Abstract
The genus Croton belongs to the Euphorbiaceae family, which comprises approximately 1300 species. Many Croton species have been used as folk medicines. This review focuses on the chemical constituents from Croton species and their relevant biological activities, covering the period from 2006 to 2018. A total of 399 new compounds, including 339 diterpenoids, were reported. Diterpenoids are characteristic components of the Croton species. These isolated compounds exhibited a broad spectrum of bioactivities, including cytotoxic, anti-inflammatory, antifungal, acetylcholinesterase inhibitory, and neurite outgrowth-promoting properties. The present review provides a significant clue for further research of the chemical constituents from the Croton species as potential medicines.
Keywords: Croton species, phytochemistry, biological activities, diterpenoids, cytotoxicity
1. Introduction
The genus Croton belongs to the Euphorbiaceae family, and contains approximately 1300 species of trees, shrubs, and herbs, which are widely distributed throughout tropical and subtropical regions of the world. Many Croton species have been used as folk medicines in Africa, south Asia, and south America, for the treatment of many diseases such as stomachache, abscesses, inflammation, and malaria [1,2,3]. The seeds of C. tiglium, which are well-known as “badou”, had been utilized as a traditional Chinese medicine to treat gastrointestinal disorders, intestinal inflammation, and rheumatism. The roots of C. crassifolius, known as “jiguxiang” in China, are mainly used as a traditional medicine for the treatment of stomachache and sore throat [3]. The genus Croton is abundant in diverse diterpenoids, including clerodane, tigliane, kaurane, labdane, cembrane, and pimarane, with a wide range of biological activities, such as cytotoxic, anti-inflammatory, and anti-microbial [1,2,3,4,5]. Due to their great structural diversity and broad relevant bioactivities, Croton species have attracted increasing research attention. Several authors have provided reviews about the chemical constituents and biological activities of Croton species. A review came out in 2006 regarding clerodane diterpenes isolated from Croton species, their 13C-NMR spectroscopic data, and biological activities [2]. In 2007, a comprehensive review on the traditional uses, chemistry, and pharmacology of Croton species was published [1]. In 2013, anticancer and antioxidant activities of extracts and pure compounds from several Croton species were reviewed [4]. Five review articles were published in recent years which focused on ethnopharmacological uses, phytochemistry, and pharmacology of a single Croton species [6,7,8,9,10]. In the last decade, there has been a dramatic progress in the chemical constituents and relevant biological activities of Croton species. However, so far, no comprehensive review has been published since 2007. In the present review, we summarize systematically the research advances on the new chemical constituents and their biological activities of Croton species reported in the literature, as found on Web of Science, Google Scholar, PubMed, and SciFinder, from 2006 to March 2018, with the aim of providing a basis for further research of natural product drug discovery.
2. Chemical Constituents
To date, 399 new compounds have been isolated and identified from Croton species, including 339 diterpenoids (1–339), seven sesquiterpenoids (340–346), one sesterterpenoid (347), one triterpenoid (348), 21 glycosides (349–369), eight alkaloids (370–377), three benzoate derivatives (378–380), three pyran-2-one derivatives (381–383), two cyclopeptide (384, 385), two tropone derivatives (386, 387), two limonoids (388, 389), and ten miscellaneous compounds (390–399). Their structures, molecular formula, names, corresponding sources, and references are summarized in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13 and Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13, Table 14, Table 15, Table 16, Table 17, Table 18, Table 19, Table 20, Table 21, Table 22, Table 23, Table 24, Table 25, Table 26 and Table 27.
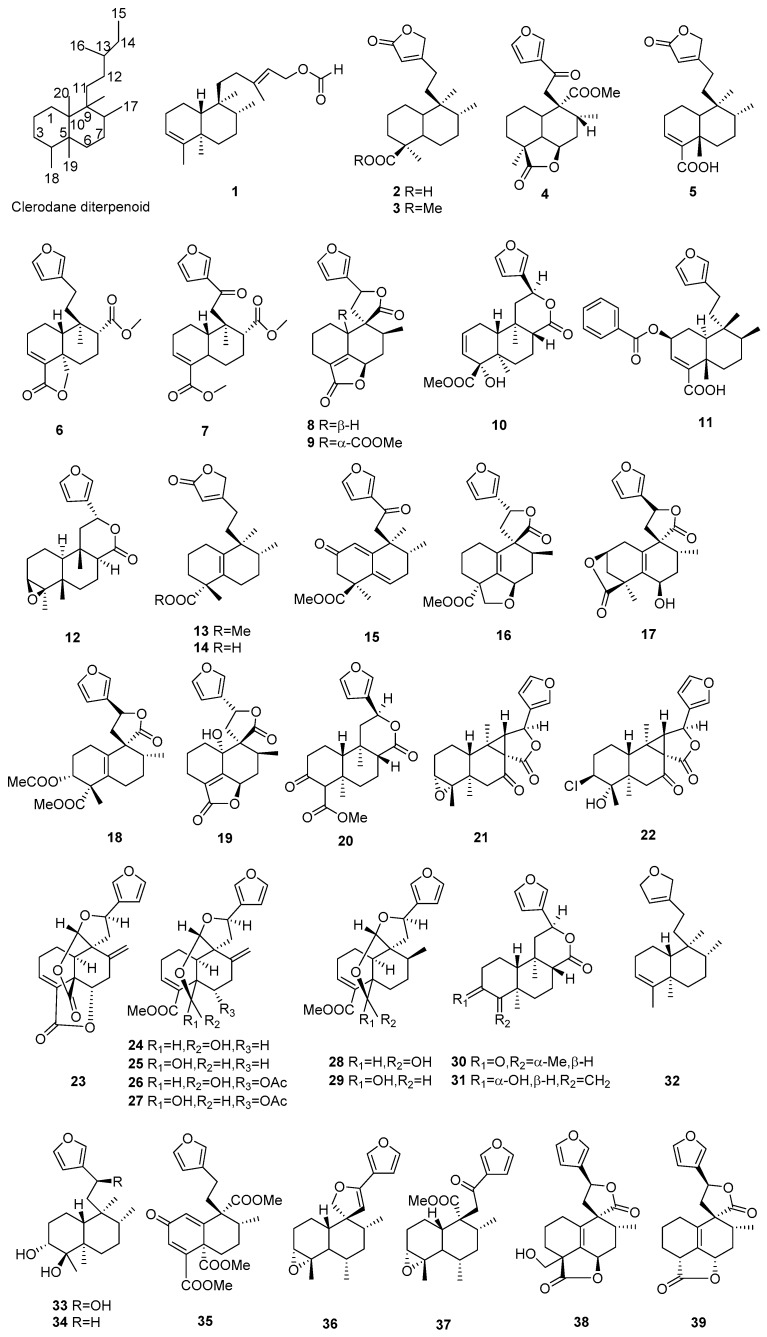
Figure 1.
Clerodane type diterpenoids from the genus Croton.
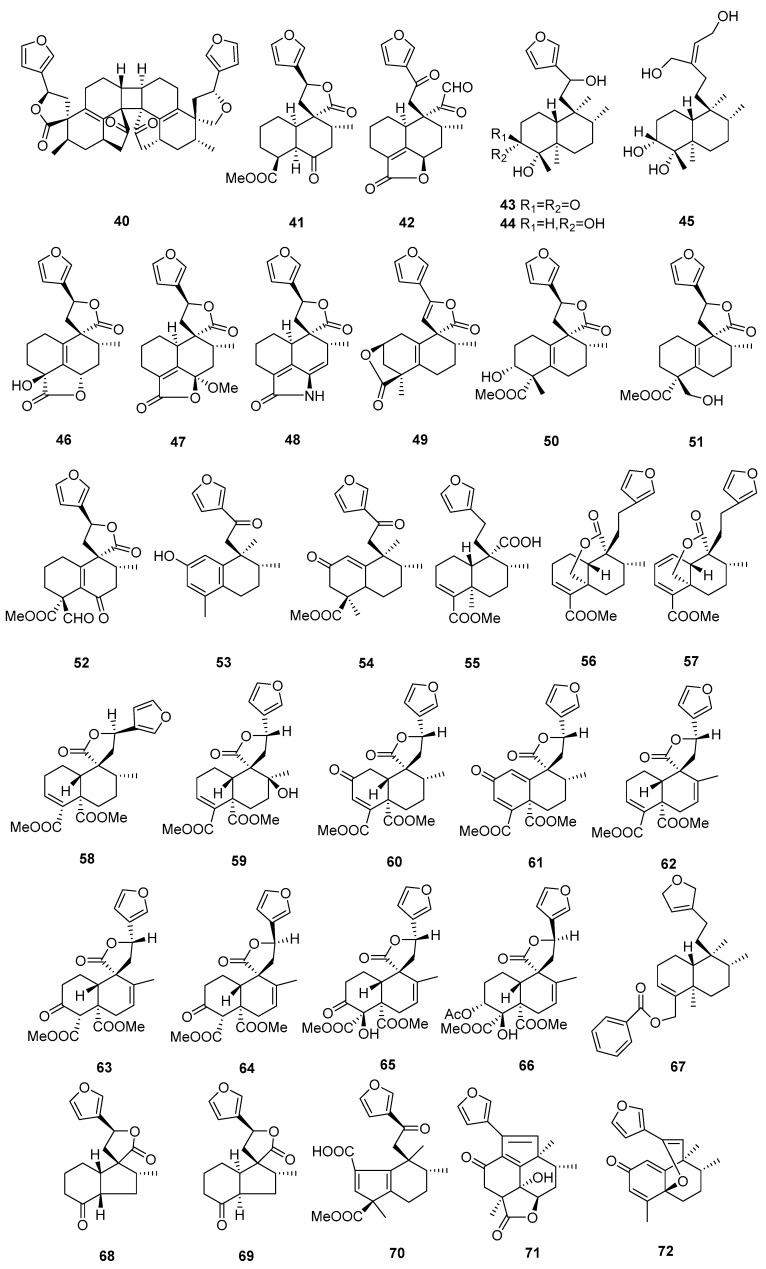
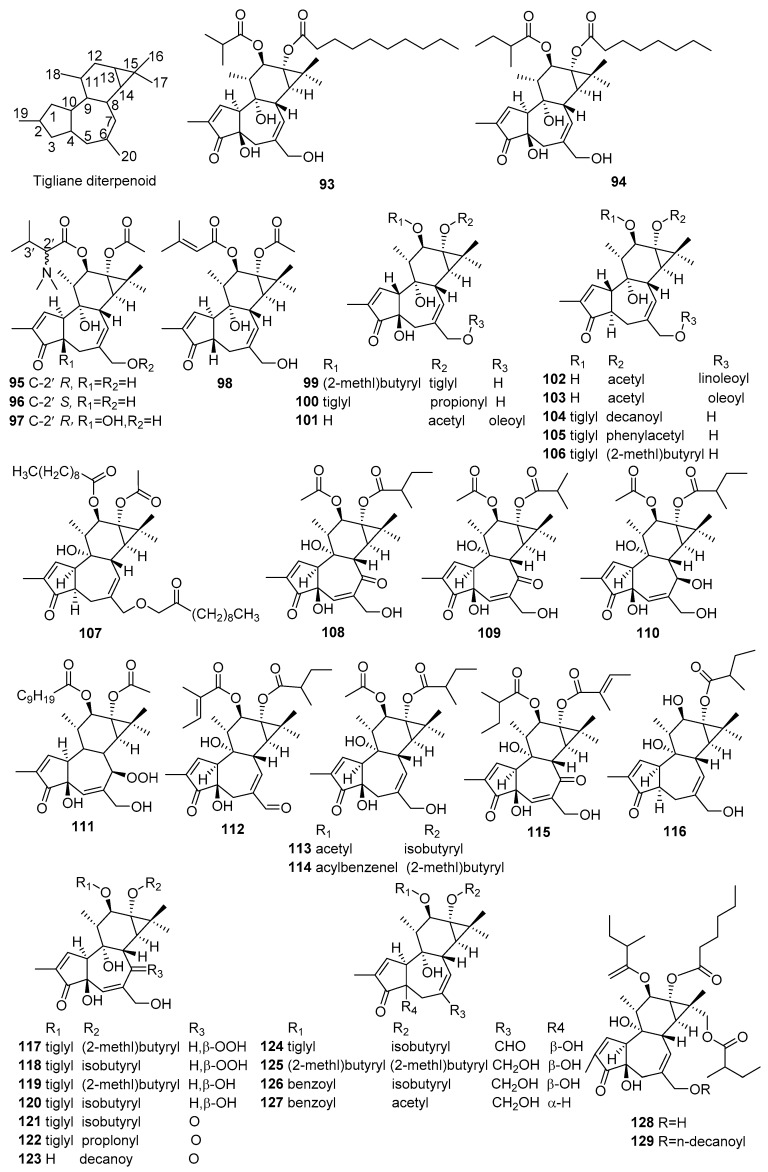
Figure 2.
Tigliane type diterpenoids from the genus Croton.
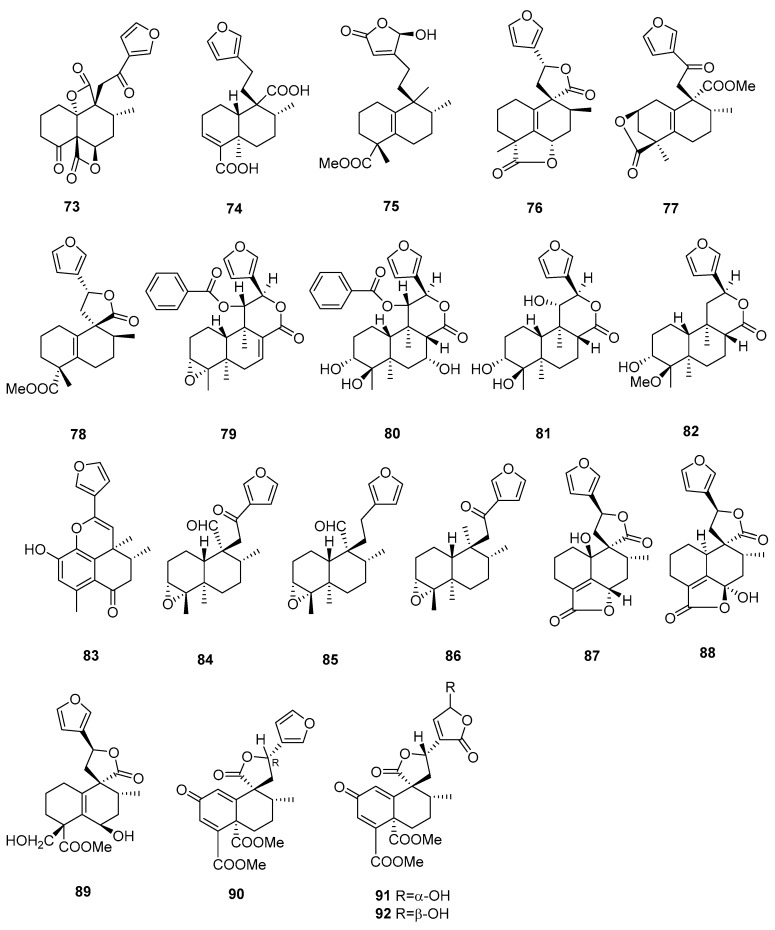
Figure 3.
Kaurane type diterpenoids from the genus Croton 1.
Figure 4.
Crotofolane type diterpenoids from the genus Croton.
Figure 5.
Labdane type diterpenoids from the genus Croton.
Figure 6.
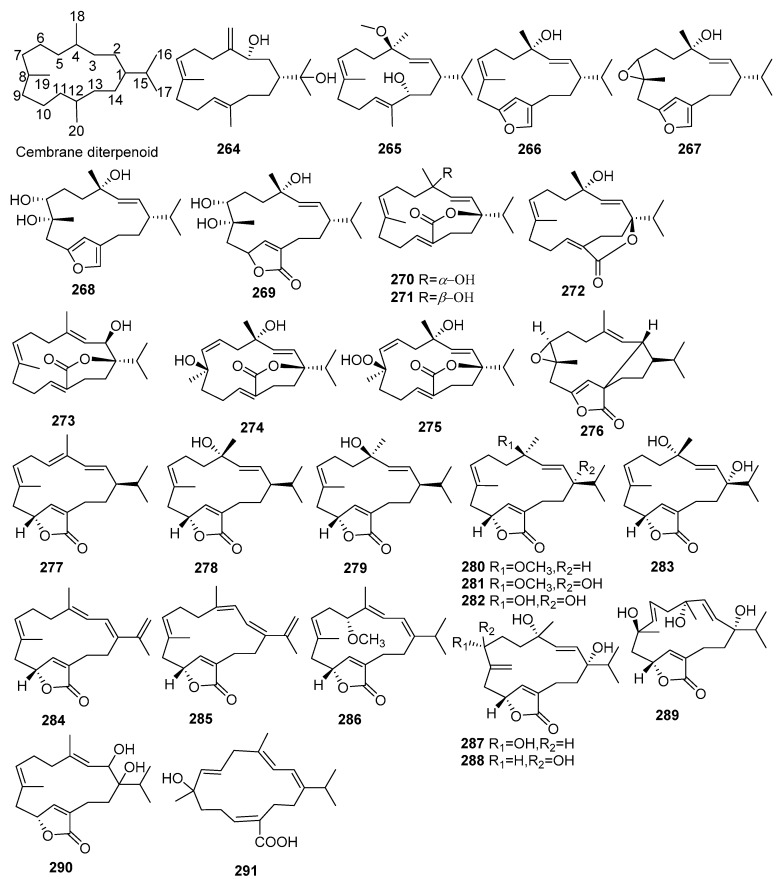
Cembrane type diterpenoids from the genus Croton.
Figure 7.
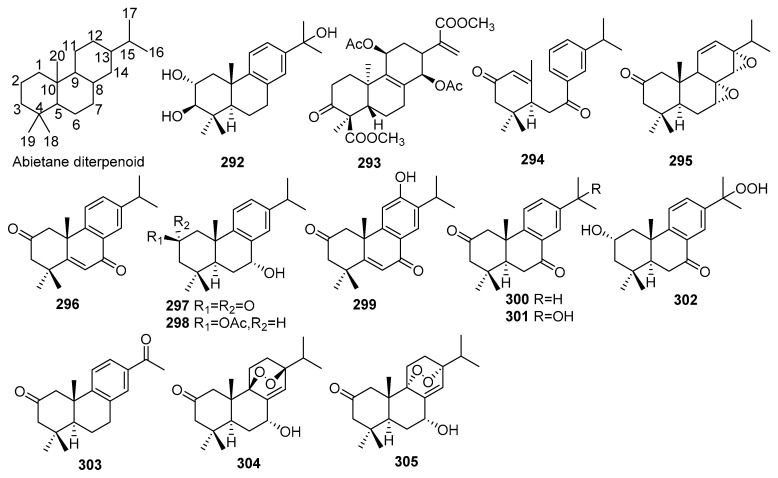
Abietane type diterpenoids from the genus Croton.
Figure 8.
