plasma C3b, sC5b-9, and CH50 levels
Plasma samples were collected at two time points: before conditioning (BF) and at the onset of complications (AF). Samples were taken at “event diagnosis” when diagnosis of TMA, VOD, and GVHD was made clinically. For patients of non-complications group, plasma of AF was collected at 100 days of post SCT for well state of patients. Plasma levels of C3b, sC5b-9, and CH50 were determined by ELISA (Xitang, Shanghai, China) according to the manufacturer’s instruction.
Assays for plasma vWF antigen and activity
Plasma vWF antigen was determined by latex immunoassay (LIA). Plasma vWF activity was determined by collagenbinding assay using rabbit-anti-human vWF antibody (DAKO, NY, USA) for detection and type III collagen from placenta (Thermo, NY, USA) for capturing.
Assay for plasma ADAMTS13 activity
Plasma ADAMTS13 activity was determined by fluorescence resonance energy transfer (FRETS)-vWF73 according to the method described previously [28, 29].
Statistical analysis
Statistical analyses were performed with SPSS 22.0 (IBM, Chicago, IL) and GraphPad Prism7 (La Jolla, CA). Differences of panel parameters among the groups were analyzed by non-parametric Kruskal-Wallis test. P values less than 0.05 were considered statistically significant between the groups.
Results
Patients
There is a total of 94 patients with male to female ratio of 53 is to 41, and the mean age is 31.4 ± 13 years old. Twenty patients had no complications, 20 had infection, 20 had 3–4 grade GVHD, 14 had VOD, and 20 had TMA. The mortality rates were 55% (TMA), 42.9% (VOD), 40% (infection), 55% (3–4 grade GVHD), and 5% (non-complications). Among 20 patients with TA-TMA, all the patients have been found with decreased hemoglobin thrombocytopenia (platelet < 50 × 109/L), schistocytes ≥ 2 per high-power field in peripheral blood, and increased lactate dehydrogenase (LDH) (18 patients > 800 U/L, 2 patients > 600 U/L). In addition, 10 patients got psychosomatic manifestations.
No obvious alterations of vWF/ADAMTS13 axis in TA-TMA
Plasma vWF antigen, vWF activity, and ADAMTS13 activity were not different among various groups except for the reduced levels of plasma vWF activity in patients with GVHD prior to conditioning and the reduced levels of ADAMTS13 activity in patients with VOD (Table 1 and Fig. 1). These results suggest that vWF and ADAMTS13 activities were not altered in TA-TMA patients, but ADAMTS13 may be important for the development of VOD after allo-SCT.
Table 1.
Plasma levels of ADAMTS13 activity, von Willebrand antigen, collagen-binding activity, complement activation fragment C3b, and sC5b-9 in patients with various complications after stem cell transplantation
| Conditions | N | ADAMTS13 (%) |
vWF Ag (%) |
vWF Ac (%) |
|||
|---|---|---|---|---|---|---|---|
| Mean | Median (95% CI) | Mean | Median (95% CI) | Mean | Median (95% CI) | ||
| TMA_BF | 20 | 83 ± 56 | 71 (56–91) | 145 ± 26 | 142.5 (130.7–155.4) | 128 ± 67 | 115.2 (92.8–142.9) |
| TMA_AF | 20 | 69 ± 43 | 55 (39–79) | 156 ± 29 | 153.5 (139.5–168.9) | 153 ± 108 | 118.4 (82.5–169.8) |
| VOD_BF | 14 | 63 ± 42 | 51 (34–77) | 163 ± 30 | 159.9 (142.3–179.8) | 174 ± 96 | 150.8 (108.4–209.9) |
| VOD_AF | 14 | 36 ± 27** | 26 (15–44)** | 164 ± 32 | 160.8 (142.1–181.9) | 215 ± 98 | 193.2 (143.5–260.0) |
| Inf_BF | 20 | 106 ± 55 | 86 (58–128) | 157 ± 42 | 149.5 (126.8–176.2) | 140 ± 124 | 93.6 (59.6–146.9) |