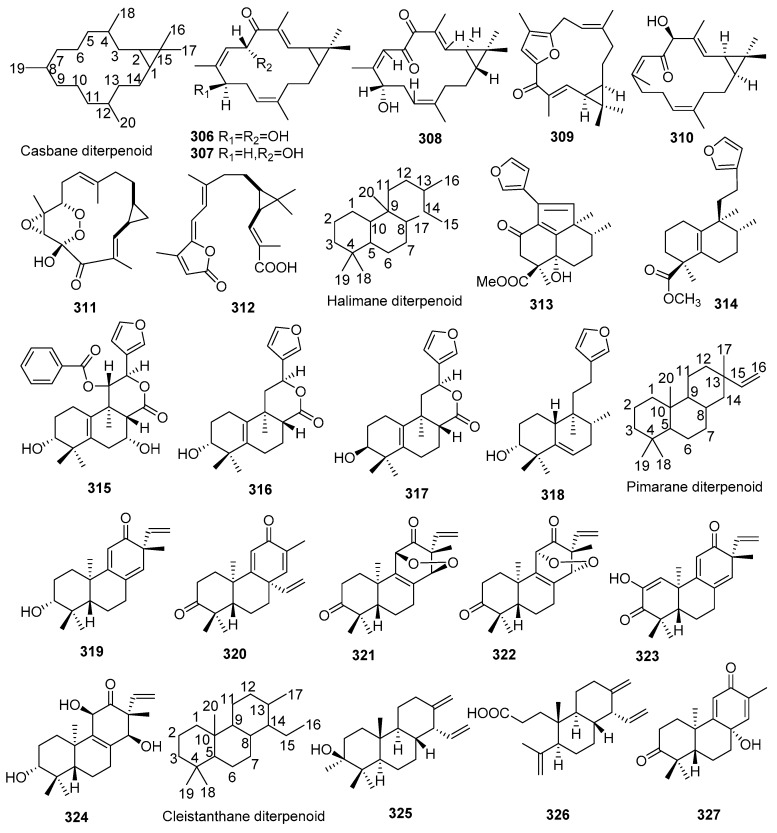
Casbane, Halimane, Pimarane and Cleistanthane type diterpenoids from the genus Croton.
Figure 9.
Grayanane, Atisane, Phytane, Laevinane type diterpenoids and Meroditerpenoids from the genus Croton.
Figure 10.
Sesquiterpenoids, Sesterterpenoid and Triterpenoid from the genus Croton.
Figure 11.
Glycosides from the genus Croton.
Figure 12.
Alkaloids from the genus Croton.
Figure 13.
Miscellaneous compounds from the genus Croton.
Table 1.
Clerodane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 1 | ent-3,13E-clerodadiene-15-formate | C21H34O2 | C. sylvaticus | [12] |
| 2 | 9-[2-(2(5H)-furanone-4-yl)ethyl]-4,8,9-trimethyl-1,2,3,4,5,6,7,8-octahydronaphthalene-4-carboxylic acid | C20H28O4 | C. crassifolius | [14] |
| 3 | 9-[2-(2(5H)-furanone-4-yl)ethyl]-4,8,9-trimethyl-1,2,3,4,5,6,7,8-octahydronaphthalene-4-carboxylic ester | C21H30O4 | C. crassifolius | [14] |
| 4 | Centrafricine I | C21H24O6 | C. mayumbensis | [19] |
| 5 | Marrubiagenin | C20H28O4 | C. glabellus | [15] |
| 6 | Methyl 15,16-epoxy-3,13(16),14-ent-clerodatrien-18,19-olide-17-carboxylate | C21H26O5 | C. oblongifolius | [29] |
| 7 | Dimethyl 15,16-epoxy-12-oxo-3,13(16),14-ent-clerodatriene-17,18-dicarboxylate | C22H28O6 | C. oblongifolius | [29] |
| 8 | Isoteucvin | C19H20O5 | C. jatrophoides | [30] |
| 9 | Jatrophoidin | C21H22O7 | C. jatrophoides | [30] |
| 10 | 8-Epicordatin | C21H26O6 | C. palanostigma | [31] |
| 11 | laevigatbenzoate | C27H31O5 | C. laevigatus | [13] |
| 12 | 3,4,15,16-diepoxy-cleroda-13(16),14-diene-12,17-olide | C20H26O4 | C. oblongifolius | [22] |
| 13 | Crassifolin A | C21H30O4 | C. crassifolius | [16] |
| 14 | Crassifolin B | C20H29O4 | C. crassifolius | [16] |
| 15 | Crassifolin C | C21H24O5 | C. crassifolius | [16] |
| 16 | Crassifolin D | C21H24O6 | C. crassifolius | [16] |
| 17 | Crassifolin E | C20H23O6 | C. crassifolius | [16] |
| 18 | Crassifolin F | C23H29O7 | C. crassifolius | [16] |
| 19 | Crassifolin G | C19H20O6 | C. crassifolius | [16] |
| 20 | Methyl 3-oxo-12-epibarbascoate | C21H26O6 | C. urucurana | [32] |
| 21 | Laevinoids A | C20H22O5 | C. laevigatus | [20] |
| 22 | Laevinoids B | C20H23O5Cl | C. laevigatus | [20] |
| 23 | Crotonolide A | C20H18O6 | C. laui | [21] |
| 24 | Crotonolide B | C21H24O6 | C. laui | [21] |
| 25 | Isocrotonolide B | C21H24O6 | C. laui | [21] |
| 26 | Crotonolide C | C23H26O8 | C. laui | [21] |
| 27 | Isocrotonolide C | C23H26O8 | C. laui | [21] |
| 28 | Crotonolide D | C21H26O6 | C. laui | [21] |
| 29 | Isocrotonolide D | C21H26O6 | C. laui | [21] |
| 30 | Crotonolide E | C20H26O4 | C. laui | [21] |
| 31 | Crotonolide F | C20H26O4 | C. laui | [21] |
| 32 | Crotonolide G | C20H32O | C. laui | [21] |
| 33 | Crotonolide H | C20H32O4 | C. laui | [21] |
| 34 | 12-Deoxycrotonolide H | C20H32O3 | C. laui | [21] |
| 35 | Crotonoligaketone | C23H26O8 | C. oligandrum | [33] |
| 36 | Crotonpene A | C20H26O3 | C. yanhuii | [23] |
| 37 | Crotonpene B | C21H28O5 | C. yanhuii | [23] |
| 38 | Crassifolin I | C20H22O6 | C. crassifolius | [34] |
| 39 | Crassifolin H | C19H20O5 | C. crassifolius | [34] |
| 40 | Crotoeurin A | C38H36O1 | C. euryphyllus | [25] |
| 41 | Crotoeurin B | C20H24O6 | C. euryphyllus | [25] |
| 42 | Crotoeurin C | C20H22O6 | C. euryphyllus | [25] |
| 43 | 3-Oxo-15,16-epoxy-4α,12-dihydroxy-ent-neo-clerodan-13(16),14-diene | C20H30O4 | C. limae | [35] |
| 44 | 15,16-Epoxy-3α,4α,12-trihydroxy-ent-neo-clerodan- 13(16),14-diene | C20H32O4 | C. limae | [35] |
| 45 | 3α,4α,15,16-Tetrahydroxy-ent-neo-cleroda-13E-ene | C20H36O4 | C. limae | [35] |
| 46 | Cracroson A | C19H21O6 | C. crassifolius | [26] |
| 47 | Cracroson B | C20H22O6 | C. crassifolius | [26] |
| 48 | Cracroson C | C19H19O4N | C. crassifolius | [26] |
| 49 | Crassifolin J | C20H20O5 | C. crassifolius | [36] |
| 60 | Crotocorylifuran-2-one | C22H24O8 | C.megalocarpoides | [27] |
| 61 | Megalocarpoidolide D | C22H22O8 | C.megalocarpoides | [27] |
| 62 | 7,8-Dehydrocrotocorylifuran | C22H24O7 | C.megalocarpoides | [27] |
| 63 | Megalocarpoidolide E | C22H24O8 | C.megalocarpoides | [27] |
| 64 | Megalocarpoidolide F | C22H24O8 | C.megalocarpoides | [27] |
| 65 | Megalocarpoidolide G | C22H24O9 | C.megalocarpoides | [27] |
| 66 | Megalocarpoidolide H | C24H28O10 | C.megalocarpoides | [27] |
| 67 | Launine K | C27H36O3 | C. laui | [37] |
| 68 | Crassin A | C17H20O4 | C. crassifolius | [17] |
| 69 | Crassin B | C17H20O4 | C. crassifolius | [17] |
| 70 | Crassin C | C21H24O6 | C. crassifolius | [17] |
| 71 | Crassin D | C20H20O5 | C. crassifolius | [17] |
| 72 | Crassin E | C19H20O3 | C. crassifolius | [17] |
| 73 | Crassin F | C19H18O7 | C. crassifolius | [17] |
| 74 | Crassin G | C20H26O5 | C. crassifolius | [17] |
| 75 | Crassin H | C21H30O5 | C. crassifolius | [17] |
| 76 | Crassifolius A | C20H22O5 | C. crassifolius | [38] |
| 77 | Crassifolius B | C21H24O6 | C. crassifolius | [38] |
| 78 | Crassifolius C | C21H26O5 | C. crassifolius | [38] |
| 79 | Crolaevinoid C | C27H28O6 | C. laevigatus | [39] |
| 80 | Crolaevinoid D | C27H32O8 | C. laevigatus | [39] |
| 81 | Crolaevinoid E | C20H28O6 | C. laevigatus | [39] |
| 82 | Crolaevinoid F | C21H30O5 | C. laevigatus | [39] |
| 83 | Norcrassifolin | C19H18O4 | C. crassifolius | [28] |
| 84 | Hypolein A | C20H26O4 | C. hypoleucus | [24] |
| 85 | Hypolein B | C20H28O3 | C. hypoleucus | [24] |
| 86 | Hypolein C | C20H28O3 | C. hypoleucus | [24] |
| 87 | Cracroson E | C19H20O6 | C. crassifolius | [40] |
| 88 | Cracroson F | C19H20O6 | C. crassifolius | [40] |
| 89 | Cracroson G | C21H26O7 | C. crassifolius | [40] |
| 90 | 12-Epi-megalocarpoidolide D | C22H22O8 | C. oligandrus | [18] |
| 91 | Crotonolins A | C22H22O10 | C. oligandrus | [18] |
| 92 | Crotonolins B | C22H22O10 | C. oligandrus | [18] |
Table 2.
Tigliane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 93 | 12-O-isobutyrylphorbol-13-decanoate | C34H52O8 | C. tiglium | [45] |
| 94 | 12-O-(2-methyl)butyrylphorbol-13-octanoate | C33H50O8 | C. tiglium | [45] |
| 95 | 12-O-[(2R)-N,N-dimethyl-3-methylbutanoyl]-4-deoxyphorbol 13-acetate | C29H43NO7 | C. ciliatoglandulifer | [41] |
| 96 | 12-O-[(2S)-N,N-dimethyl-3-methylbutanoyl]-4-deoxyphorbol 13-acetate | C29H43NO7 | C. ciliatoglandulifer | [41] |
| 97 | 12-O-[(2R)-N,N-Dimethyl-3-methylbutanoyl]phorbol 13-acetate | C29H43NO8 | C. ciliatoglandulifer | [41] |
| 98 | 12-O-[3-Methyl-2-butenoyl]-4-deoxyphorbol 13-acetate | C27H36NO7 | C. ciliatoglandulifer | [41] |
| 99 | 12-O-(2-methyl)butyrylphorbol-13-tiglate | C30H42O8 | C. tiglium | [46] |
| 100 | 12-O-tiglylphorbol-13-propionate | C28H38O8 | C. tiglium | [46] |
| 101 | 13-O-acetylphorbol-20-oleate | C40H62O8 | C. tiglium | [46] |
| 106 | 12-O-tiglyl-4-deoxy-4α-phorbol-13-(2-methyl)butyrate | C30H42O7 | C. tiglium | [46] |
| 107 | Alienusolin | C42H66O8 | C. alienus | [42] |
| 108 | 12-O-acetyl-5,6-didehydro-7-oxophorbol-13-yl 2-methylbutanoate | C27H36O9 | C. tiglium | [47] |
| 109 | 12-O-acetyl-5,6-didehydro-7-oxophorbol-13-yl2-methylpropanoate | C26H34O9 | C. tiglium | [47] |
| 110 | 12-Oacetyl-5,6-didehydro-6,7-dihydro-7-hydroxyphorbol-13-yl 2-methylbutanoate | C27H38O9 | C. tiglium | [47] |
| 111 | 12-O-decanoyl-7-hydroperoxy-phorbol-5-ene-13-acetate | C32H42O10 | C. mauritianus | [43] |
| 112 | 20-deoxy-20-oxophorbol12-tiglate 13-(2-methyl)butyrate | C30H40O8 | C. tiglium | [48] |
| 113 | 12-O-acetylphorbol-13-isobutyrate | C26H36O8 | C. tiglium | [48] |
| 114 | 12-O-benzoylphorbol-13-(2-methyl)butyrate | C32H40O8 | C. tiglium | [48] |
| 115 | 12-O-tiglyl-7-oxo-5-ene-phorbol-13-(2-methyl)butyrate | C30H40O9 | C. tiglium | [48] |
| 116 | 13-O-(2-metyl)butyryl-4-deoxy-4a-phorbol | C25H36O6 | C. tiglium | [48] |
| 117 | Crotignoid A | C30H42O10 | C. tiglium | [49] |
| 118 | Crotignoid B | C29H40O10 | C. tiglium | [49] |
| 119 | Crotignoid C | C30H42O9 | C. tiglium | [49] |
| 120 | Crotignoid D | C29H40O9 | C. tiglium | [49] |
| 121 | Crotignoid E | C29H38O9 | C. tiglium | [49] |
| 122 | Crotignoid F | C28H36O9 | C. tiglium | [49] |
| 123 | Crotignoid G | C30H44O8 | C. tiglium | [49] |
| 124 | Crotignoid H | C29H38O8 | C. tiglium | [49] |
| 125 | Crotignoid I | C30H44O8 | C. tiglium | [49] |
| 126 | Crotignoid J | C31H38O8 | C. tiglium | [49] |
| 127 | Crotignoid K | C29H34O7 | C. tiglium | [49] |
| 128 | Crotusin A | C36H54O10 | C. caudatus | [44] |
| 129 | Crotusin B | C46H72O11 | C. caudatus | [44] |
| 130 | Crotusin C | C36H52O11 | C. caudatus | [44] |
| 131 | 12-O-tiglylphorbol-4-deoxy- 4β-phorbol-13-acetate | C27H36O7 | C. tiglium | [50] |
| 132 | 12-O-tiglylphorbol-4-deoxy-4β-phorbol-13-hexadecanoate | C41H64O7 | C. tiglium | [50] |
| 133 | 13-O-acetylphorbol-4-deoxy-4β-phorbol-20-oleate | C40H62O7 | C. tiglium | [50] |
| 134 | 13-O-acetylphorbol-4-deoxy-4β-phorbol-20-linoleate | C40H60O7 | C. tiglium | [50] |
| 135 | 4-deoxy-20-oxophorbol 12-tiglyl 13-acetate | C27H34O7 | C. tiglium | [51] |
| 136 | 7-oxo-5-ene-phorbol-13-(2-methylbutyrate) | C25H34O8 | C. tiglium | [51] |
| 137 | 7-hydroxyl-phorbol-5-ene-13-(2-methyl)butyrate | C25H36O8 | C. tiglium | [51] |
Table 3.
Kaurane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 149 | Caracasine | C21H30O3 | C. caracasana | [53] |
| 150 | Caracasine acid | C20H28O3 | C. caracasana | [53] |
| 151 | Kongensin A | C22H30O5 | C. kongensis | [56] |
| 152 | Kongensin B | C22H30O6 | C. kongensis | [56] |
| 153 | Kongensin C | C20H28O5 | C. kongensis | [56] |
| 154 | Kongensin D | C20H28O4 | C. kongensis | [57] |
| 155 | Kongensin E | C26H36O7 | C. kongensis | [57] |
| 156 | Kongensin F | C24H34O5 | C. kongensis | [58] |
| 157 | Crotonkinin A | C20H30O2 | C. tonkinensis | [62] |
| 158 | Crotonkinin B | C22H32O4 | C. tonkinensis | [62] |
| 159 | 14-epi-hyalic acid | C20H28O4 | C. argyrophylloides | [63] |
| 160 | 14-[(2-methylbutanoyl)oxy]-3,4-seco-ent-kaura-4(19),16-dien-3-oic acid | C25H39O4 | C. megistocarpus | [54] |
| 161 | 14-{[(2Z)-2-methylbut-2-enoyl]oxy}-3,4-seco-ent-kaura-4(19),16-dien-3-oic acid | C25H37O4 | C. megistocarpus | [54] |
| 162 | ent-11β-acetoxykaur-16-en-18-ol | C22H34O3 | C. tonkinensis | [64] |
| 163 | ent-11α-hydroxy-18-acetoxykaur-16-ene | C22H34O3 | C. tonkinensis | [64] |
| 164 | ent-14β-hydroxy-18-acetoxykaur-16-ene | C22H34O3 | C. tonkinensis | [64] |
| 165 | ent-7α-hydroxy-18-acetoxykaur-16-ene | C22H34O3 | C. tonkinensis | [64] |
| 166 | ent-14S*-hydroxykaur-16-en-19-oic acid | C20H30O3 | C. pseudopulchellus | [65] |
| 167 | ent-14S*,17-dihydroxykaur-15-en-19-oic acid | C20H30O4 | C. pseudopulchellus | [65] |
| 168 | ent-3,4-seco-17-oxo-kaur-4(19),15(16)-dien-3-oic acid | C20H28O3 | C. oblongifolius | [55] |
| 169 | Crotonkinin C | C22H30O5 | C. tonkinensis | [66] |
| 170 | Crotonkinin D | C24H34O6 | C. tonkinensis | [66] |
| 171 | Crotonkinin E | C24H34O5 | C. tonkinensis | [66] |
| 172 | Crotonkinin F | C24H34O5 | C. tonkinensis | [66] |
| 173 | Crotonkinin G | C23H36O5 | C. tonkinensis | [66] |
| 174 | Crotonkinin H | C22H36O4 | C. tonkinensis | [66] |
| 175 | Crotonkinin I | C24H36O5 | C. tonkinensis | [66] |
| 176 | Crotonkinin J | C23H34O5 | C. tonkinensis | [66] |
| 177 | 14β-hydroxy-3-oxo-ent-kaur-16-ene | C20H30O2 | C. kongensis | [67] |
| 178 | Kongeniod A | C21H30O3 | C. kongensis | [59] |
| 179 | Kongeniod B | C21H30O4 | C. kongensis | [59] |
| 180 | Kongeniod C | C23H32O5 | C. kongensis | [59] |
| 181 | 15-oxo-17(10′-α-pinenyl)-kauran-18-oic acid | C30H44O3 | C. limae | [35] |
| 182 | Micansinoic acid | C40H58O7 | C. micans | [60] |
| 183 | Isomicansinoic acid | C40H58O7 | C. micans | [60] |
| 184 | Dimethylester of micansinoic | C42H62O7 | C. micans | [60] |
| 185 | Methyl-micansinoic acid | C41H60O7 | C. micans | [60] |
| 186 | Ethyl-micansinoic acid | C42H62O7 | C. micans | [60] |
| 187 | Crotonkinensin C | C40H62O8 | C. tonkinensis | [61] |
| 188 | Crotonkinensin D | C44H66O10 | C. tonkinensis | [61] |
Table 4.
Crotofolane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 189 | Crotocascarin A | C25H32O7 | C. cascarilloides | [68] |
| 190 | Crotocascarin B | C25H32O7 | C. cascarilloides | [68] |
| 191 | Crotocascarin C | C25H32O8 | C. cascarilloides | [68] |
| 192 | Crotocascarin D | C25H32O6 | C. cascarilloides | [68] |
| 193 | Crotocascarin E | C26H34O8 | C. cascarilloides | [68] |
| 194 | Crotocascarin F | C24H30O7 | C. cascarilloides | [68] |
| 195 | Crotocascarin G | C24H30O7 | C. cascarilloides | [68] |
| 196 | Crotocascarin H | C24H30O8 | C. cascarilloides | [68] |
| 197 | Crotocascarin α | C24H32O8 | C. cascarilloides | [68] |
| 198 | Crotocascarin β | C24H32O7 | C. cascarilloides | [68] |
| 199 | (5β,6β)-5,6: 13,16-diepoxycrotofola-4(9),10(18),13,15-tetraen-1-one | C20H22O3 | C. argyrophyllus | [72] |
| 200 | (5β,6β)-5,6: 13,16-diepoxy-2-epicrotofola-4(9),10(18),13,15-tetraen-1-one | C20H22O3 | C. argyrophyllus | [72] |
| 201 | (5β,6β)-5,6: 13,16-diepoxy-16-hydroxycrotofola-4(9),10(18),13,15-tetraen-1-one | C20H22O4 | C. argyrophyllus | [72] |
| 202 | (5β,6β)-5,6: 13,16-diepoxy-16-hydroxy-2-epi-crotofola-4(9),10(18),13,15-tetraen-1-one | C20H22O4 | C. argyrophyllus | [72] |
| 203 | Crotocarasin A | C20H22O4 | C. caracasanus | [73] |
| 204 | Crotocarasin B | C20H22O4 | C. caracasanus | [73] |
| 205 | Crotocarasin C | C22H26O5 | C. caracasanus | [73] |
| 206 | Crotocarasin D | C22H26O5 | C. caracasanus | [73] |
| 207 | EBC-162 | C20H24O2 | C. insularis | [74] |
| 208 | EBC-233 | C20H24O4 | C. insularis | [74] |
| 209 | EBC-300 | C20H24O4 | C. insularis | [74] |
| 210 | EBC-240 | C20H26O5 | C. insularis | [74] |
| 211 | EBC-241 | C20H26O5 | C. insularis | [74] |
| 212 | Crotocascarin I | C20H24O5 | C. cascarilloides | [69] |
| 213 | Crotocascarin J | C20H24O6 | C. cascarilloides | [69] |
| 214 | Crotocascarin K | C20H24O5 | C. cascarilloides | [69] |
| 215 | Crotocascarin γ | C19H24O6 | C. cascarilloides | [69] |
| 216 | Crotocascarin L | C22H26O7 | C. cascarilloides | [70] |
| 217 | Crotocascarin M | C21H26O6 | C. cascarilloides | [70] |
| 218 | Crotocascarin N | C20H22O6 | C. cascarilloides | [70] |
| 219 | Crotocascarin O | C25H34O9 | C. cascarilloides | [70] |
| 220 | Crotocascarin P | C25H34O8 | C. cascarilloides | [70] |
| 221 | Crotocascarin Q | C25H32O7 | C. cascarilloides | [70] |
| 222 | Neocrotocascarin | C25H32O8 | C. cascarilloides | [70] |
| 223 | Crotodichogamoin A | C20H22O4 | C. dichogamus | [75] |
| 224 | Crotodichogamoin B | C20H22O2 | C. dichogamus | [75] |
| 225 | Cascarinoid A | C28H31NO5 | C. cascarilloides | [71] |
| 226 | Cascarinoid B | C28H31NO5 | C. cascarilloides | [71] |
| 227 | Cascarinoid C | C28H31NO6 | C. cascarilloides | [71] |
Table 5.
Labdane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 228 | Labdinine N | C20H34O3 | C. laui | [76] |
| 229 | ent-12,15-dioxo-3,4-seco-4,8,13-labdatrien-3-oic acid | C20H28O4 | C. stipuliformis | [78] |
| 230 | ent-12,15-epoxy-3,4-seco-4,8,12,14-labdatetraen-3-oic acid | C20H28O3 | C. stipuliformis | [78] |
| 231 | ent-15-nor-14-oxo-3,4-seco-4,8,12(E)-labdatrien-3-oic acid | C19H28O3 | C. stipuliformis | [78] |
| 232 | ent-12,15-dioxo-8,13-labdadien-3a-ol | C20H28O3 | C. stipuliformis | [78] |
| 233 | Crotonlaevin A | C18H30O4 | C. laevigatus | [79] |
| 234 | Crotonlaevin B | C20H32O5 | C. laevigatus | [79] |
| 235 | Crotonlaevin C | C21H34O5 | C. laevigatus | [79] |
| 236 | Crotonlaevin D | C18H30O3 | C. laevigatus | [79] |
| 237 | Crotonlaevin E | C20H32O5 | C. laevigatus | [79] |
| 238 | Crotonlaevin F | C22H34O6 | C. laevigatus | [79] |
| 239 | Crotonlaevin G | C22H36O5 | C. laevigatus | [79] |
| 240 | Crotonlaevin H | C22H36O5 | C. laevigatus | [79] |
| 241 | Crotonlaevin I | C20H34O4 | C. laevigatus | [79] |
| 242 | Crotonlaevin J | C20H30O3 | C. laevigatus | [79] |
| 243 | Crotonlaevin K | C20H28O3 | C. laevigatus | [79] |
| 244 | Crotonlaevin L | C20H30O4 | C. laevigatus | [79] |
| 245 | Crotonlaevin M | C20H30O4 | C. laevigatus | [79] |
| 246 | Crotonlaevin N | C20H30O3 | C. laevigatus | [79] |
| 247 | Crotonlaevin O | C20H30O3 | C. laevigatus | [79] |
| 248 | Crotonlaevin P | C20H30O3 | C. laevigatus | [79] |
| 249 | Crotonolide I | C20H34O3 | C. laui | [21] |
| 250 | Crotonolide J | C19H30O3 | C. laui | [21] |
| 251 | Launine A | C19H32O3 | C. laui | [82] |
| 252 | Launine B | C19H32O4 | C. laui | [82] |
| 253 | Launine C | C20H34O3 | C. laui | [82] |
| 254 | Launine D | C20H34O3 | C. laui | [82] |
| 255 | Launine E | C20H32O5 | C. laui | [82] |
| 256 | Launine F | C20H32O5 | C. laui | [82] |
| 257 | Launine G | C20H30O4 | C. laui | [82] |
| 258 | Launine H | C20H30O4 | C. laui | [82] |
| 259 | Launine I | C20H34O3 | C. laui | [82] |
| 260 | 15,16-epoxy-4-hydroxy-labda-13(16),14-dien-3,12-dione | C20H28O4 | C. jacobinensis | [77] |
| 261 | Crotondecalvatin A | C29H42O4 | C. decalvatus | [80] |
| 262 | Crotondecalvatin B | C30H42O6 | C. decalvatus | [80] |
| 263 | Bicrotonol A | C40H68O4 | C. crassifolius | [81] |
Table 6.
Cembrane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 264 | Launine O | C20H34O2 | C. laui | [76] |
| 265 | Launine P | C21H36O2 | C. laui | [76] |
| 266 | Furanocembranoid 1 | C20H30O2 | C. oblongifolius | [83] |
| 267 | Furanocembranoid 2 | C20H30O3 | C. oblongifolius | [83] |
| 268 | Furanocembranoid 3 | C20H32O4 | C. oblongifolius | [83] |
| 269 | Furanocembranoid 4 | C20H32O5 | C. oblongifolius | [83] |
| 270 | Laevigatlactone A | C20H30O3 | C. laeVigatus | [84] |
| 271 | Laevigatlactone C | C20H30O3 | C. laeVigatus | [84] |
| 272 | Laevigatlactone B | C20H30O3 | C. laeVigatus | [84] |
| 273 | Laevigatlactone D | C20H30O3 | C. laeVigatus | [84] |
| 274 | Laevigatlactone E | C20H30O4 | C. laeVigatus | [84] |
| 275 | Laevigatlactone F | C20H30O5 | C. laeVigatus | [84] |
| 276 | (+)-[1R*,2S*,7S*,8S*,12R*]-7,8-Epoxy-2,12-cyclocembra-3E,10Zdien-20,10-olide | C20H28O3 | C. gratissimus | [85] |
| 277 | (+)-[1R*,10R*]-Cembra-2E,4E,7E,11Z-tetraen-20,10-olide | C20H28O2 | C. gratissimus | [85] |
| 278 | (+)-[1R*,4S*,10R*]-4-Hydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O3 | C. gratissimus | [85] |
| 279 | (−)-[1R*,4R*,10R*]-4-Hydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O3 | C. gratissimus | [85] |
| 280 | (−)-(1R*,4R*,10R*)-4-Methoxycembra-2E,7E,11Z-trien-20,10-olide | C21H32O3 | C. gratissimus | [86] |
| 281 | (−)-(1S*,4R*,10R*)-1-Hydroxy-4-methoxycembra-2E,7E,11Ztrien-20,10-olide | C21H32O4 | C. gratissimus | [86] |
| 282 | (−)-(1S*,4S*,10R*)-1,4-Dihydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O4 | C. gratissimus | [86] |
| 283 | (−)-(1S*,4S*,10R*)-1,4-Dihydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O4 | C. gratissimus | [86] |
| 284 | (+)-(10R*)-Cembra-1E,3E,7E,11Z,16-pentaen-20,10-olide | C20H26O | C. gratissimus | [86] |
| 285 | (+)-(10R*)-Cembra-1Z,3Z,7E,11Z,15-pentaen-20,10-olide | C20H26O | C. gratissimus | [86] |
| 286 | (+)-(5R*,10R*)-5-Methoxycembra-1E,3E,7E,11Z,15-pentaen-20,10-olide | C21H30O3 | C. gratissimus | [86] |
| 287 | (+)-(1S*,4S*,7R*,10R*)-1,4,7-Trihydroxycembra-2E,8(19),11Z-trien-20,10-olide | C20H30O5 | C. gratissimus | [86] |
| 288 | (−)-(1S*,4S*,7S*,10R*)-1,4,7-Trihydroxycembra-2E,8(19),11Z-trien-20,10-olide | C20H30O3 | C. gratissimus | [86] |
| 289 | (+)-(1S*,4R*,8S*,10R*)-1,4,8-Trihydroxycembra-2E,6E,11Z-trien-20,10-olide | C20H30O5 | C. gratissimus | [86] |
| 290 | Cembranoid 1 | C20H30O4 | C. longissimus | [87] |
| 291 | Cembranoid 2 | C20H30O3 | C. longissimus | [87] |
| 281 | (−)-(1S*,4R*,10R*)-1-Hydroxy-4-methoxycembra-2E,7E,11Ztrien-20,10-olide | C21H32O4 | C. gratissimus | [86] |
| 282 | (−)-(1S*,4S*,10R*)-1,4-Dihydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O4 | C. gratissimus | [86] |
| 283 | (−)-(1S*,4S*,10R*)-1,4-Dihydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O4 | C. gratissimus | [86] |
| 284 | (+)-(10R*)-Cembra-1E,3E,7E,11Z,16-pentaen-20,10-olide | C20H26O | C. gratissimus | [86] |
| 285 | (+)-(10R*)-Cembra-1Z,3Z,7E,11Z,15-pentaen-20,10-olide | C20H26O | C. gratissimus | [86] |
| 286 | (+)-(5R*,10R*)-5-Methoxycembra-1E,3E,7E,11Z,15-pentaen-20,10-olide | C21H30O3 | C. gratissimus | [86] |
| 287 | (+)-(1S*,4S*,7R*,10R*)-1,4,7-Trihydroxycembra-2E,8(19),11Z-trien-20,10-olide | C20H30O5 | C. gratissimus | [86] |
| 288 | (−)-(1S*,4S*,7S*,10R*)-1,4,7-Trihydroxycembra-2E,8(19),11Z-trien-20,10-olide | C20H30O3 | C. gratissimus | [86] |
| 289 | (+)-(1S*,4R*,8S*,10R*)-1,4,8-Trihydroxycembra-2E,6E,11Z-trien-20,10-olide | C20H30O5 | C. gratissimus | [86] |
| 290 | Cembranoid 1 | C20H30O4 | C. longissimus | [87] |
| 291 | Cembranoid 2 | C20H30O3 | C. longissimus | [87] |
Table 7.
Abietane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 292 | Isolophanthin E | C20H30O3 | C. megalocarpoides | [27] |
| 293 | rel-(1R,4aR,5R,8R)-methyl-7-(1-(methoxycarbonyl)vinyl)-5,8-diacetoxy-1,2,3,4a,5,6,7,8,9,10,10a-dodecahydro-1,4a-dimethyl-2-oxophenanthrene-1-carboxylate | C26H34O9 | C. argyrophylloides | [63] |
| 294 | Crotontomentosin A | C20H26O2 | C. caudatus | [88] |
| 295 | Crotontomentosin B | C20H30O3 | C. caudatus | [88] |
| 296 | Crotontomentosin D | C20H24O2 | C. caudatus | [88] |
| 297 | Crotontomentosin C | C20H28O2 | C. caudatus | [88] |
| 298 | Crotontomentosin E | C22H32O3 | C. caudatus | [88] |
| 299 | Crotolaevigatone A | C20H24O3 | C. laevigatus | [89] |
| 300 | Crotolaevigatone B | C20H26O2 | C. laevigatus | [89] |
| 301 | Crotolaevigatone C | C20H26O3 | C. laevigatus | [89] |
| 302 | Crotolaevigatone D | C20H28O4 | C. laevigatus | [89] |
| 303 | Crotolaevigatone E | C19H24O2 | C. laevigatus | [89] |
| 304 | Crotolaevigatone F | C20H30O4 | C. laevigatus | [89] |
| 305 | Crotolaevigatone G | C20H30O4 | C. laevigatus | [89] |
Table 8.
Casbane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 306 | 1,4-dihydroxy-2E,6E,12E-trien-5-one-casbane | C20H30O3 | C. nepetaefolius | [90] |
| 307 | 4-hydroxy-2E,6E,12E-5-one-casbane | C20H29O3 | C. nepetaefolius | [90] |
| 308 | 1-hydroxy-(2E,6Z,12E)-casba-2,6,12-triene-4,5-dione | C20H28O3 | C. argyrophyllus | [91] |
| 309 | 6E,12E-casba-1,3,6,12-tetraen-1,4-epoxy-5-one | C20H26O2 | C. argyrophyllus | [91] |
| 310 | (2E,5β,6E,12E)-5-hydroxycasba-2,6,12-trien-4-one | C20H30O2 | C. argyrophyllus | [72] |
| 311 | EBC-324 | C20H28O5 | C. insularis | [92] |
| 312 | EBC-329 | C20H26O4 | C. insularis | [92] |
Table 9.
Halimane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 313 | Crassifoliusin A | C21H24O5 | C. crassifolius | [95] |
| 314 | Crotontomentosin F | C21H30O3 | C. caudatus | [88] |
| 315 | Crolaevinoid A | C27H30O7 | C. laevigatus | [39] |
| 316 | Crolaevinoid B | C20H26O4 | C. laevigatus | [39] |
| 317 | Crothalimene A | C20H26O4 | C. dichogamus | [75] |
| 318 | Crothalimene B | C20H30O2 | C. dichogamus | [75] |
Table 10.
Pimarane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 319 | ent-3β-hydroxypimara-8(14),9,15-trien-12-one | C20H28O2 | C. insularis | [98] |
| 320 | EBC-316 | C20H26O2 | C. insularis | [99] |
| 321 | EBC-325 | C20H26O4 | C. insularis | [99] |
| 322 | EBC-326 | C20H26O4 | C. insularis | [99] |
| 323 | EBC-327 | C20H24O3 | C. insularis | [99] |
| 324 | EBC-345 | C20H30O4 | C. insularis | [99] |
Table 11.
Cleistanthane type diterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 325 | 3-hydroxycleistantha-13(17),15-diene | C20H32O | C. oblongifolius | [93] |
| 326 | 3,4-seco-cleistantha-4(18),13(17),15-trien-3-oic acid | C20H30O2 | C. oblongifolius | [93] |
| 327 | rel-(5β,8α,10α)-8-hydroxy-13-methylpodocarpa-9(11),13-diene-3,12-dione | C18H25O3 | C. regelianus | [94] |
Table 12.
Grayanane type diterpenoids from the genus Croton.
Table 13.
Atisane type diterpenoids from the genus Croton.
Table 14.
Phytane type diterpenoids from the genus Croton.
Table 15.
Laevinane type diterpenoids from the genus Croton.
Table 16.
Meroditerpenoids from the genus Croton.
Table 17.
Sesquiterpenoids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 340 | 6α -methoxy-cyperene | C16H26O | C. muscicarpa | [103] |
| 341 | rel-(1R,4S,6R,7S,8αR)-decahydro-1-(hydroxymethyl)-4,9,9-trimethyl-4,7-(epoxymethano)azulen-6-ol | C15H26O3 | C. regelianus | [94] |
| 342 | Blumenol A | C13H20O3 | C. pedicellatus | [104] |
| 343 | Crocrassins A | C15H24O3 | C. crassifolius | [105] |
| 344 | Crocrassins B | C16H26O3 | C. crassifolius | [105] |
| 345 | 1,3,5-cadinatriene-(7R,10S)-diol | C15H25O2 | C. dichogamus | [75] |
| 346 | Cracroson H | C15H22O3 | C. crassifolius | [40] |
Table 18.
Sesterterpenoid from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 347 | Pseudopulchellol | C25H40O | C.pseudopulchellus | [106] |
Table 19.
Triterpenoid from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 348 | 3α-hydroxy-urs-12,15-dien | C30H48O | C. bonplandianum | [107] |
Table 20.
Glycosides from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 349 | Cyperenoic acid-9-O-β-d-glucopyranoside | C21H32O8 | C. crassifolius | [108] |
| 350 | 3-O-β-d-xylopyranosylspathodic acid | C35H56O9 | C. lachnocarpus | [109] |
| 351 | Helichrysoside-3′-methylether | C31H28O14 | C. zambesicus | [110] |
| 352 | Crotonionoside A | C29H42O11 | C. cascarilloides | [111] |
| 353 | Crotonionoside B | C30H44O12 | C. cascarilloides | [111] |
| 354 | Crotonionoside C | C24H42O12 | C. cascarilloides | [111] |
| 355 | Crotonionoside D | C31H46O14 | C. cascarilloides | [111] |
| 356 | Crotonionoside E | C35H52O16 | C. cascarilloides | [111] |
| 357 | Crotonionoside F | C24H42O11 | C. cascarilloides | [111] |
| 358 | Crotonionoside G | C29H40O11 | C. cascarilloides | [111] |
| 359 | Oblongionoside A | C24H42O12 | C. oblongifolius | [112] |
| 360 | Oblongionoside B | C24H42O12 | C. oblongifolius | [112] |
| 361 | Oblongionoside C | C24H44O11 | C. oblongifolius | [112] |
| 362 | Oblongionoside D | C24H44O11 | C. oblongifolius | [112] |
| 363 | Oblongionoside E | C19H36O8 | C. oblongifolius | [112] |
| 364 | Oblongionoside F | C19H36O8 | C. oblongifolius | [112] |
| 365 | Blumenol A glucoside | C19H30O8 | C. pedicellatus | [10] |
| 366 | Sparsioside | C53H102O10 | C. sparsiorus | [113] |
| 367 | 3,12-dioxo-15,16-epoxy-4α-hydroxy-6-(β-glucopyranosyl)-ent-neo-clerodan-13(16),14-diene | C26H38O10 | C. limae | [35] |
| 368 | Isocrotofolane glucoside | C26H38O9 | C. cascarilloides | [69] |
| 369 | 2-methoxyphenol-β-d-(6-O-β-d-apiofuranosyl) glucopyranoside | C18H26O11 | C. cascarilloides | [69] |
Table 21.