| Inf_AF | 20 | 106 ± 43 | 95 (74–121) | 166 ± 53 | 151.1 (117.2–194.7) | 235 ± 106 | 208.2 (161.7–268.0) |
| GVHD_BF | 20 | 73 ± 39 | 61 (44–84) | 155 ± 30 | 152.1 (138.1–167.5) | 92 ± 107 | 50.1 (65.6–85.5)* |
| GVHD_AF | 20 | 81 ± 52 | 62 (41–93) | 172 ± 24 | 170.7 (160.2–182.0) | 171 ± 120 | 112.3 (65.6–192.4) |
| None_BF | 20 | 88 ± 56 | 74 (56–98) | 159 ± 23 | 157.7 (147.5–168.7) | 148 ± 170 | 83.3 (48.0–144.8) |
| None_AF | 20 | 104 ± 72 | 86 (65–114) | 168 ± 20 | 167.0 (157.9–178.6) | 169 ± 98 | 130.0 (86.3–195.7) |
| Conditions | C3b (ng/mL) |
sC5b-9 (ng/mL) |
CH50 (U/L) |
|||
|---|---|---|---|---|---|---|
| Mean | Median (95% CI) | Mean | Median (95% CI) | Mean | Median (95% CI) | |
| TMA_BF | 166 ± 98 | 138 (102–188) | 673 ± 150 | 656 (584–736) | 61.8 ± 23.0 | 60.3 (41.1–77.5) |
| TMA_AF | 455 ± 224*** | 394 (300–519)*** | 1058 ± 276*** | 1025 (907–1158)*** | 128.4 ± 37.3** | 128.4 (113.5–142.3)** |
| VOD_BF | 212 ± 96 | 193 (150–250) | 454 ± 197 | 420 (333–529) | 77.9 ± 31.24 | 71.0 (55.6–105.3) |
| VOD_AF | 248 ± 112 | 227 (177–292) | 673 ± 256 | 622 (484–799) | 87.98 ± 44.0 | 99.5 (42.1–116.2) |
| Inf_BF | 213 ± 161 | 154 (102–232) | 616 ± 224 | 577 (483–689) | 77.0 ± 30.5 | 79.2 (51.9–100.3) |
| Inf_AF | 202 ± 125 | 153 (102–231) | 723 ± 348 | 657 (536–806) | 60.4 ± 35.4 | 53.0 (31.7–93.2) |
| GVHD_BF | 200 ± 120 | 163 (116–227) | 559 ± 185 | 521 (427–636) | 63.5 ± 24.7 | 64.4 (46.4–71.7) |
| GVHD_AF | 299 ± 143** | 252 (182–348)** | 550 ± 167 | 522 (442–615) | 64.7 ± 31.5 | 58.0 (48.4–85.3) |
| None_BF | 211 ± 146 | 173 (128–235) | 558 ± 197 | 515 (415–638) | 68.1 ± 33.9 | 67.5 (44.7–90.6) |
| None_AF | 154 ± 88 | 130 (97–174) | 640 ± 155 | 621 (550–700) | 54.5 ± 34.3 | 44.8 (28.2–85.2) |
BF and AF indicate before and after complication occurred, respectively
TMA thrombotic microangiopathy, VOD venous occlusive disease, Infinfection, GVHD graft-versus-host hyperreactivity disease, None no complication controls
p < 0.05;
p < 0.01;
p < 0.001
Fig. 1.
Plasma levels of vWF antigen, collagen-binding activity, and ADAMTS13 activity in patients with TA-TMA and other complications following allo-SCT. Plasma levels of vWF antigen (a), collagen-binding activity (b), and ADAMTS13 activity toward FRETS-vWF73 (c) were determined in patients with TMA (n = 20), VOD (n = 14), infection (n = 20), and GVHD (n = 20), as well as in those without complications (n = 20) before conditioning (BF) and during the complications (AF) or no complications (None_AF). P value > 0.05 is not statistically significant, p values < 0.05 (one star) and < 0.01 (two star) is considered to be statistically significant
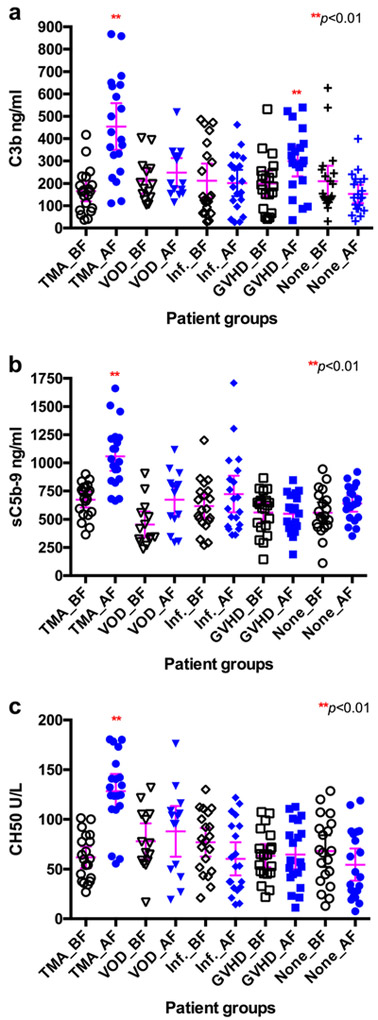
Plasma C3b, sC5b-9, and CH50 levels were significantly increased in patients with TA-TMA
When compared with those before conditioning or those with no complications following allo-SCT, plasma C3b, sC5b-9, and CH50 levels were significantly increased in patients with TA-TMA (p < 0.001). We also found C3b level increased in patients with GVHD (p < 0.01). No statistically significant difference among all other groups was detected (all p values > 0.05) (Table 1 and Fig. 2). These results demonstrated that plasma C3b, sC5b-9, and CH50 levels may be a specific biomarker for TA-TMA following allo-SCT and C3b may also be a biomarker of GVHD.