Alkaloids from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 370 | Crotamide A | C36H65NO | C. sparsiflorus | [114] |
| 371 | Crotamide B | C38H69NO | C. sparsiflorus | [114] |
| 372 | Crotonine | C12H14N2O4 | C. tiglium | [97] |
| 373 | Crotonimide A | C16H20N2O3 | C. pullei | [115] |
| 374 | Crotsparsidine | C17H17O3N | C. sparsiflorus | [96] |
| 375 | Crotonimide C. | C20H20N2O3 | C. alienus | [42] |
| 376 | 6-Hydroxy-1-methyl-2-dimethyl-3,4-tetrahydro-b-carbo-line | C14H19N2O | C. heliotropiifolius | [116] |
| 377 | N-trans-feruloyl-3,5-dihydroxyindolin-2-one | C20H20N2O6 | C. echioides | [117] |
Table 22.
Benzoate derivatives from the genus Croton.
Table 23.
Pyran-2-one derivatives from the genus Croton.
Table 24.
Cyclicpeptides from the genus Croton.
Table 25.
Tropone derivatives from the genus Croton.
Table 26.
Limonoids from the genus Croton.
Table 27.
Miscellaneous compounds from the genus Croton.
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 390 | Crotoncaudatin | C22H22O9 | C. caudatus | [127] |
| 391 | 8S-(−)-8-(4-hydroxy-3-methoxybenzoyl)-dihydrofuran-8(8′H)-one | C20H30O2 | C. kongensis | [67] |
| 392 | Lobaceride | C35H58O6 | C. lobatus | [129] |
| 393 | Laevifolin A | C29H38O4 | C. laevifolius | [128] |
| 394 | Laevifolin B | C29H38O4 | C. laevifolius | [128] |
| 395 | 2,6-Dimethyl-1-oxo-4-indanecarboxylic acid | C12H12O3 | C. steenkampianus | [102] |
| 396 | 3(3′-Methoxy-5′-phenylfuran-2′-yl)propan-1-ol | C14H16O3 | C. oblongifolius | [22] |
| 397 | Sparsifol | C7H15O6 | C. sparsiflorus | [96] |
| 398 | Sparsioamide | C43H81NO5 | C. sparsiflorus | [113] |
| 399 | hexyl Z-ferulate | C16H22O4 | C. laevigatus | [89] |
2.1. Diterpenoids
Phytochemical investigations on Croton species revealed the predominant secondary metabolites as diterpenoids, including clerodane, tigliane, kaurane, crotofolane, labdane, cembrane, abietane, casbane, halimane, pimarane, cleistanthane, grayanane, atisane, phytane, and laevinane diterpenoids. Three hundred & thirty-nine new diterpenoids (1–339) were reported from Croton species.
2.1.1. Clerodanes
Ninety-two new clerodane diterpenoids (1–92) were isolated from Croton species, including two clerodane diterpenoid with acyclic at C-9s, eight clerodane diterpenoids with butenolide at C-9, and 82 furan-clerodane diterpenoids [11]. Their structures, molecular formula, names, corresponding sources, and references are listed in Figure 1 and Table 1. Two new clerodane diterpenoids with acyclic side chain at C-9, ent-3,13E-clerodadiene-15-formate (1) and 3α,4α,15,16-tetrahydroxy-ent-neo-cleroda-13E-ene (45), were isolated from the roots of C. sylvaticus [12] and the roots of C. limae [13], respectively. Eight new clerodane diterpenoids with butenolide at C-9 (2, 3, 5, 13, 14, 75, 91, 92) were obtained from three Croton species (C. crassifolius, C. glabellus, and C. oligandrus) [14,15,16,17,18]. Furan-clerodane diterpenoids are abundant in Croton species, and 82 new ones were isolated from different Croton species. For example, Centrafricine I (4) from C. mayumbensis was a new furan-clerodane diterpenoid with a 6, 18-γ-lactone ring [19]. Two novel rearranged ent-clerodane diterpenoids Laevinoids A, B (21, 22) containing an unusual 3/5 bicyclic ring were obtained from the branches and leaves of C. laevigatus; 22 represents the first chlorinated example of the clerodane family [20]. Compounds (23–27) bearing a C-19/C-20 six-membered ring were identified from C. laui [21]. Phytochemical investigations on three Croton species (C. oblongifolius, C. yanhuii, and C. hypoleucus) afforded six new furan-clerodanoids (12, 36, 37, 84–86) with a 3,4-epoxy moiety [22,23,24]. Crotoeurins A–C (40–42) were found from the twigs and leaves of C. euryphyllus. Among them, crotoeurin A (40) was a nor-clerodane diterpenoid dimer with a unique cyclobutane ring via a [2 + 2] cycloaddition [25]. Three new furan-clerodane diterpenoids, cracroson A–C (46–48) were obtained from C. crassifolius, while cracroson C (48) represents the first example of a clerodane diterpenoid alkaloid [26]. Twelve new ent-clerodanoids (55, 66) were isolated from the roots of C. megalocarpoides. Among them, compounds (58–66) possessed 9, 12-γ-lactone ring [27]. Investigation on the roots of C. crassifolius afforded eight new clerodanoids, crassins A−H (68–75). Among them, crassins A–B (68, 69) represents ring B rearranged clerodanoids, whereas crassins C (70) was ring A rearranged one [17]. One new nor-clerodane diterpenoid, norcrassifolin (83), with a 1,12-lactone six-membered ring, was isolated from C. crassifolius [28].
2.1.2. Tiglianes
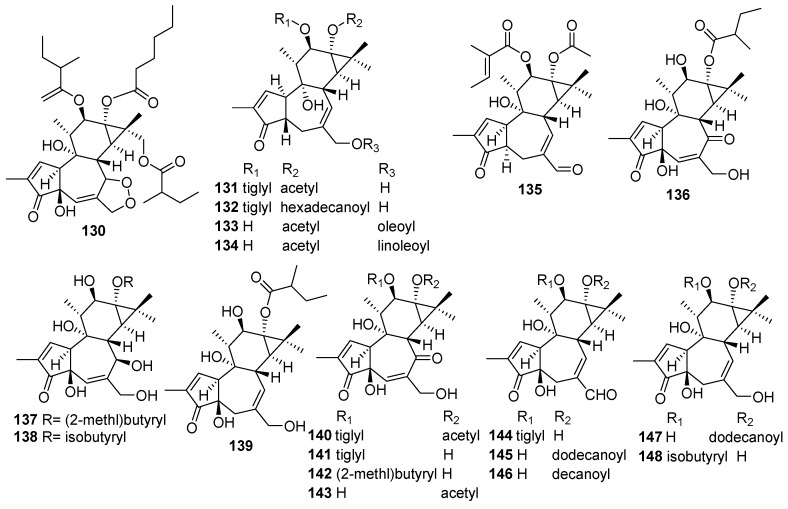
Fifty-six new tigliane diterpenoids (93–148) were reported from Croton species. Their structures, molecular formula, names, corresponding sources, and references are collected in Figure 2 and Table 2. Investigations on the aerial parts of C. ciliatoglandulifer produced four new tiglianoids (95–98). Among them, tiglianoids (95–97) possess a N,N-dimethyl moiety at 2′position [41]. Alienusolin (107) and compound (111) were obtained from the roots and the leaves of C. alienus and the leaves of C. mauritianus, respectively [42,43]. The twigs and leaves of C. caudatus produced three new tiglianoids, crotusins A–C (128–130) [44]. Tigliane diterpenoids were abundant in C. tiglium, other 47 new ones (93, 94, 99–106, 108–110, 112–127, 131–148) were isolated from C. tiglium [45,46,47,48,49,50,51,52]. Among them, compound (112) was the first tiglianoid with the C20-aldehyde group [48].
2.1.3. Kauranes
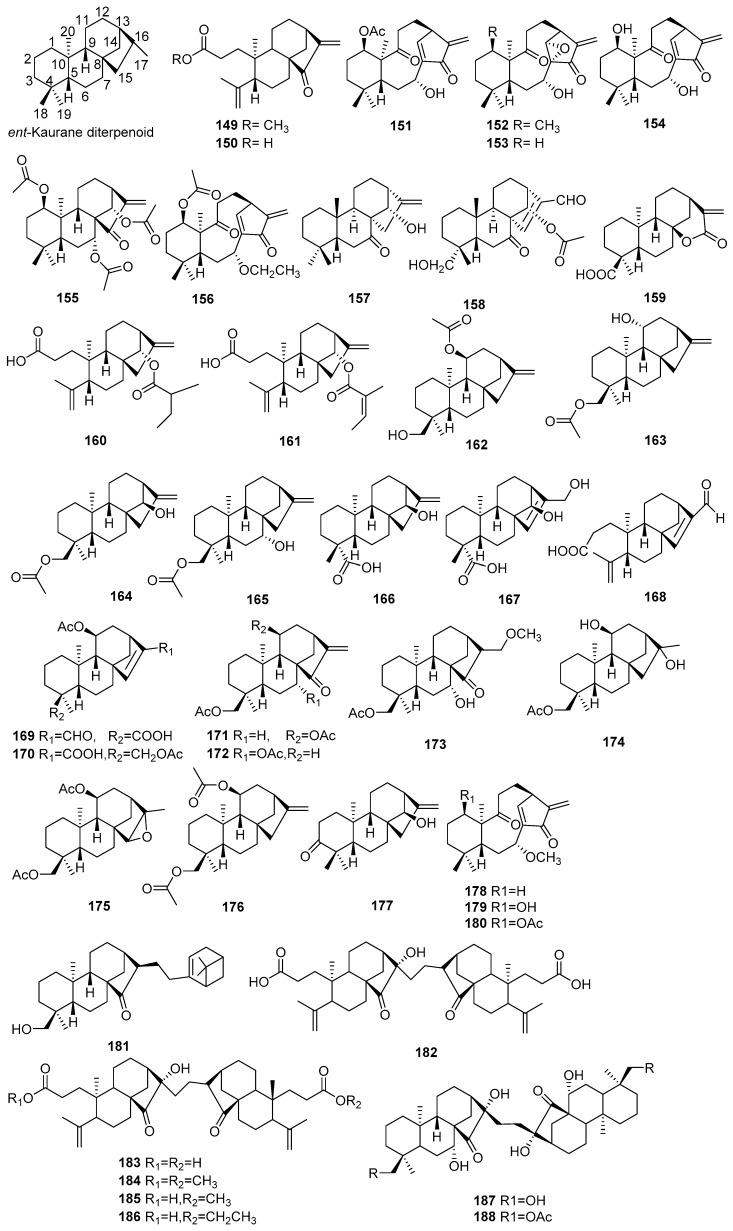
Fourty new kaurane diterpenoids (149–188) were found from Croton species. Their structures, molecular formula, names, corresponding sources, and references are listed in Figure 3 and Table 3. Five new 3,4-seco ent-kauranes (149–150, 160–161, 168) were isolated from C. caracasana [53], C. megistocarpus [54], and C. oblongifolius [55], respectively. Investigations on C. kongensis afforded eight new 8,9-seco-ent-kaurane diterpenes (151–154, 156, 178–180) [56,57,58,59]. Compound 181, one new kaurane bearing a monoterpene unit at C-16, was found from C. limae [35]. From the stems of C. micans, five new 3,4-seco-ent-kaurene dimers (182–186) were isolated [60], while other two dimeric ent-kaurane diterpenoids (187–188) were elucidated from C. tonkinensis [61].
2.1.4. Crotofolanes
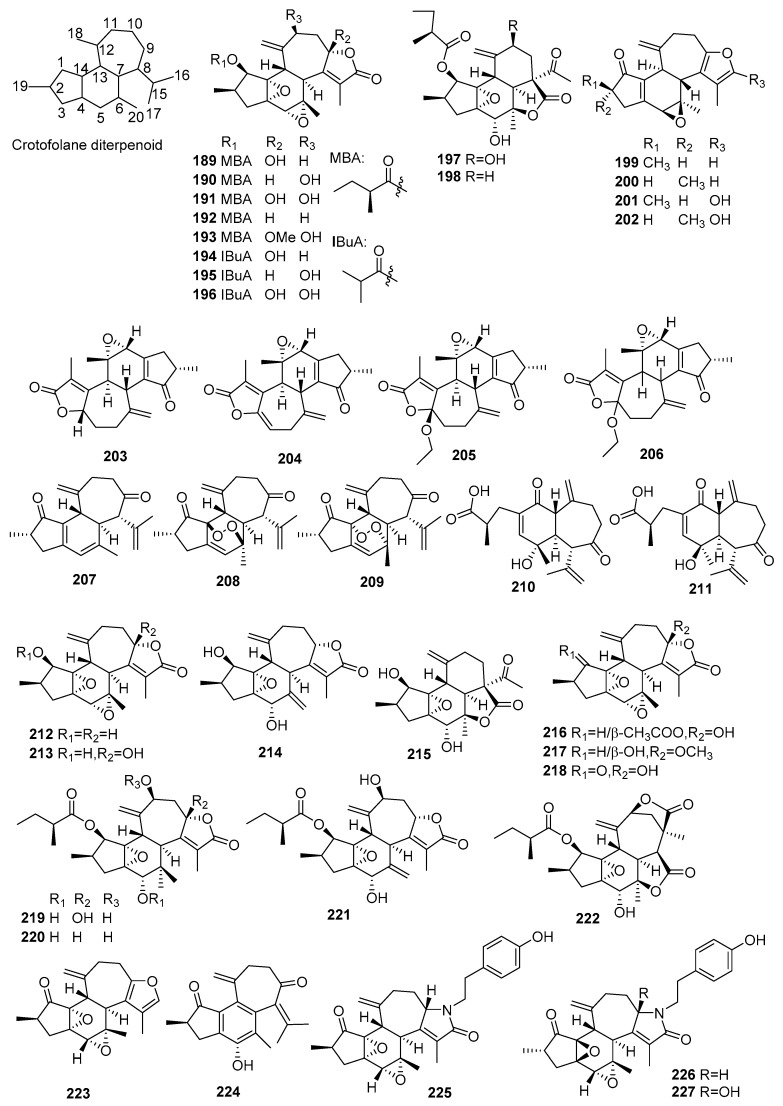
Thirty-nine new crotofolane diterpenoids (189–227) were obtained from Croton species. Their structures, molecular formula, names, corresponding sources, and references are summarized in Figure 4 and Table 4. Twenty-four new crotofolane diterpenoids (189–198, 212–222, 225–227) were isolated from C. caracasanus [68,69,70,71]. Among them, three new crotofolane diterpenoid alkaloids, cascarinoids A–C (225–227), were firstly found. Investigations on C. argyrophyllus gave four new crotofolanes (199–202) [72]. Crotocarasin A–D (203–206) were isolated from the stems of C. caracasanus [73]. Five new 1, 14-seco-crotofolanes from C. insularis were obtained [74], while C. dichogamus yielded crotodichogamoin A–B (223–224) [75].
2.1.5. Labdanes
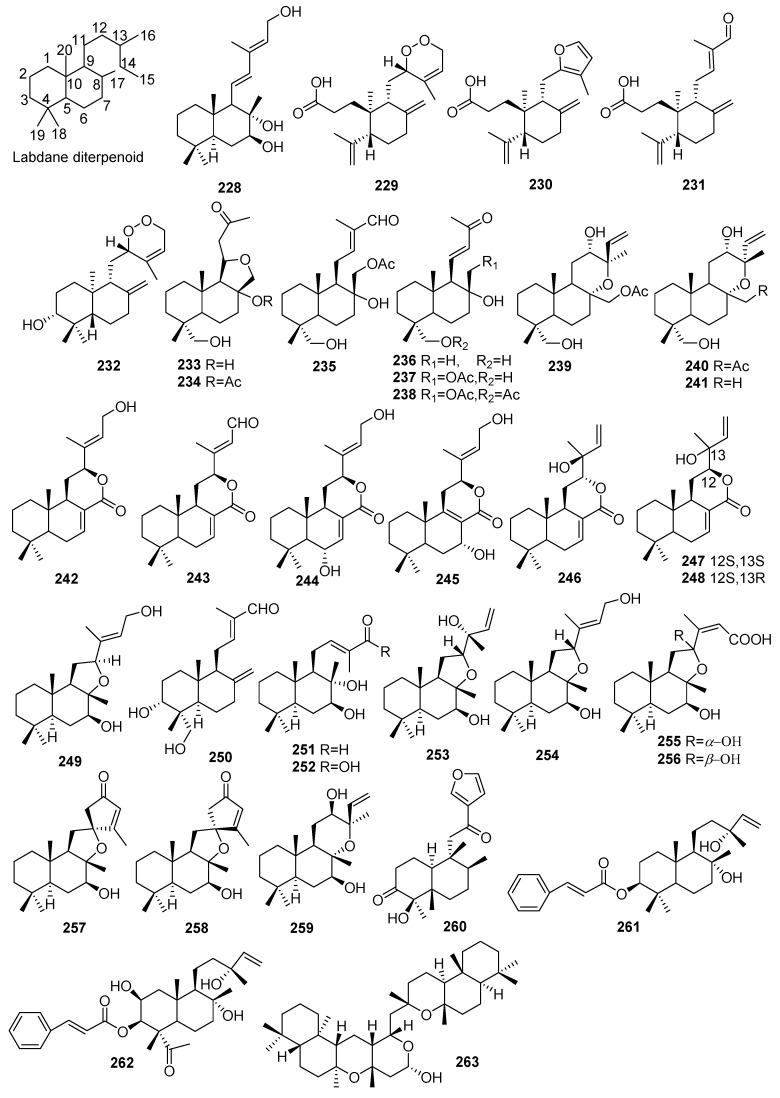
Thirty-six new labdane diterpenoids (228–263) were isolated from Croton species. Their structures, molecular formula, names, corresponding sources, and references are collected in Figure 5 and Table 5 12 new labdanes (228, 249–259) were isolated from C. laui [21,76,77]. From the leaves of C. stipuliformis, three 3,4-seco-ent-labdanes (229–231) and one ent-labdane (232) were obtained [78]. Investigation of C. laevigatus led to the isolation of 16 new labdanes (233–248). Among them, crotonlaevins A–B (233, 234), represents rare labdanes with a dodecahydronaphtho [1,2-c] furan moiety [79]. Three new labdane diterpenoids (260–262) were found from C. jacobinensis [77] and C. decalvatus [80], respectively. Bicrotonol A (263), one dimeric labdane-type diterpenoid, was obtained from the roots of C. crassifolius [81].
2.1.6. Cembranes
A total of 28 new cembrane diterpenoids (264–291) were obtained from Croton species. Their structures, molecular formula, names, corresponding sources, and references are listed in Figure 6 and Table 6. launine O-P (264, 265), two new cembranes, were reported from the aerial parts of C. laui [76]. Investigations on the stem bark of C. oblongifolius afforded four new furanocembranoids (266–269) [83]. laevigatlactones A–F (270–275), six new cembranoids possessing a rare six-membered lactone moiety attached to C-1 and C-20, were firstly isolated from C. laevigatus [84]. 14 new cembranoids (276–289) were found from C. gratissimus [85,86]. Among them, compound 276 was first example of a 2,12-cyclocembranolide. The leaves of C. longissimus produced two new cembranes (290, 291) [87].
2.1.7. Abietanes
Fourteen new abietane diterpenoids (292–305) were isolated from Croton species. Their structures, molecular formula, names, corresponding sources, and references are collected in Figure 7 and Table 7. Two new abietanes (292, 293) were obtained from C. megalocarpoides [27], and C. argyrophylloides [63], respectively. Investigation of C. caudatus led to the isolation of 5 new abietanes (294–298). Among them, crotontomentosin A (294) was a 9,10-seco abietane [88]. Crotolaevigatones A–G (299–305), 7 new abietanes were found from the twigs and leaves of C. laevigatus, and compounds (304, 305) possessed a 9,13-epidioxy moiety [89].
2.1.8. Casbanes
Seven new casbane diterpenoids (306–312) were found from Croton species. Their structures, molecular formula, names, corresponding sources, and references are summarized in Figure 8 and Table 8. Five new casbane s (306–310) were reported from C. nepetaefolius [90], and C. argyrophyllus [72,91], respectively. Investigations on the stem bark of C. insularis afforded two new casbanes, EBC-324 (311) and EBC-329 (312). Among them, EBC-329 (312) represented the first natural seco-casbane diterpene, while EBC-324 (311) was the first endoperoxide casbane [92].
2.1.9. Halimanes
Six new halimane diterpenoids (313–318) were reported from Croton species. Their structures, molecular formula, names, corresponding sources, and references are collected in Figure 8 and Table 9. Investigations on the stem bark of C. oblongifolius afforded two new cleistanthanes (325, 326). Among them, compound 326 was a 3,4-seco cleistanthane [93]. One new bis-nor-cleistanthane diterpenoid (327), was found from the twigs and leaves of C. caudatus [94].
2.1.10. Pimaranes
Six new pimarane diterpenoids (319–324) were obtained from Croton species. Their structures, molecular formula, names, corresponding sources, and references are listed in Figure 8 and Table 10. All six new pimaranes (319–324) were isolated from C. insularis [96,97]. Among them, compound 319 was an important biosynthetic intermediate.
2.1.11. Cleistanthanes
Three new cleistanthane diterpenoids (325–327) were ioslated from Croton species. Their structures, molecular formula, names, corresponding sources, and references are collected in Figure 8 and Table 11. Investigations on the stem bark of C. oblongifolius afforded two new cleistanthanes (325, 326). Among them, compound 326 was a 3,4-seco cleistanthane [93]. One new bis-nor-cleistanthane diterpenoid (327), was found from the twigs and leaves of C. caudatus [94].
2.1.12. Grayananes, Atisanes, Phytanes, Laevinanes and Meroditerpenoids
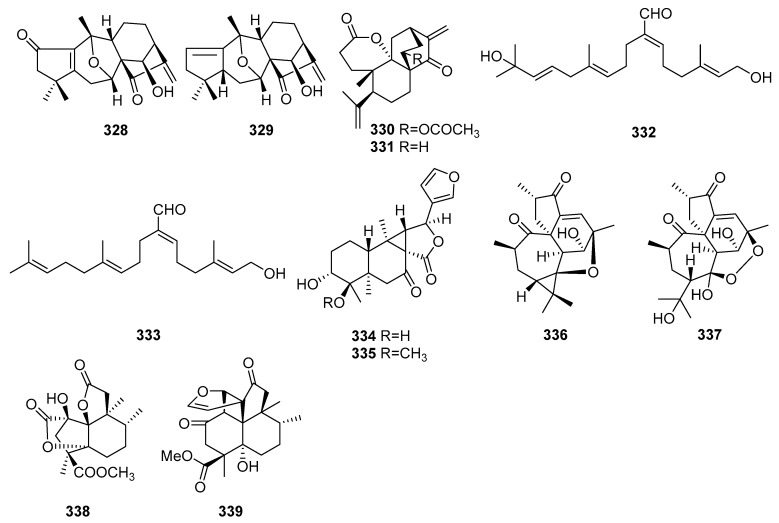
From the leaves of C. tonkinensis, two new rare grayanane diterpenoids, crotonkinensins A (328) and B (329), were isolated [100]. Two new 3,4-seco atisane diterpenoids, crotobarin (330) from C. barorum and crotogoudin (331) from C. goudotii, were found [101]. Investigations on the aerial parts of C. laui gave two new phytane diterpenoids (332, 333) [37]. Two new laevinane diterpenoids, crolaevinoid G (334) and H (335), were obtained [39]. Two new meroditerpenoids, steenkrotin A (336) and B (337), containing new carbon skeletons, were isolated from the leaves of C. steenkampianus [102]. From the the roots of C. crassifolius, two new meroditerpenoids, norcrassin A (338) and cracroson D (339), were reported [35,69]. Among them, norcrassin A (338) possessing a new carbon skeleton with a 5/5/5/6 tetracyclic system, was a C16 tetranorditerpenoid, while cracroson D (339) featured a new skeleton with a rare cyclobutane ring. Their structures, molecular formula, names, corresponding sources, and references are listed in Figure 9 and Table 12, Table 13, Table 14, Table 15 and Table 16.
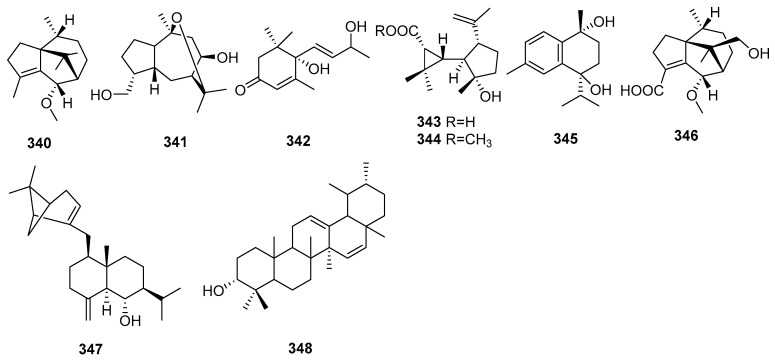
2.2. Sesquiterpenoids, Sesterterpenoids and Triterpenoids
Seven new sesquiterpenoids (340–346), one sesterterpenoid (347) and one triterpenoid (348) were ioslated from Croton species. Their structures, molecular formula, names, corresponding sources, and references are summarized in Figure 10 and Table 17, Table 18 and Table 19. From C. muscicarpa, one new patchoulane sesquiterpenoid (340) was obtained [103]. A guaiane sesquiterpenoid (341) was isolated from C. regelianus [94]. Investigations on the leaves of C. pedicellatus afforded a bis-nor-sesquiterpenoid (342) [104]. Two rare sesquiterpenoid, Crocrassins A (343) and B (344) having cyclopropylcyclopentane moiety, were reported [105]. Other two sesquiterpenoids, 1,3,5-cadinatriene-(7R,10S)-diol (345) and cracroson H (346) were found from C. dichogamus [75], and C. crassifolius [40], respectively. One rare sesterterpenoid, pseudopulchellol (347), was isolated from the leaves of C. pseudopulchellus [106]. From the root of C. bonplandianum, a new ursane triterpenoid (348) was obtained [107].
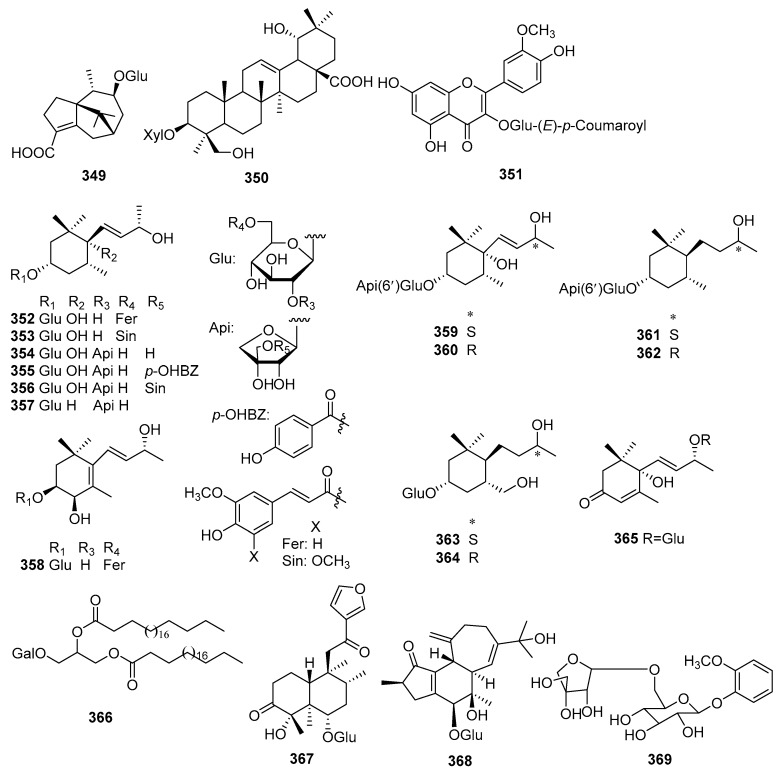
2.3. Glycosides
Twenty-one new glycosides (349–369) were ioslated from Croton species. Their structures, molecular formula, names, corresponding sources, and references are collected in Figure 11 and Table 20. From C. crassifolius, a patchoulane sesquiterpenoid glycoside (349), an isocrotofolane glucoside (368), and a phenolic glycoside (369) were reported [69,108]. Compound 350, isolated from C. lachnocarpus, was the first triterpenoid glucoside reported from the genus Croton [109]. A new flavone glucoside (351) was found from the leaves of C. zambesicus [110]. Investigations on the leaves of C. cascarilloides and C. oblongifolius afforded 13 new megastigmane glycosides, crotonionosides A–G (352–358) and Oblongionosides A–F (359–364) [111,112]. One new bis-nor-sesquiterpenoid glycoside (365) was isolated from C. pedicellatus [104]. One new diglyceride galactoside (366) and one new clerodane glucoside (367) were obtained from C. sparsiorus [113], and C. limae [35], respectively.
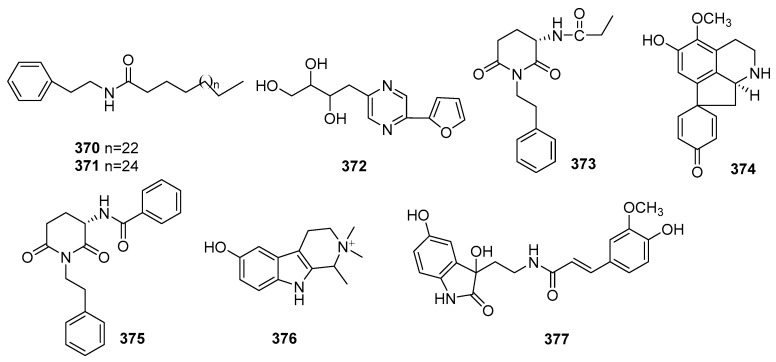
2.4. Alkaloids
Eight new alkaloids (370–377) were reported from Croton species. Their structures, molecular formula, names, corresponding sources, and references are listed in Figure 12 and Table 21. From C. sparsiflorus, two new amide alkaloids crotamides A (370) and B (371), and one new proaporphine alkaloid, crotsparsidine (374) were isolated [96,114]. One new pyrazine derivative, crotonine (372) was obtained from the leaves of C. tiglium [97]. Investigations on C. cascarilloides afforded a new glutarimide alkaloid, crotonimide C (375) [42]. Other three new alkaloids (373, 376–377) were found from C. pullei, C. heliotropiifolius, and C. echioides, respectively [115,116,117].
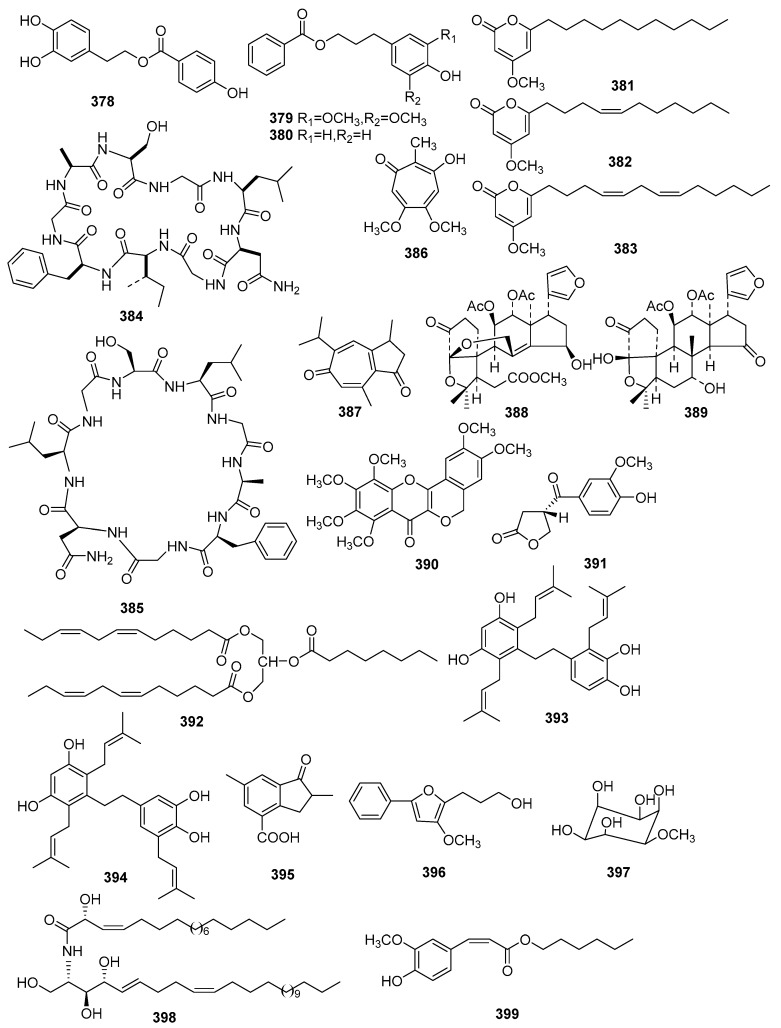
2.5. Benzoate Derivatives, Pyran-2-One Derivatives, Cyclicpeptides, Tropone Derivatives and Limonoids
Three benzoate derivatives (378–380) were isolated from C. sylvaticus and C. hutchinsonianus [118,119]. Investigations on C. crassifolius afforded three new pyran-2-one derivatives, crotonpyrone A (381), B (382) and C (383) [120,121]. Two cyclicpeptides (384, 385) were obtained from C. gossypifolius and C. urucurana [122,123], while two tropone derivatives (386, 387) were isolated from C. zehntneri and C. argyroglossum [124,125]. From the root bark of C. jatrophoides, two new limonoids, musidunin (388) and musiduol (389), were found [126]. Their structures, molecular formula, names, corresponding sources, and references are collected in Figure 13 and Table 22, Table 23, Table 24, Table 25 and Table 26.
2.6. Miscellaneous Compounds
Flavonoids, lignans, and other types of 10 compounds were also isolated from Croton species. Their structures, molecular formula, names, corresponding sources, and references are collected in Figure 13 and Table 27. From the stems of C. caudatus, one new flavone, crotoncaudatin (390), was isolated [127]. A new nor-lignan (391) was obtained from the twigs and leaves of C. kongensis [67]. Investigations on C. laevifolius gave two new prenylated dihydrostilbenes, laevifolin A (393), B (394) and one new aromatic compound (399) [89,128]. A long chain linear ester, lobaceride (392) was isolated from the twigs and leaves of C. lobatus [129]. One indanone derivative (395) was found from the leaves of C. steenkampianus [102], while a trisubstituted furan derivative (396) was isolated from the bark of C. oblongifolius [22]. From C. sparsiflorus, an inositol, sparsifol (397), and a sphingolipid, sparsioamide (398), were obtained [96,113].
3. Biological Activities
Compounds isolated from Croton species exert a wide range of biological activities, including cytotoxic, anti-inflammatory, antifungal, acetylcholinesterase inhibitory, and neurite outgrowth-promoting activities.
3.1. Cytotoxic Activity
The anti-tumor activity of many plants from the Croton species have been reported. Therefore, the cytotoxicity of the isolated compounds is the most commonly studied bioactivity. The cytotoxic activities of the isolated compounds from the Croton species are listed in Table 28. Four new tigliane diterpene esters (135–137, 139) from the leaves of C. tiglium, exhibited most potent cytotoxic activity against K562 cell line with IC50 values of 0.03, 0.03, 0.07 and 0.05 μM, respectively [51].
Table 28.
Cytotoxic activity of compounds from the genus Croton.