Fig. 2.
Plasma levels of C3b, sC5b-9, and CH50 in patients with TA-TMA and other complications following allo-SCT. Plasma levels of C3b (a), sC5b-9 (b), and CH50 (c) before conditioning (BF) and during the complications (AF) including TMA (n = 20), VOD (n = 14), infection (n = 20), and GVHD (n = 20) or no complications during the follow-up as controls (None_AF, n = 20) in patients with TMA. P values < 0.05 and < 0.01 were considered to be statistically significant (**p < 0.01: compared to None_AF group)
Discussion
TA-TMA is a potentially fatal syndrome with a similar clinical feature to atypical hemolytic uremic syndrome (aHUS) and TTP. The pathogenesis of TA-TMA is not fully understood. In the present study, we find that plasma levels of C3b, sC5b-9, and CH50 are significantly increased in patients with TA-TMA compared with those with other complications or no complications following allo-SCT.
Factor H autoantibodies or gene variations or mutations are present in some patients with TA-TMA after SCT [12, 30]. Our findings suggest a role of complement activation in pathogenesis of TA-TMA following allo-SCT. Therapeutic intervention with anti-complement monoclonal antibody may be helpful in reducing morbidity and mortality.
Prompt and accurate diagnosis of TA-TMA following allo-SCT remains to be a challenge [5, 31]. Two different definitions for TA-TMA are proposed by the Blood and Marrow Transplants Clinical Trials Network (CTN) and International Working Group (IWG) [32, 33]. These different diagnostic criteria poss a further challenge for assessing the sensitivity and specificity for a given diagnostic test. In our study, we adopted Cho’s diagnostic criteria, which does not require the presence of renal or neurologic findings for diagnosis [22]. The mortality rate in our cohort with TA-TMA is nearly 55%, which is similar to what has been reported in the literature [1, 6, 32, 34–36]. The high mortality rate of TA-TMA patients calls for an urgent need of early biomarkers for diagnosis, thereby a specifically targeted therapy should be given for these patients.
In summary, plasma C3b levels are significantly increased in TA-TMA and GVHD patients, while sC5b-9 and CH50 increased specifically in TA-TMA. Plasma ADAMTS13 activity is significantly reduced in patients with VOD, additionally, the elevated levels of plasma C3b, sC5b-9, and CH50 levels may be useful biomarkers for the early diagnosis of TA-TMA and GVHD. Our findings suggest the rationale for potential benefit of early use of anti-complement therapy in patients with probable TA-TMA. A multi-central trial will be critical to determine the efficacy of such an intervention to reduce the mortality rate associated with complications following allo-SCT.
Acknowledgments
This work was supported by grants from the Jiangsu Province of China (BE2016665, ZDRCA2016047 and RC2011105), National Nature Science Foundation of China (81270591 and 81670132), Jiangsu Provincial Special Program of Social Development (SBE2016740635), Jiangsu Provincial Special Program of Medical Science (BL2012005), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and NIH R01HL115187-01A1 (to XLZ).
Footnotes
Compliance with ethical standards All the patients provided written informed consent for the protocol, which was approved by our hospital’s Ethics Committee.
Conflict of interest XLZ is a member of the speakers’ bureau and has received research support from Alexion and served as a consultant for Ablynx. All other authors declare no conflict of interest.