| Compounds | Tumor Cell Line | Activity (IC50) | Ref |
|---|---|---|---|
| Methyl 15,16-epoxy-3,13(16),14-ent-clerodatrien-18,19-olide-17-carboxylate (6) | HuCCA-1 | 36.0 μg/mL | [29] |
| KB | 26.0 μg/mL | [29] | |
| HeLa | 30.0 μg/mL | [29] | |
| MDA-MB231 | 29.0 μg/mL | [29] | |
| T47D | 10.0 μg/mL | [29] | |
| Dimethyl-15,16-epoxy-12-oxo-3,13 (16)14-ent-clerodatriene-17,18-dicarboxylate (7) | HuCCA-1 | 39.0 μg/mL | [29] |
| KB | 27.0 μg/mL | [29] | |
| HeLa | 29.0 μg/mL | [29] | |
| MDA-MB231 | 27.0 μg/mL | [29] | |
| T47D | 25.0 μg/mL | [29] | |
| Laevigatbenzoate (8) | HeLa | 45.4 μM | [13] |
| Crotonolide A (23) | HL-60 | 9.42 μM | [21] |
| P-388 | 7.45 μM | [21] | |
| 15-oxo-17(10′-α-pinenyl)-kauran-18-oic acid (181) | HCT-116 | 7.14 μg/mL | [35] |
| OVCAR-8 | 8.19 μg/mL | [35] | |
| SF-295 | >10.0 μg/mL | [35] | |
| Launine K (67) | HeLa | 14.5 μM | [37] |
| MCF-7 | 62.5 μM | [37] | |
| Crassin H (75) | HL-60 | 11.8 ± 2.1 μM | [17] |
| A549 | 5.2 ± 0.4 μM | [17] | |
| Crassifolius A (76) | Hep3B | 17.91 μM | [38] |
| HepG2 | 42.04 μM | [38] | |
| Cracroson D (339) | T24 | 14.48 ± 0.65 μM | [40] |
| A549 | 25.64 ± 2.14 μM | [40] | |
| Cracroson E (87) | T24 | 22.99 ± 1.76 μM | [40] |
| A549 | 51.88 ± 14.07μM | [40] | |
| Hela | 3.9 μM | [48] | |
| DU145 | 7.2 μM | [48] | |
| A549 | 5.8 μM | [48] | |
| SGC-7091 | 13 μM | [48] | |
| H1975 | 10 μM | [48] | |
| HL60 | 12 μM | [48] | |
| 293T | 291.6 μM | [48] | |
| LX-2 | >500.0 μM | [48] | |
| 12-O-benzoylphorbol-13-(2-methyl)butyrate (114) | K562 | 15 μM | [48] |
| MOLT-4 | 12 μM | [48] | |
| U937 | 17 μM | [48] | |
| MCF-7 | 20 μM | [48] | |
| Hela | 4.6 μM | [48] | |
| DU145 | 4.3 μM | [48] | |
| A549 | 6.9 μM | [48] | |
| SGC-7091 | 10 μM | [48] | |
| H1975 | 3.3 μM | [48] | |
| HL60 | 6.8 μM | [48] | |
| 293T | 420.4 μM | [48] | |
| LX-2 | >500.0 μM | [48] | |
| 12-O-tiglyl-7-oxo-5-ene-phorbol-13-(2-methyl)butyrate (115) | K562 | 17 μM | [48] |
| MOLT-4 | 4.8 μM | [48] | |
| U937 | 21 μM | [48] | |
| MCF-7 | 20 μM | [48] | |
| Hela | 5.0 μM | [48] | |
| DU145 | 10 μM | [48] | |
| A549 | 19 μM | [48] | |
| SGC-7091 | 23 μM | [48] | |
| H1975 | 10 μM | [48] | |
| HL60 | 10 μM | [48] | |
| 293T | 455.3 μM | [48] | |
| LX-2 | >500.0 μM | [48] | |
| 13-O-(2-metyl)butyryl-4-deoxy-4a-phorbol (116) | K562 | 8.0 μM | [48] |
| MOLT-4 | 9.9 μM | [48] | |
| U937 | 18 μM | [48] | |
| MCF-7 | 24 μM | [48] | |
| H1975 | 10 μM | [48] | |
| HL60 | 10 μM | [48] | |
| 293T | 455.3 μM | [48] | |
| LX-2 | >500.0 μM | [48] | |
| Hela | 10 μM | [48] | |
| DU145 | 10 μM | [48] | |
| A549 | 4.5 μM | [48] | |
| SGC-7091 | 5.4 μM | [48] | |
| H1975 | 3.3 μM | [48] | |
| HL60 | 9.8 μM | [48] | |
| 293T | 191.0 μM | [48] | |
| LX-2 | >500.0 μM | [48] | |
| Crotignoid A (117) | HL-60 | 1.61 μM | [49] |
| A549 | 2.85 μM | [49] | |
| Crotignoid B (118) | HL-60 | 22.1 μM | [49] |
| A549 | 31.0 μM | [49] | |
| Crotignoid C (119) | HL-60 | 32.3 μM | [49] |
| A549 | 5.03 μM | [49] | |
| Crotignoid D (120) | HL-60 | 19.8 μM | [49] |
| A549 | 10.2 μM | [49] | |
| Crotignoid F (122) | HL-60 | 44.6 μM | [49] |
| A549 | 6.96 μM | [49] | |
| Crotignoid G (123) | HL-60 | 22.1 μM | [49] |
| A549 | 3.89 μM | [49] | |
| Crotignoid H (124) | HL-60 | 9.97 μM | [49] |
| A549 | 8.08 μM | [49] | |
| Crotignoid I (125) | HL-60 | 14.8 μM | [49] |
| A549 | 24.4 μM | [49] | |
| Crotignoid J (126) | HL-60 | 14.2 μM | [49] |
| A549 | 29.5 μM | [49] | |
| Crotusin A (128) | HL-60 | 12.53 ± 0.37 μM | [44] |
| SMMC-7721 | 7.06 ± 0.72 μM | [44] | |
| A549 | 9.69 ± 0.41 μM | [44] | |
| MCF-7 | 9.56 ± 0.76 μM | [44] | |
| SW480 | 14.88 ± 0.43 μM | [44] | |
| Crotusin B (129) | HL-60 | 19.39 ± 0.46 μM | [44] |
| SMMC-7721 | 21.13 ± 0.29 μM | [44] | |
| A549 | 14.66 ± 1.66 μM | [44] | |
| MCF-7 | 1.49 ± 0.23 μM | [44] | |
| SW480 | 31.21 ± 3.20 μM | [44] | |
| Crotusin C (130) | HL-60 | 4.19 ± 0.15 μM | [44] |
| SMMC-7721 | 3.87 ± 0.12 μM | [44] | |
| A549 | 2.44 ± 0.35 μM | [44] | |
| MCF-7 | 0.49 ± 0.04 μM | [44] | |
| SW480 | 2.89 ± 0.01 μM | [44] | |
| 12-O-tiglylphorbol-4-deoxy-4β-phorbol-13-acetate (131) | SNU387 | 59.5 ± 2.1 μM | [50] |
| SNU398 | 43.7 ± 1.5 μM | [50] | |
| 12-O-tiglylphorbol-4-deoxy-4β-phorbol-13-hexadecanoate (132) | SNU387 | 30.2 ± 1.4 μM | [50] |
| SNU398 | 91.2 ± 3.7 μM | [50] | |
| 13-O-acetylphorbol-4-deoxy-4β-phorbol-20-oleate (133) | SNU387 | 1.9 ± 0.2 μM | [50] |
| SNU398 | 13.5 ± 1.1 μM | [50] | |
| 13-O-acetylphorbol-4-deoxy-4β-phorbol-20-linoleate (134) | SNU387 | 0.71 ± 0.08 μM | [50] |
| SNU398 | 18.2 ± 1.7 μM | [50] | |
| 4-deoxy-20-oxophorbol 12-tiglyl 13-acetate (135) | K562 | 0.03 μM | [51] |
| A549 | 6.88 μM | [51] | |
| Huh-7 | 3.85 μM | [51] | |
| 7-oxo-5-ene-phorbol-13-(2-methylbutyrate) (136) | K562 | 0.03 μM | [51] |
| A549 | 6.33 μM | [51] | |
| Huh-7 | 20.9 μM | [51] | |
| 7-hydroxyl-phorbol-5-ene-13-(2-methyl)butyrate (137) | K562 | 0.07 μM | [51] |
| A549 | 8.86 μM | [51] | |
| Huh-7 | 11.6 μM | [51] | |
| 13-O-(2-metyl)butyryl-phorbol (139) | K562 | 0.05 μM | [51] |
| A549 | 43.5 μM | [51] | |
| Huh-7 | 34.2 μM | [51] | |
| 7-keto-12-O-tiglylphorbol-13-acetate (140) | HL-60 | 6.22 ± 3.24 μg/mL | [52] |
| A549 | 18.0 ± 9.48 μg/mL | [52] | |
| Phorbol-13-isobutyrate (148) | HL-60 | 0.22 ± 0.15 μg/mL | [52] |
| 14-epi-hyalic acid (159) | HL-60 | 8.2 μM | [63] |
| Kongeniod A (178) | HL-60 | 1.27 ± 0.24 μM | [59]] |
| A549 | 5.74 ± 0.25 μM | [59] | |
| Kongeniod B (179) | HL-60 | 0.47 ± 0.04 μM | [59] |
| A549 | 3.25 ± 0.91 μM | [59] | |
| Kongeniod C (180) | HL-60 | 0.58 ± 0.17 μM | [59] |
| Crotonkinensin D (188) | MCF-7 | 9.4 ± 1.7 μM | [61] |
| MCF-7/TAMR | 2.6 ± 0.9 μM | [61] | |
| MCF-7/ADR | 18.9 ± 0.6 μM | [61] | |
| MDA-MB-231 | 22.0 ± 0.9 μM | [61] | |
| EBC-162 (207) | HL-60 | 15 μg/mL | [74] |
| HT29 | 15 μg/mL | [74] | |
| MCF-7 | 30 μg/mL | [74] | |
| MM96 | 10 μg/mL | [74] | |
| NNF | 20 μg/mL | [74] | |
| K562 | 50 μg/mL | [74] | |
| EBC-233 (208) | HL-60 | 10 μg/mL | [74] |
| HT29 | 80 μg/mL | [74] | |
| MCF-7 | 20 μg/mL | [74] | |
| MM96 | 6 μg/mL | [74] | |
| NNF | 50 μg/mL | [74] | |
| K562 | 50 μg/mL | [74] | |
| EBC-300 (209) | HL-60 | 35 μg/mL | [74] |
| HT29 | 100 μg/mL | [74] | |
| MCF-7 | 100 μg/mL | [74] | |
| MM96 | 80 μg/mL | [74] | |
| NNF | 80 μg/mL | [74] | |
| K562 | 100 μg/mL | [74] | |
| EBC-240 (210) | HL-60 | 45 μg/mL | [74] |
| HT29 | 80 μg/mL | [74] | |
| MCF-7 | 50 μg/mL | [74] | |
| MM96 | 12 μg/mL | [74] | |
| NNF | 80 μg/mL | [74] | |
| K562 | 60 μg/mL | [74] | |
| EBC-241 (211) | HL-60 | 40 μg/mL | [74] |
| HT29 | 80 μg/mL | [74] | |
| MCF-7 | 40 μg/mL | [74] | |
| MM96 | 12 μg/mL | [74] | |
| NNF | 75 μg/mL | [74] | |
| K562 | 60 μg/mL | [74] | |
| Furanocembranoid 1 (266) | BT474 | 7.8 μg/mL | [83] |
| CHAGO | 7.0 μg/mL | [83] | |
| Hep-G2 | 5.6 μg/mL | [83] | |
| KATO-3 | 5.9 μg/mL | [83] | |
| SW-620 | 6.3 μg/mL | [83] | |
| Furanocembranoid 2 (267) | BT474 | 9.5 μg/mL | [83] |
| CHAGO | >10 μg/mL | [83] | |
| Hep-G2 | >10 μg/mL | [83] | |
| KATO-3 | 6.8 μg/mL | [83] | |
| SW-620 | 9.9 μg/mL | [83] | |
| Furanocembranoid 3 (268) | BT474 | 9.6 μg/mL | [83] |
| CHAGO | 7.1 μg/mL | [83] | |
| Hep-G2 | 5.7 μg/mL | [83] | |
| KATO-3 | 8.2 μg/mL | [83] | |
| SW-620 | 5.6 μg/mL | [83]] | |
| Furanocembranoid 4 (269) | BT474 | 9.6 μg/mL | [83] |
| CHAGO | 9.3 μg/mL | [83] | |
| Hep-G2 | 6.1 μg/mL | [83] | |
| KATO-3 | 8.1 μg/mL | [83] | |
| SW-620 | 6.0 μg/mL | [83] | |
| Laevigatlactone B (272) | Hela | 38.4 μM | [84] |
| (+)-[1R*,2S*,7S*,8S*,12R*]-7,8-Epoxy-2,12-cyclocembra-3E,10Zdien-20,10-olide (276) | PEO1 | 132 nM | [85] |
| PEO1TaxR | 200 nM | [85] | |
| (+)-[1R*,4S*,10R*]-4-Hydroxycembra-2E,7E,11Z-trien-20,10-olide (278) | PEO1 | 125 nM | [85] |
| PEO1TaxR | 135 nM | [85] | |
| Crotontomentosin A (294) | Hela | 24.0 ± 2.6 μM | [88] |
| Hep G2 | 87.9 ± 4.5 μM | [88] | |
| MDA-MB-231 | 54.1 ± 2.1 μM | [88] | |
| A549 | 40.6 ± 3.9 μM | [88] | |
| Crotontomentosin B (295) | Hela | >100 μM | [88] |
| Hep G2 | 28.1 ± 2.1 μM | [88] | |
| MDA-MB-231 | 28.7 ± 3.4 μM | [88] | |
| A549 | 29.1 ± 5.2 μM | [88] | |
| Crotontomentosin C (297) | Hela | 47.9 ± 3.3 μM | [88] |
| Hep G2 | 83.3 ± 5.3 μM | [88] | |
| MDA-MB-231 | >100 μM | [88] | |
| A549 | >100 μM | [88]] | |
| Crotontomentosin D (296) | Hela | 59.7 ± 4.5 μM | [88] |
| Hep G2 | >100 μM | [88] | |
| MDA-MB-231 | 49.3 ± 2.8 μM | [88] | |
| A549 | >100 μM | [88] | |
| Crotolaevigatone B (300) | A549 | 21.2 μM | [89] |
| MDA-MB-231 | 33.4 μM | [89] | |
| Crotolaevigatone G (305) | A549 | 25.6 μM | [89] |
| MDA-MB-231 | 32.7 μM | [89] | |
| EBC-324 (311) | MCF-7 | 40 μM | [92] |
| NFF | 50 μM | [92] | |
| K562 | 6 μM | [92] | |
| EBC-329 (312) | MCF-7 | 13 μM | [92] |
| NFF | 40 μM | [92] | |
| K562 | 0.6 μM | [92] | |
| ent-3β-hydroxypimara-8(14),9,15-trien-12-one (319) | NFF | 23 μg/mL | [98] |
| Hela | 13 μg/mL | [98] | |
| HT 29 | 13 μg/mL | [98] | |
| MCF-7 | 16 μg/mL | [98] | |
| MM96L | 2.8 μg/mL | [98] | |
| K562 | 17 μg/mL | [98] | |
| EBC-325 (321) | MCF-7 | 20 μM | [99] |
| NFF | 6 μM | [99] | |
| K562 | 3 μM | [99] | |
| EBC-326 (322) | MCF-7 | 14 μM | [99] |
| NFF | 6 μM | [99] | |
| K562 | 6 μM | [99] | |
| EBC-327 (323) | MCF-7 | 10 μM | [99] |
| NFF | 10 μM | [99] | |
| K562 | 10 μM | [99] | |
| 3-hydroxycleistantha-13(17),15-diene (325) | KATO-3 | 6.0 μg/mL | [93] |
| SW-620 | >10 μg/mL | [93] | |
| BT474 | 6.1 μg/mL | [93] | |
| Hep-G2 | 0.5 μg/mL | [93] | |
| CHAGO | 5.5 μg/mL | [93] | |
| 3,4-seco-cleistantha-4(18),13(17),15-trien-3-oic acid (326) | KATO-3 | 9.6 μg/mL | [93] |
| SW-620 | >10 μg/mL | [93] | |
| BT474 | 10 μg/mL | [93] | |
| Hep-G2 | 8.6 μg/mL | [93] | |
| CHAGO | >10 μg/mL | [93] | |
| Crotobarin (330) | KB | 2.5 ± 0.10 μM | [101] |
| HT29 | 2.1 ± 0.60 μM | [101] | |
| A549 | 0.79 ± 0.15 μM | [101] | |
| HL60 | 0.56 ± 0.02 μM | [101] | |
| Crotogoudin (331) | KB | 1.5 ± 0.03 μM | [101] |
| HT29 | 1.9 ± 0.25 μM | [101] | |
| A549 | 0.54 ± 0.02 μM | [101] | |
| HL60 | 0.49 ± 0.01 μM | [101] | |
| Crotonpyrone A (381) | Hela | 10.21 μg/mL | [120] |
| NCI-446 | 6.59 μg/mL | [120] | |
| Crotonpyrone B (382) | Hela | 9.54 μg/mL | [120] |
| [1−9-NαC]-crourorb A1 (385) | NCI-ADR/RES | 4.8 μM | [123] |
3.2. Anti-Inflammatory Activity
Bioassay-guided fractionation of the aerial parts of C. ciliatoglandulifer led to the isolation of tigliane diterpenoids 95, 97, which inhibited the enzymes cyclooxygenases-1 (IC50, 0.001, and 1.0 μM, respectively) and cyclooxygenases-2 (IC50, 2.2 μM, for compound 95) [41]. A tigliane diterpenoid (114) was isolated from the branches and leaves of C. tiglium, which displayed moderate inhibition of the enzymes COX-1 and COX-2, with IC50 values of 0.14 and 8.5 μM, respectively [48]. crotonkinin A (157), isolated from C. tonkinensis, showed anti-inflammatory effect on LPS-induced iNOS-dependent NO production and NOX-dependent ROS production in microglial cells (IC50, 46.2 ± 3.1 μM in NOS; maximum inhibition of NOX activity at 50 μM, 11.2%) [62]. Eight ent-kauranes (169–176) from C. tonkinensis exhibited the anti-inflammatory potential for inhibition of superoxide Anion generation and elastase release. Among them, crotonkinins F (172) displayed significant inhibition of superoxide anion generation (IC50, 2.88 ± 0.52 μM) and elastase release (IC50, 4.44 ± 1.45 μM) [66]. Labdane diterpenoids 251, 254 and 257, 258, isolated from the aerial parts of C. laui, were found to show anti-inflammatory activities in LPS-stimulated RAW 264.7 cells with IC50 values in the range 42.73–93.04 μM [82]. Two grayanane diterpenoids, crotonkinensins A (328) and B (329) from the leaves of C. tonkinensis, were reported to decrease the LPS-induced COX-2 promoter activity in Raw 264.7 cells with IC50 values of 7.14 ± 0.2 and 5.49 ± 0.2 μM, respectively [100]. Two benzoate derivatives (379, 380) were obtained from C. hutchinsonianus. Compound 379 showed significant activity against COX-1 (IC50, 4.95 ± 0.58 μg/mL) and COX-2 (IC50, 2.11 ± 1.3 μg/mL), while compound 380 (IC50, 1.88 ± 0.17 μg/mL) preferentially inhibited COX-2 [119].
3.3. Antifungal Activity
Two benzoate derivatives (379–380) were isolated from C. hutchinsonianus, and exhibited antifungal activity against Candida albicans (IC50, 11.41 ± 1.44, and 5.36 ± 0.01 μg/mL, respectively) [119]. Ursane triterpenoid (348) from the root of C. bonplandianum, displayed the antifungal activity against Calletotricheme camellie (IC50, 10 μg/mL), Fussarium equisitae (IC50, <15 μg/mL), Alterneria alternata (IC50, 10 μg/mL), Curvularia eragrostidies (IC50, <10 μg/mL) and Colletorichum gloeosporiodes (IC50, 15 μg/mL) [107].
3.4. Acetylcholinesterase Inhibitory Activity
An indole alkaloid derivative 376, isolated from the leaves of C. heliotropiifolius, exhibited the acetylcholinesterase inhibitory activity with IC50 values of 17.8 ± 0.6 μM [116]. Compund 378 from C. sylvaticus, also displayed the same activity [118].
3.5. Neurite Outgrowth-Promoting Activity
Two clerodane diterpenoids, crotonpenes A (36) and B (37) were isolated from C. yanhuii, which markedly increased the NGF (20 ng/mL)-induced proportion of neurite bearing cells by 59%, and 47% at 15 μM, respectively [23]. Crotoeurins A–C (40–42) obtained from C. euryphyllus, were found to display neurite outgrowth-promoting activity on NGF mediated PC12 cells at concentration of 10 μM. The percentages of neurite-bearing cells were 9.72%, 14.90%, and 7.14%, respectively [25].
3.6. Other Activities
Besides the above activities, other biological activities have also been reported. Crotonolide G (32), from the aerial parts of C. laui, was found to exhibit potent antibacterial activity (MIC, 43.4 μM) against four strains of Gram-positive bacteria, namely, Staphylococcus aureus, Staphylococcus epidermidis, Micrococcus luteus, and Bacillus subtilis [21]. Crassifolin H (39) was obtained from roots of C. crassifolius as an angiogenic inhibitor by reducing vessel formation to 59.3% at 15 μg/mL [34]. Tigliane diterpene (111) was isolated from the leaves of C. mauritianus, which inhibited chikungunya virus-induced cell death in cell culture with EC50s of 4.0 ± 0.8 μM [43]. The leaves of C. tiglium yielded two tigliane diterpenoids (135, 136), which displayed significant antitubercular activities with MIC values of 19.5, and 20.9 μM, respectively [51]. Compounds (162–165) were four ent-kaurane diterpenes from C. tonkinensis, which significantly stimulated differentiation in osteoblasts [64]. From the twigs and leaves of C. cascarilloides, two crotofolane diterpenoid alkaloids cascarinoids B–C (226, 227) were obtained, both of which displayed moderate activities against the ConA-induced proliferation of T lymphocyte cells and/or LPS-induced proliferation of B lymphocyte cells with IC50 values ranging from 6.14 to 16.27 μM [71]. Meroditerpenoid (336), from C. steenkampianus, showed antiplasmodial activities of 15.8 (D10), 9.1 (W2), and 9.4 (Dd2) μM [102]. Indole alkaloid (377) was found in C. mauritianus with antioxidant activity (IC50, 30.0.0 ± 0.7 μM) by the DPPH radical scavenging assay [117]. Bioactivity-guided fractionation of the root bark of C. jatrophoides resulted in the isolation of musidunin (388) and musiduol (389), both of which showed insect antifeedant activities (PC50, 3 μg/mL, PC95, 10 μg/mL; PC50, 4 μg/mL, PC95, 20 μg/mL, respectively) against the second-instar larvae of Pectinophora gossypiella in a leaf disk assay [126].
4. Conclusions
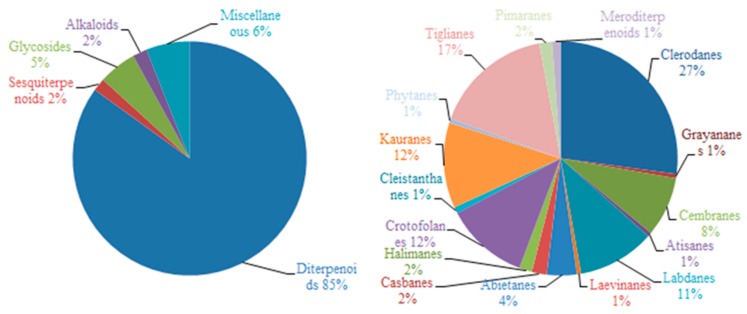
In the present review, we systematically summarized the chemical constituents and biological activity studies of Croton species covering from 2006 to 2018. To date, a total of 399 new compounds were reported from Croton species, which included 339 diterpenoids, seven sesquiterpenoids, 21 glycosides, eight alkaloids, and 24 miscellaneous compounds (Figure 14). Obviously, diterpenoids are characteristic components for Croton species. The diterpenoids with clerodane, tigliane, kaurane, crotofolane, labdane, and cembrane skeletons are among the most studied diterpenoids isolated from Croton species (Figure 14). Although the current studies have shown that these isolated compounds from Croton species possessed diversified biological activities, many compounds have never been biologically tested. Moreover, most studies conducted so far have focused mainly on in vitro cytotoxic assays. Further studies on the mechanism of actions and the structure activity relationship are needed in order to provide a better understanding of the chemical constituents from Croton species as potential medicines. Increasing interest in the chemistry and pharmaceutics of Croton species may promote new progress in finding and developing novel compounds.
Figure 14.
The percentage of each type of compounds (left), the percentage of each type of diterpenoids (right) from Croton Species.
Author Contributions
W.-H.X. classified the chemical constituents and drafted the structural formulas, wrote the manuscript; W-Y L. collected literatures; Q.L. managed references, overall responsibility.
Funding
We are thankful for financial supports from the Natural Science Foundation of China (NSFC) (21362035); The Initial Foundation of Scientific Research for the introduction of talents from Southwest Forestry University for Wen-Hui Xu (20130916); and Opening Research Foundation from Key Laboratory for Forest Resources Conservation and Utilization in the Southwest Mountains of China, Ministry of Education, Southwest Forestry University (KLE201807).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Salatino A., Salatino M.L.F., Negri G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae) J. Braz. Chem. Soc. 2007;18:11–33. doi: 10.1590/S0103-50532007000100002. [DOI] [Google Scholar]
- 2.Júnior S.F.P., Conserva L.M., Filho J.M.B. Clerodane diterpenes from Croton species: Distribution and a compilation of their 13C-NMR spectral data. Nat. Prod. Commun. 2006;1:319–344. [Google Scholar]
- 3.Wu X.A., Zhao Y.M. Advance on chemical composition and pharmacological action of Croton L. Nat. Prod. Res. Dev. 2004;16:467–472. [Google Scholar]
- 4.Nath R., Roy S., De B., Choudhury M.D. Anticancer and antioxidant activity of Croton: A review. Int. J. Pharm. Pharm. Sci. 2013;5:63–70. [Google Scholar]
- 5.Premprasert C., Tewtrakul S., Plubrukarn A., Wungsintaweekul J. Anti-inflammatory activity of diterpenes from Croton stellatopilosus on LPS-induced RAW264. 7 cells. J. Nat. Med. 2013;67:174–181. doi: 10.1007/s11418-012-0668-5. [DOI] [PubMed] [Google Scholar]
- 6.Maroyi A. Ethnopharmacological uses, phytochemistry, and pharmacological properties of Croton macrostachyus Hochst. Ex Delile: A Comprehensive Review. Evid Based. Compl. Alt. 2017;20:1–17. doi: 10.1155/2017/1694671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maroyi A. Ethnomedicinal uses and pharmacological activities of Croton megalobotrys Müll Arg: A systematic review. Trop. J. Pharm. Res. 2017;16:2535–2543. [Google Scholar]
- 8.Maroyi A. Traditional usage, phytochemistry and pharmacology of Croton sylvaticus Hochst. ex C. Krauss. Asian Pac. J. Trop. Med. 2017;10:423–429. doi: 10.1016/j.apjtm.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Dutta S., Chaudhuri T.K. Pharmacological aspect of Croton bonplandianus Baill: A comprehensive review. J. Pharmacogn. Phytochem. 2018;7:811–813. [Google Scholar]
- 10.Ghosh T., Biswas M.K., Roy P., Guin C. A review on traditional and pharmacological uses of Croton bonplandianum with special reference to phytochemical aspect. Eur. J. Med. Plant. 2018;22:1–10. doi: 10.9734/EJMP/2018/40697. [DOI] [Google Scholar]
- 11.Li R., Morris-Natschke S.L., Lee K.H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016;33:1166–1226. doi: 10.1039/C5NP00137D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndunda B., Langat M.K., Midiwo J.O., Omosa L.K. Diterpenoid derivatives of Kenyan croton sylvaticus. Nat. Prod. Commun. 2015;10:557–578. [PubMed] [Google Scholar]
- 13.Zou G.A., Zhang H.W., Aisa H.A., Yang J.S., Peng C.Z., Zou Z.M. Laevigatbenzoate from Croton laevigatus vahl. J. Nat. Med. 2011;65:391–394. doi: 10.1007/s11418-010-0503-9. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y., Zhang L., Wen X.Q., Zeng X.J., Rui W., Cen Y.Z. Two new diterpenoids from croton crassifolius. J. Asian. Nat. Prod. Res. 2012;14:785–788. doi: 10.1080/10286020.2012.694872. [DOI] [PubMed] [Google Scholar]
- 15.García A., Ramírez-Apan T., Cogordan J.A., Delgado G. Absolute configuration assignments by experimental and theoretical approaches of ent-labdane-and cis-ent-clerodane-type diterpenes isolated from Croton glabellus. Can. J. Chem. 2006;84:1593–1602. doi: 10.1139/v06-164. [DOI] [Google Scholar]
- 16.Wang G.C., Li J.G., Li G.Q., Xu J.J., Wu X., Ye W.C., Li Y.L. Clerodane diterpenoids from Croton crassifolius. J. Nat. Prod. 2012;75:2188–2192. doi: 10.1021/np300636k. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Q.Q., Tang S., Song W.B., Wang W.Q., Huang M., Xuan L.J. Crassins A-H, diterpenoids from the roots of Croton crassifolius. J. Nat. Prod. 2017;80:254–260. doi: 10.1021/acs.jnatprod.6b00425. [DOI] [PubMed] [Google Scholar]
- 18.Guetchueng S.T., Nahar L., Ritchie K.J., Ismail F.M.D., Evans A.R., Sarker S.D. Ent-clerodane diterpenes from the bark of Croton oligandrus Pierre ex Hutch. and assessment of their cytotoxicity against human cancer cell lines. Molecules. 2018;23:410. doi: 10.3390/molecules23020410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamale S.C., Koudou J., Samb A., Heitz A., Teulade J.C. Structural elucidation of a new furoclerodane from stem barks of croton mayumbensis J. Leonard extracts. Int. J. Phys. Sci. 2009;4:96–100. [Google Scholar]
- 20.Wang G.C., Zhang H., Liu H.B., Yue J.M. Laevinoids A and B: Two diterpenoids with an unprecedented backbone from Croton laevigatus. Org. Lett. 2013;15:4880–4883. doi: 10.1021/ol402318m. [DOI] [PubMed] [Google Scholar]
- 21.Liu C.P., Xu J.B., Zhao J.X., Xu C.H., Dong L., Ding J., Yue J.M. Diterpenoids from Croton laui and their cytotoxic and antimicrobial activities. J. Nat. Prod. 2014;77:1013–1020. doi: 10.1021/np500042c. [DOI] [PubMed] [Google Scholar]
- 22.Pudhom K., Sommit D. Clerodane diterpenoids and a trisubstituted furan from Croton oblongifolius. Phytochem. Lett. 2011;4:147–150. doi: 10.1016/j.phytol.2011.02.004. [DOI] [Google Scholar]
- 23.Sun Y., Wang M., Ren Q., Li S., Xu J., Ohizumi Y., Xie C., Jin D.Q., Guo Y. Two novel clerodane diterpenenes with NGF-potentiating activities from the twigs of Croton yanhuii. Fitoterapia. 2014;95:229–233. doi: 10.1016/j.fitote.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Velázquez-Jiménez R., Vargas-Mendoza D., Gayosso-de-Lucio J.A., González-Montiel S., Villagómez-Ibarra J.R. Three novel epoxy-clerodanes bearing a furan ring from Croton hypoleucus. Phytochem. Lett. 2018;24:21–26. doi: 10.1016/j.phytol.2018.01.001. [DOI] [Google Scholar]
- 25.Pan Z., Ning D., Wu X., Huang S., Li D., Lv S. New clerodane diterpenoids from the twigs and leaves of Croton euryphyllus. Bioorg. Med. Chem. Lett. 2015;46:329–1332. doi: 10.1016/j.bmcl.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 26.Qiu M., Cao D., Gao Y., Li S., Zhu J., Yang B., Zhou L., Zhou Y., Jin J., Zhao Z. New clerodane diterpenoids from Croton crassifolius. Fitoterapia. 2016;108:81–86. doi: 10.1016/j.fitote.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Ndunda B., Langat M.K., Mulholland D.A., Eastman H., Jacob M.R., Khan S.I., Walker L.A., Muhammad I., Kerubo L.O., Midiwo J.O. New ent-Clerodane and abietane diterpenoids from the Roots of Kenyan Croton megalocarpoides Friis & M. G. Gilbert. Planta Med. 2016;82:1079–1086. doi: 10.1055/s-0042-108857. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z.X., Li H.H., Fan G.X., Li Z.Y., Dong L.L., Li H.Y., Fei D.Q. A novel norclerodane diterpenoid from the roots of Croton crassifolius. Nat. Prod. Commun. 2015;10:1917–1918. [PubMed] [Google Scholar]
- 29.Youngsa-ad W., Ngamrojanavanich N., Mahidol C., Ruchirawat S., Prawat H., Kittakoop P. Diterpenoids from the roots of Croton oblongifolius. Planta Med. 2007;73:1491–1494. doi: 10.1055/s-2007-990247. [DOI] [PubMed] [Google Scholar]
- 30.Mbwambo Z., Foubert K.M., Kapingu M., Magadula J., Moshi M., Lemiere F., Goubitz K., Fraanje J., Peschar R., Vlietinck A., et al. New furanoditerpenoids from croton jatrophoides. Planta Med. 2008;75:262–267. doi: 10.1055/s-0028-1088383. [DOI] [PubMed] [Google Scholar]
- 31.Brasil D.S.B., Müller A.H., Guilhon G.M.S.P., Alves C.N., Peris G., Llusard R., Moliner V. Isolation, X-ray crystal structure and theoretical calculations of the new compound 8-Epicordatin and identification of others terpenes and steroids from the bark and leaves of Croton palanostigma klotzsch. J. Braz. Chem. Soc. 2010;21:731–739. doi: 10.1590/S0103-50532010000400021. [DOI] [Google Scholar]
- 32.Pizzolatti M.G., Bortoluzzi A.J., Brighente I.M.C., Zuchinalli A., Carvalho F.K., Candido A.C.S., Peresb M.T.L.P. Clerodane diterpenes from bark of Croton urucurana Baillon. J. Braz. Chem. Soc. 2013;24:609–614. [Google Scholar]
- 33.Abega D.F., Kapche D.W., Ango P.Y., Mapitse R., Yeboah S.O., Ngadjui B.T. Chemical constituents of croton oligandrum (euphorbiaceae) Z. Naturforsch. C. 2014;69:181–185. doi: 10.5560/znc.2013-0207. [DOI] [PubMed] [Google Scholar]
- 34.Huang W.H., Li G.Q., Li J.G., Wu X., Ge W., Chung H.Y., Ye W.C., Li Y.L., Wang G.C. Two new clerodane diterpenoids from Croton crassifolius. Heterocycles. 2014;89:1585–1593. [Google Scholar]
- 35.Sousa A.H., Junior J.N.S., Guedes M.L.S., Braz-Filho R., Costa-Lotufo L.V., Araujo A.J., Silveira E.R., Lima M.A.S. New terpenoids from Croton limae (Euphorbiaceae) J. Braz. Chem. Soc. 2015;26:1565–1572. [Google Scholar]
- 36.Wang J.J., Chung H.Y., Zhang Y.B., Li G.Q., Li Y.L., Huang W.H., Wang G.C. Diterpenoids from the roots of Croton crassifolius and their anti-angiogenic activity. Phytochemistry. 2016;12:270–275. doi: 10.1016/j.phytochem.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Yang L., Zhang Y.-B., Wu Z.-N., Chen N.-H., Jiang S.-Q., Jiang L., Li Y.-L., Wang G.-C. Three new diterpenoids from Croton laui. Chem. Lett. 2016;45:1235–1237. doi: 10.1246/cl.160632. [DOI] [PubMed] [Google Scholar]
- 38.Tian J.L., Yao G.D., Wang Y.X., Gao P.Y., Wang D., Li L.Z., Lin B., Huang X.X., Song S.J. Cytotoxic clerodane diterpenoids from Croton crassifolius. Bioorg. Med. Chem. Lett. 2017;27:1237–1242. doi: 10.1016/j.bmcl.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J.S., Tang Y.Q., Huang J.L., Li W., Zou Y.H., Tang G.H., Liu B., Yin S. Bioactive diterpenoids from Croton laevigatus. Phytochemistry. 2017;144:151–158. doi: 10.1016/j.phytochem.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Qiu M., Jin J., Zhou L., Zhou W., Liu Y., Tan Q., Cao D., Zhao Z. Diterpenoids from Croton crassifolius include a novel skeleton possibly generated via an intramolecular [2 + 2]-photocycloaddition reaction. Phytochemistry. 2018;145:103–110. doi: 10.1016/j.phytochem.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Rios M.Y., Aguilar-Guadarrama A.B. Nitrogen-containing phorbol esters from Croton ciliatoglandulifer and their effects on cyclooxygenases-1 and-2. J. Nat. Prod. 2006;69:887–890. doi: 10.1021/np0504311. [DOI] [PubMed] [Google Scholar]
- 42.Ndunda B., Langat M.K., Wanjohi J.M., Midiwo J.O., Kerubo L.O. Alienusolin, a new 4α-deoxyphorbol ester derivative, and crotonimide C, a new glutarimide alkaloid from the Kenyan Croton alienus. Planta Med. 2013;79:1762–1766. doi: 10.1055/s-0033-1351044. [DOI] [PubMed] [Google Scholar]
- 43.Corlay N., Delang L., Girard-Valenciennes E., Neyts J., Clerc P., Smadja J., Gueritte F., Leyssen P., Litaudon M. Tigliane diterpenes from Croton mauritianus as inhibitors of chikungunya virus replication. Fitoterapia. 2014;97:87–91. doi: 10.1016/j.fitote.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y.Y., Yang K.X., Yang X.W., Khan A., Liu L., Wang B., Zhao Y.L., Liu Y.P., Li Y., Luo X.D. New cytotoxic tigliane diterpenoids from Croton caudatus. Planta Med. 2016;82:729–733. doi: 10.1055/s-0042-102539. [DOI] [PubMed] [Google Scholar]
- 45.Jiang L., Zhang Y.B., Jiang S.Q., Zhou Y.D., Luo D., Niu Q.W., Qian Y.R., Li Y.L., Wang G.C. Phorbol ester-type diterpenoids from the twigs and leaves of Croton tiglium. J. Asian Nat. Prod. Res. 2017;19:1191–1197. doi: 10.1080/10286020.2017.1307836. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X.L., Wang L., Li F., Yu K., Wang M.K. Cytotoxic phorbol esters of Croton tiglium. J. Nat. Prod. 2013;76:858–864. doi: 10.1021/np300832n. [DOI] [PubMed] [Google Scholar]
- 47.Ren F.X., Ren F.Z., Yang Y., Yu N.J., Zhang Y., Zhao Y.M. Tigliane diterpene esters from the leaves of Croton tiglium. Helv. Chim. Acta. 2014;97:1014–1019. doi: 10.1002/hlca.201300408. [DOI] [Google Scholar]
- 48.Wang J.F., Yang S.H., Liu Y.Q., Li D.X., He W.J., Zhang X.X., Liu Y.H., Zhou X.J. Five new phorbol esters with cytotoxic and selective anti-inflammatory activities from Croton tiglium. Bioorg. Med. Chem. Lett. 2015;25:1986–1989. doi: 10.1016/j.bmcl.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Zhang D.-D., Zhou B., Yu J.-H., Xu C.-H., Ding J., Zhang H., Yue J.-M. Cytotoxic tigliane-type diterpenoids from Croton tiglium. Tetrahedron. 2015;71:9638–9644. doi: 10.1016/j.tet.2015.10.070. [DOI] [Google Scholar]
- 50.Zhang X.-L., Khan A.-A., Wang L., Yu K., Li F., Wang M.-K. Four new phorbol diesters from Croton tiglium and their cytotoxic activities. Phytochem. Lett. 2016;16:82–86. doi: 10.1016/j.phytol.2016.03.008. [DOI] [Google Scholar]
- 51.Zhao B.Q., Peng S., He W.J., Liu Y.H., Wang J.F., Zhou X.J. Antitubercular and cytotoxic tigliane-type diterpenoids from Croton tiglium. Bioorg. Med. Chem. Lett. 2016;26:4996–4999. doi: 10.1016/j.bmcl.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Du Q., Zhao Y., Liu H., Tang C., Zhang M., Ke C., Ye Y. Isolation and structure characterization of cytotoxic phorbol esters from the seeds of Croton tiglium. Planta Med. 2017;83:1361–1367. doi: 10.1055/s-0043-110227. [DOI] [PubMed] [Google Scholar]
- 53.Suirez A.I., Chavez K., Monache F.D., Vasquez L., Delannoy D.M., Orsini G., Compagnone R.S. New 3,4-seco ent-kaurenes from Croton caracasana flowers. Nat. Prod. Commun. 2008;3:319–322. [Google Scholar]
- 54.Mora S., Castro V., Poveda L., Chavarría M., Murillo R. Two new 3,4-seco-ent-kaurenes and other constituents from the costa rican endemic species Croton megistocarpus. Helv. Chim. Acta. 2011;94:1888–1892. doi: 10.1002/hlca.201100127. [DOI] [Google Scholar]
- 55.Suwancharoen S., Chonvanich O., Roengsumran S., Pornpakakul S. Seco-kaurane skeleton diterpenoids from Croton oblongifolius. Chem. Nat. Compd. 2012;48:583–586. doi: 10.1007/s10600-012-0317-y. [DOI] [Google Scholar]