References
- 1.Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM, Goebel J, Dixon BP (2015) A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev 29(3):191–204. doi: 10.1016/j.blre.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, Myers K, Grimley M, Bleesing J, El-Bietar J, Wallace G, Chima RS, Paff Z, Laskin BL (2014) Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood 124(4):645–653. doi: 10.1182/blood-2014-03-564997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Changsirikulchai S, Myerson D, Guthrie KA, McDonald GB, Alpers CE, Hingorani SR (2009) Renal thrombotic microangiopathy after hematopoietic cell transplant: role of GVHD in pathogenesis. Clin J Am Soc Nephrol 4(2):345–353. doi: 10.2215/CJN.02070508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siami K, Kojouri K, Swisher KK, Selby GB, George JN, Laszik ZG (2008) Thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation: an autopsy study. Transplantation 85(1): 22–28. doi: 10.1097/01.tp.0000297998.33418.7e [DOI] [PubMed] [Google Scholar]
- 5.George JN (2008) Hematopoietic stem cell transplantation-associated thrombotic microangiopathy: defining a disorder. Bone Marrow Transplant 41(11):917–918. doi: 10.1038/bmt.2008.7 [DOI] [PubMed] [Google Scholar]
- 6.Laskin BL, Goebel J, Davies SM, Jodele S (2011) Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood 118(6):1452–1462. doi: 10.1182/blood-2011-02-321315 [DOI] [PubMed] [Google Scholar]
- 7.Thachil J (2009) Nitric oxide in transplantation-related thrombotic microangiopathy. Bone Marrow Transplant 43(6):513–514. doi: 10.1038/bmt.2008.350 [DOI] [PubMed] [Google Scholar]
- 8.Carroll MC, Isenman DE (2012) Regulation of humoral immunity by complement. Immunity 37(2):199–207. doi: 10.1016/j.immuni.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vorup-Jensen T, Boesen T (2011) Protein ultrastructure and the nanoscience of complement activation. Adv Drug Deliv Rev 63(12):1008–1019. doi: 10.1016/j.addr.2011.05.023 [DOI] [PubMed] [Google Scholar]
- 10.Wehner J, Morrell CN, Reynolds T, Rodriguez ER, Baldwin WM 3rd (2007) Antibody and complement in transplant vasculopathy. Circ Res 100(2):191–203. doi: 10.1161/01.RES.0000255032.33661.88 [DOI] [PubMed] [Google Scholar]
- 11.Holmes LV, Strain L, Staniforth SJ, Moore I, Marchbank K, Kavanagh D, Goodship JA, Cordell HJ, Goodship TH (2013) Determining the population frequency of the CFHR3/CFHR1 deletion at 1q32. PLoS One 8(4):e60352. doi: 10.1371/journal.pone.0060352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jodele S, Zhang K, Zou F, Laskin B, Dandoy CE, Myers KC, Lane A, Meller J, Medvedovic M, Chen J, Davies SM (2016) The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood 127(8):989–996. doi: 10.1182/blood-2015-08-663435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laskin BL, Maisel J, Goebel J, Yin HJ, Luo G, Khoury JC, Davies SM, Jodele S (2013) Renal arteriolar C4d deposition: a novel characteristic of hematopoietic stem cell transplantation-associated thrombotic microangiopathy Transplantation 96(2):217–223. doi: 10.1097/TP0b013e31829807aa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Fontbrune FS, Galambrun C, Sirvent A, Huynh A, Faguer S, Nguyen S, Bay JO, Neven B, Moussi J, Simon L, Xhaard A, Resche-Riggon M, O'Meara A, Fremeaux-Bacchi V, Veyradier A, Socie G, Coppo P, de Latour RP (2015) Use of eculizumab in patients with allogeneic stem cell transplant-associated thrombotic microangiopathy: a study from the SFGM-TC. Transplantation 99(9):1953–1959. doi: 10.1097/TP.0000000000000601 [DOI] [PubMed] [Google Scholar]
- 15.Jodele S, Fukuda T, Vinks A, Mizuno K, Laskin BL, Goebel J, Dixon BP, Teusink A, Pluthero FG, Lu L, Licht C, Davies SM (2014) Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant 20(4):518–525. doi: 10.1016/j.bbmt.2013.12.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peffault de Latour R, Xhaard A, Fremeaux-Bacchi V, Coppo P, Fischer AM, Helley D, Socie G (2013) Successful use of eculizumab in a patient with post-transplant thrombotic microangiopathy. Br J Haematol 161(2):279–280. doi: 10.1111/bjh.12202 [DOI] [PubMed] [Google Scholar]
- 17.George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB (2004) Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: a diagnostic dilemma. Transfusion 44(2):294–304 [DOI] [PubMed] [Google Scholar]
- 18.Uderzo C, Bonanomi S, Busca A, Renoldi M, Ferrari P, Iacobelli M, Morreale G, Lanino E, Annaloro C, Volpe AD, Alessandrino P, Longoni D, Locatelli F, Sangalli H, Rovelli A (2006) Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation 82(5):638–644. doi: 10.1097/01.tp.0000230373.82376.46 [DOI] [PubMed] [Google Scholar]
- 19.George JN, Nester CM (2014) Syndromes of thrombotic microangiopathy. N Engl J Med 371(7):654–666. doi: 10.1056/NEJMra1312353 [DOI] [PubMed] [Google Scholar]
- 20.Zheng XL (2015) ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med 66:211–225. doi: 10.1146/annurev-med-061813-013241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peyvandi F, Siboni SM, Lambertenghi Deliliers D, Lavoretano S, De Fazio N, Moroni B, Lambertenghi Deliliers G, Mannuccio Mannucci P (2006) Prospective study on the behaviour of the metalloprotease ADAMTS13 and of von Willebrand factor after bone marrow transplantation. Br J Haematol 134(2):187–195. doi: 10.1111/j.1365-2141.2006.06126.x [DOI] [PubMed] [Google Scholar]
- 22.Cho BS, Yahng SA, Lee SE, Eom KS, Kim YJ, Kim HJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS, Park CW (2010) Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation 90(8):918–926. doi: 10.1097/TP.0b013e3181f24e8d [DOI] [PubMed] [Google Scholar]
- 23.Tutschka PJ, Copelan EA, Klein JP (1987) Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood 70(5):1382–1388 [PubMed] [Google Scholar]
- 24.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, Storer B, Hegenbart U, Somlo G, Chauncey T, Bruno B, Appelbaum FR, Blume KG, Forman SJ, McSweeney P, Storb R (2003) Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood 102(9):3447–3454. doi: 10.1182/blood-2002-09-2955 [DOI] [PubMed] [Google Scholar]
- 25.Jones RJ, Lee KSK, Beschormer WE (1987) Venoocclusive disease of the 1120 liver following bone marrow transplantation. Transplantation 44(1121):778–783 [DOI] [PubMed] [Google Scholar]
- 26.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 15(6):825–828 [PubMed] [Google Scholar]
- 27.Zhang L, Lawson HL, Harish VC, Huff JD, Knovich MA, Owen J (2006) Creation of a recombinant peptide substrate for fluorescence resonance energy transfer-based protease assays. Anal Biochem 358(2):298–300. doi: 10.1016/j.ab.2006.06.022 [DOI] [PubMed] [Google Scholar]
- 28.Raife TJ, Cao W, Atkinson BS, Bedell B, Montgomery RR, Lentz SR, Johnson GF, Zheng XL (2009) Leukocyte proteases cleave von Willebrand factor at or near the ADAMTS13 cleavage site. Blood 114(8):1666–1674. doi: 10.1182/blood-2009-01-195461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols WG (2003) Management of infectious complications in the hematopoietic stem cell transplant recipient. J Intensive Care Med 18(6):295–312. doi: 10.1177/0885066603258009 [DOI] [PubMed] [Google Scholar]
- 30.Jodele S, Licht C, Goebel J, Dixon BP, Zhang K, Sivakumaran TA, Davies SM, Pluthero FG, Lu L, Laskin BL (2013) Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood 122(12):2003–2007. doi: 10.1182/blood-2013-05-501445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi CM, Schmaier AH, Snell MR, Lazarus HM (2009) Thrombotic microangiopathy in haematopoietic stem cell transplantation: diagnosis and treatment. Drugs 69(2):183–198. doi: 10.2165/00003495-200969020-00004 [DOI] [PubMed] [Google Scholar]
- 32.Batts ED, Lazarus HM (2007) Diagnosis and treatment of transplantation-associated thrombotic microangiopathy: real progress or are we still waiting? Bone Marrow Transplant 40(8):709–719. doi: 10.1038/sj.bmt.1705758 [DOI] [PubMed] [Google Scholar]
- 33.Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, Ferrara J, Soiffer R, Giralt S (2005) Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 11 (8):571–575. doi: 10.1016/j.bbmt.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 34.Noris M, Mescia F, Remuzzi G (2012) STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol 8(11):622–633. doi: 10.1038/nrneph.2012.195 [DOI] [PubMed] [Google Scholar]
- 35.George JN, Terrell DR, Vesely SK, Kremer Hovinga JA, Lammle B (2012) Thrombotic microangiopathic syndromes associated with drugs, HIV infection, hematopoietic stem cell transplantation and cancer. Presse Med 41(3 Pt 2):e177–e188. doi: 10.1016/j.lpm.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 36.Nakamae H, Yamane T, Hasegawa T, Nakamae M, Terada Y, Hagihara K, Ohta K, Hino M (2006) Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am J Hematol 81 (7):525–531. doi: 10.1002/ajh.20648 [DOI] [PubMed] [Google Scholar]