- 56.Chen W., Yang X.D., Zhao J.F., Yang J.H., Zhang H.B., Li Z.Y., Li L. Three new, 1-oxygenated ent-8,9-secokaurane diterpenes from Croton kongensis. Helv. Chim. Acta. 2006;89:537–541. doi: 10.1002/hlca.200690057. [DOI] [Google Scholar]
- 57.Chen W., Yang X.D., Zhao J.F., Zhang H.B., Li L. Two new, 1-oxygenated ent-kaurane-type diterpenes from Croton kongensis. Helv. Chim. Acta. 2007;90:1554–1558. doi: 10.1002/hlca.200790162. [DOI] [Google Scholar]
- 58.Yang X.-D., Chen W., Zhao J.-F., Yang L.-J., Zhang H.-B., Li L. Ent-kaurane diterpenes and phenolic compounds from Croton kongensis (Euphorbiaceae) Biochem. Syst. Ecol. 2009;37:237–240. doi: 10.1016/j.bse.2009.03.007. [DOI] [Google Scholar]
- 59.Shi S.Q., Fan Y.Y., Xu C.H., Ding J., Wang G.W., Yue J.M. Cytotoxic 8,9-seco-ent-kaurane diterpenoids from Croton kongensis. J. Asian Nat. Prod. Res. 2017 doi: 10.1080/10286020.2017.1373100. [DOI] [PubMed] [Google Scholar]
- 60.Mateu E., Chavez K., Riina R.S., Compagnone R., Monache F.D., Suárez A.I. New 3,4-seco-ent-kaurene dimers from Croton micans. Nat. Prod. Commun. 2012;7:5–8. [PubMed] [Google Scholar]
- 61.Thuong P.T., Pham T.H., Le T.V., Dao T.T., Dang T.T., Nguyen Q.T., Oh W.K. Symmetric dimers of ent-kaurane diterpenoids with cytotoxic activity from Croton tonkinensis. Bioorg. Med. Chem. Lett. 2012;22:1122–1124. doi: 10.1016/j.bmcl.2011.11.116. [DOI] [PubMed] [Google Scholar]
- 62.Kuo P.C., Shen Y.C., Yang M.L., Wang S.H., Thang T.D., Dung N.X., Chiang P.-C., Lee K.-H., Lee E.-J., Wu T.-S. Crotonkinins A and B and related diterpenoids from Croton tonkinensis as anti-inflammatory and antitumor agents. J. Nat. Prod. 2007;70:1906–1909. doi: 10.1021/np070383f. [DOI] [PubMed] [Google Scholar]
- 63.Santos H.S., Barros F.W., Albuquerque M.R., Bandeira P.N., Pessoa C., Braz-Filho R., Monte F.J.Q., Leal-Cardoso J.H., Lemos T.L.G. Cytotoxic diterpenoids from croton argyrophylloides. J. Nat. Prod. 2009;72:1884–1887. doi: 10.1021/np900250k. [DOI] [PubMed] [Google Scholar]
- 64.Dao T.T., Lee K.Y., Jeong H.M., Nguyen P.H., Tran T.L., Thuong P.T., Nguyen B.T., Oh W.K. Ent-kaurane diterpenoids from Croton tonkinensis stimulate osteoblast differentiation. J. Nat. Prod. 2011;74:2526–2531. doi: 10.1021/np200667f. [DOI] [PubMed] [Google Scholar]
- 65.Langat M.K., Crouch N.R., Pohjala L., Tammela P., Smith P.J., Mulholland D.A. Ent-kauren-19-oic acid derivatives from the stem bark of Croton pseudopulchellus Pax. Phytochem. Lett. 2012;5:414–418. doi: 10.1016/j.phytol.2012.03.002. [DOI] [Google Scholar]
- 66.Kuo P.C., Yang M.L., Hwang T.L., Lai Y.Y., Li Y.C., Thang T.D., Wu T.S. Anti-inflammatory diterpenoids from Croton tonkinensis. J. Nat. Prod. 2013;76:230–236. doi: 10.1021/np300699f. [DOI] [PubMed] [Google Scholar]
- 67.Sun L., Meng Z., Li Z., Yang B., Wang Z., Ding G., Xiao W. Two new natural products from Croton kongensis Gagnep. Nat. Prod. Res. 2014;28:563–567. doi: 10.1080/14786419.2013.867856. [DOI] [PubMed] [Google Scholar]
- 68.Kawakami S., Toyoda H., Harinantenaina L., Matsunami K., Otsuka H., Shinzato T., Takeda Y., Kawahata M., Yamaguchid K. Eight new diterpenoids and two new nor-diterpenoids from the stems of Croton cascarilloides. Chem. Pharm. Bull. 2013;61:411–418. doi: 10.1248/cpb.c12-01002. [DOI] [PubMed] [Google Scholar]
- 69.Kawakami S., Matsunami K., Otsuka H., Inagaki M., Takeda Y., Kawahata M., Yamaguchic K. Crotocascarins I-K: Crotofolane-type diterpenoids, crotocascarin γ, isocrotofolane glucoside and phenolic glycoside from the leaves of Croton cascarilloides. Chem. Pharm. Bull. 2015;63:1047–1054. doi: 10.1248/cpb.c15-00635. [DOI] [PubMed] [Google Scholar]
- 70.Kawakami S., Inagaki M., Matsunami K., Otsuka H., Kawahata M., Yamaguchi K. Crotofolane-type diterpenoids, crotocascarins L–Q, and a rearranged crotofolane-type diterpenoid, neocrotocascarin, from the Stems of Croton cascarilloides. Chem. Pharm. Bull. 2016;64:1492–1498. doi: 10.1248/cpb.c16-00500. [DOI] [PubMed] [Google Scholar]
- 71.Gao X.H., Xu Y.S., Fan Y.Y., Gan L.S., Zuo J.P., Yue J.M. Cascarinoids A-C, a class of diterpenoid alkaloids with unpredicted conformations from Croton cascarilloides. Org. Lett. 2018;20:228–231. doi: 10.1021/acs.orglett.7b03592. [DOI] [PubMed] [Google Scholar]
- 72.Filho F.A.S., Junior J.N.S., Braz-Filho R., Simone C.A., Silveira E.R., Lima M.A.S. Crotofolane-and casbane-type diterpenes from Croton argyrophyllus. Helv. Chim. Acta. 2013;96:1146–1154. doi: 10.1002/hlca.201200347. [DOI] [Google Scholar]
- 73.Chávez K., Compagnoneb R.S., Riina R., Briceño A., González T., Squitieri E., Landaetab C., Soscúnd H., Suáreza A.I. Crotofolane diterpenoids from Croton caracasanus. Nat. Prod. Commun. 2013;8:1679–1682. [PubMed] [Google Scholar]
- 74.Maslovskaya L.A., Savchenko A.I., Pierce C.J., Gordon V.A., Reddell P.W., Parsons P.G., Williams C.M. Unprecedented 1,14-seco-crotofolanes from Croton insularis: Oxidative cleavage of crotofolin C by a putative homo-baeyer-villiger rearrangement. Chem. Eur. J. 2014;20:14226–14230. doi: 10.1002/chem.201404250. [DOI] [PubMed] [Google Scholar]
- 75.Aldhaher A., Langat M., Ndunda B., Chirchir D., Midiwo J.O., Njue A., Schwikkard S., Carew M., Mulholland D. Diterpenoids from the roots of Croton dichogamus Pax. Phytochemistry. 2017;144:1–8. doi: 10.1016/j.phytochem.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 76.Yang L., Wu Z.N., Zhang Y.B., Chen N.H., Zhuang L., Li Y.L., Wang G.C. Three new diterpenoids from Croton laui Merr. et Metc. Nat. Prod. Res. 2017;31:1028–1033. doi: 10.1080/14786419.2016.1266350. [DOI] [PubMed] [Google Scholar]
- 77.Bernardino A.C.S.S., Teixeira A.M.R., de Menezes J.E.S.A., Pinto C.C.C., Santos H.S., Freire P.T.C., Coutinho H.D.M., Sena Junior D.M., Bandeira P.N., Braz-Filho R. Spectroscopic and microbiological characterization of labdane diterpene 15,16-epoxy-4-hydroxy-labda-13(16),14-dien-3,12-dione isolated from the stems of Croton jacobinensis. J. Mol. Struct. 2017;1147:335–344. doi: 10.1016/j.molstruc.2017.06.084. [DOI] [Google Scholar]
- 78.Ramos F., Takaishi Y., Kashiwada Y., Osorio C., Duque C., Acuna R., Fujimoto Y. Ent-3,4-seco-labdane and ent-labdane diterpenoids from Croton stipuliformis (Euphorbiaceae) Phytochemistry. 2008;69:2406–2410. doi: 10.1016/j.phytochem.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 79.Huang H.-L., Qi F.-M., Yuan J.-C., Zhao C.-G., Yang J.-W., Fang F.-H., Wu Q.-X., Gao K., Yuan C.-S. Labdane-type diterpenoids from Croton laevigatus. RSC Adv. 2014;4:39530–39540. doi: 10.1039/C4RA04863F. [DOI] [Google Scholar]
- 80.Pompimon W., Udomputtimekakul P., Apisantiyakom S., Baison W., Penlap N., Chaibun S., Nuntasaen N. Two new labdane-type diterpenoids cinnamate from Croton decalvatus Esser. Nat. Prod. Res. 2017 doi: 10.1080/14786419.2017.1408089. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z.X., Wu P.Q., Li H.H., Qi F.M., Fei D.Q., Hu Q.L., Liu Y.H., Huang X.L. Norcrassin A, a novel C16 tetranorditerpenoid, and bicrotonol A, an unusual dimeric labdane-type diterpenoid, from the roots of Croton crassifolius. Org. Biomol. Chem. 2018;16:1745–1750. doi: 10.1039/C7OB02991H. [DOI] [PubMed] [Google Scholar]
- 82.Yang L., Zhang Y.B., Chen L.F., Chen N.H., Wu Z.N., Jiang S.Q., Jiang L., Li G.Q., Li Y.L., Wang G.C. New labdane diterpenoids from Croton laui and their anti-inflammatory activities. Bioorg. Med. Chem. Lett. 2016;26:4687–4691. doi: 10.1016/j.bmcl.2016.08.052. [DOI] [PubMed] [Google Scholar]
- 83.Pudhom K., Vilaivan T., Ngamrojanavanich N., Dechangvipart S., Sommit D., Petsom A., Roengsumran S. Furanocembranoids from the stem bark of Croton oblongifolius. J. Nat. Prod. 2007;70:659–661. doi: 10.1021/np060520t. [DOI] [PubMed] [Google Scholar]
- 84.Zou G.A., Gang D., Su Z.-H., Yang J.-S., Zhang H.-W., Peng C.-Z., Aisa H.A., Zou Z.-M. Lactonecembranoids from Croton laewigatus. J. Nat. Prod. 2010;73:792–795. doi: 10.1021/np100044t. [DOI] [PubMed] [Google Scholar]
- 85.Mulholland D.A., Langat M.K., Crouch N.R., Coley H.M., Mutambi E.M., Nuzillard J.M. Cembranolides from the stem bark of the southern african medicinal plant, Croton gratissimus (Euphorbiaceae) Phytochemistry. 2010;71:1381–1386. doi: 10.1016/j.phytochem.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 86.Langat M.K., Crouch N.R., Smith P.J., Mulholland D.A. Cembranolides from the leaves of Croton gratissimus. J. Nat. Prod. 2011;74:2349–2355. doi: 10.1021/np2002012. [DOI] [PubMed] [Google Scholar]
- 87.Kawakami S., Matsunami K., Otsuka H., Lhieochaiphant D., Lhieochaiphant S. Two new cembranoids from the leaves of Croton longissimus Airy Shaw. J. Nat. Med. 2013;67:410–414. doi: 10.1007/s11418-012-0693-4. [DOI] [PubMed] [Google Scholar]
- 88.Song J.T., Han Y., Wang X.L., Shen T., Lou H.X., Wang X.N. Diterpenoids from the twigs and leaves of Croton caudatus var. tomentosus. Fitoterapia. 2015;107:54–59. doi: 10.1016/j.fitote.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 89.Song J.-T., Liu X.-Y., Li A.-L., Wang X.-L., Shen T., Ren D.-M., Lou H.-X., Wang X.-N. Cytotoxic abietane-type diterpenoids from twigs and leaves of Croton laevigatus. Phytochem. Lett. 2017;22:241–246. doi: 10.1016/j.phytol.2017.10.007. [DOI] [Google Scholar]
- 90.Santos H.S., Mesquita F.M.R., Lemos T.L.G., Monte F.J.Q., Braz-Filho R. Diterpenos casbanos e acetofenonas de Croton nepetaefolius (Euphorbiaceae) Quim. Nova. 2008;31:601–604. doi: 10.1590/S0100-40422008000300026. [DOI] [Google Scholar]
- 91.e Silva-Filho F.A., Braz-Filho R., Silveira E.R., Lima M.A. Structure elucidation of casbane diterpenes from Croton argyrophyllus. Magn. Reson. Chem. 2011;49:370–373. doi: 10.1002/mrc.2752. [DOI] [PubMed] [Google Scholar]
- 92.Maslovskaya L.A., Savchenko A.I., Krenske E.H., Gordon V.A., Reddell P.W., Pierce C.J., Parsons P.G., Williams C.M. Croton insularis introduces the seco-casbane class with EBC-329 and the first casbane endoperoxide EBC-324. Chem. Commun. 2014;50:12315–12317. doi: 10.1039/C4CC05413J. [DOI] [PubMed] [Google Scholar]
- 93.Roengsumran S., Pata P., Ruengraweewat N., Tummatorn J., Pornpakakul S., Sangvanich P., Puthong S., Petsom A. New cleistanthane diterpenoids and 3,4-seco-cleistanthane diterpenoids from croton oblongifolius. Chem. Nat. Compd. 2009;45:641–646. doi: 10.1007/s10600-009-9442-7. [DOI] [Google Scholar]
- 94.Torres M.C.M., Braz-Filho R., Silveira E.R., Diniz J.C., Viana F.A., Pessoa O.D.L. Terpenoids from Croton regelianus. Helv. Chim. Acta. 2010;93:375–381. doi: 10.1002/hlca.200900201. [DOI] [Google Scholar]
- 95.Zhang Z.-X., Li H.-H., Qi F.-M., Xiong H.-Y., Dong L.-L., Fan G.-X., Fei D.-Q. A new halimane diterpenoid from Croton crassifolius. Bull. Korean Chem. Soc. 2014;35:1556–1558. doi: 10.5012/bkcs.2014.35.5.1556. [DOI] [Google Scholar]
- 96.Maslovskaya L.A., Savchenko A.I., Gordon V.A., Reddell P.W., Pierce C.J., Parsons P.G., Williams C.M. Isolation and confirmation of the proposed cleistanthol biogentic link from Croton insularis. Org. Lett. 2011;13:1032–1035. doi: 10.1021/ol103083m. [DOI] [PubMed] [Google Scholar]
- 97.Maslovskaya L.A., Savchenko A.I., Gordon V.A., Reddell P.W., Pierce C.J., Parsons P.G., Williams C.M. EBC-316, 325–327, and 345: New pimarane diterpenes from Croton insularis found in the Australian rainforest. Aust. J. Chem. 2015;68:652–659. doi: 10.1071/CH14550. [DOI] [Google Scholar]
- 98.Thuong P.T., Dao T.T., Pham T.H., Nguyen P.H., Le T.V., Lee K.Y., Oh W.-K. Crotonkinensins A and B, diterpenoids from the Vietnamese medicinal plant Croton tonkinensis. J. Nat. Prod. 2009;72:2040–2042. doi: 10.1021/np900215r. [DOI] [PubMed] [Google Scholar]
- 99.Rakotonandrasana O.L., Raharinjato F.H., Rajaonarivelo M., Dumontet V., Martin M.-T., Bignon J., Rasoanaivo P. Cytotoxic 3,4-seco-atisane diterpenoids from Croton barorum and Croton goudotii. J. Nat. Prod. 2010;73:1730–1733. doi: 10.1021/np1005086. [DOI] [PubMed] [Google Scholar]
- 100.Adelekan A.M., Prozesky E.A., Hussein A.A., Urena L.D., Rooyen P.H., Liles D.C., Meyer J.J.M., Rodrfguez B. Bioactive diterpenes and other constituents of Croton steenkampianus. J. Nat. Prod. 2008;71:1919–1922. doi: 10.1021/np800333r. [DOI] [PubMed] [Google Scholar]
- 101.Barreto M.B., Gomes C.L., Freitas J.V.B.D., Pinto F.D.C.L., Silveira E.R., Gramosa N.V., Torres D.S.C. Flavonoides e terpenoides de Croton muscicarpa (euphorbiaceae) Quim. Nova. 2013;36:675–679. doi: 10.1590/S0100-40422013000500011. [DOI] [Google Scholar]
- 102.Lopes E.L., Neto M.A., Silveira E.R., Pessoa O.D.L., Braz-Filho R. Flavonoides e sesquiterpenos de Croton pedicellatus kunth. Quim. Nova. 2012;35:2169–2172. doi: 10.1590/S0100-40422012001100012. [DOI] [Google Scholar]
- 103.Zhang Z.-X., Li H.-H., Qi F.-M., Dong L.-L., Hai Y., Fan G.-X., Fei D.-Q. Crocrassins A and B: Two novel sesquiterpenoids with an unprecedented carbon skeleton from Croton crassifolius. RSC Adv. 2014;4:30059–30061. doi: 10.1039/C4RA03798G. [DOI] [Google Scholar]
- 104.Langat M.K., Crouch N.R., Nuzillard J.-M., Mulholland D.A. Pseudopulchellol: A unique sesquiterpene-monoterpene derived C-25 terpenoid from the leaves of Croton pseudopulchellus Pax (Euphorbiaceae) Phytochem. Lett. 2018;23:38–40. doi: 10.1016/j.phytol.2017.11.008. [DOI] [Google Scholar]
- 105.Ghosh P., Mandal A., Rasul M.G. A new bioactive ursane-type triterpenoid from Croton bonplandianum Bail. J. Chem. Sci. 2013;125:359–364. doi: 10.1007/s12039-013-0387-9. [DOI] [Google Scholar]
- 106.Yuan Q.Q., Song W.B., Wang W.Q., Xuan L.J. A new patchoulane-type sesquiterpenoid glycoside from the roots of Croton crassifolius. Nat. Prod. Res. 2017;31:289–293. doi: 10.1080/14786419.2016.1233413. [DOI] [PubMed] [Google Scholar]
- 107.Pan Z.H., Ning D.S., Liu J.L., Pan B., Li D.P. A new triterpenoid saponin from the root of Croton lachnocarpus Benth. Nat. Prod. Res. 2014;28:48–51. doi: 10.1080/14786419.2013.838238. [DOI] [PubMed] [Google Scholar]
- 108.Aderogba M.A., McGaw L.J., Bezabih M., Abegaz B.M. Isolation and characterisation of novel antioxidant constituents of Croton zambesicus leaf extract. Nat. Prod. Res. 2011;25:1224–1233. doi: 10.1080/14786419.2010.532499. [DOI] [PubMed] [Google Scholar]
- 109.Kawakami S., Matsunami K., Otsuka H., Shinzato T., Takeda Y. Crotonionosides A-G: Megastigmane glycosides from leaves of Croton cascarilloides Rauschel. Phytochemistry. 2011;72:147–153. doi: 10.1016/j.phytochem.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 110.Takeshige Y., Kawakami S., Matsunami K., Otsuka H., Lhieochaiphant D., Lhieochaiphant S. Oblongionosides A-F, megastigmane glycosides from the leaves of Croton oblongifolius Roxburgh. Phytochemistry. 2012;80:132–136. doi: 10.1016/j.phytochem.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 111.Mehmood R., Bibi A., Malik A. New secondary metabolites from Croton sparsiflorus Morong. Turk. J. Chem. 2013;37:111–118. [Google Scholar]
- 112.Mehmood R., Imran M., Safder M., Anjum S., Malik A. Structural determination of crotamides A and B, the new amides from Croton sparsiflorus. J. Asian Nat. Prod. Res. 2010;12:662–665. doi: 10.1080/10286020.2010.489896. [DOI] [PubMed] [Google Scholar]
- 113.Mehmood R., Malik A. New secondary metabolites from Croton sparsiflorus. Z. Naturforsch. B. 2011;66:857–860. doi: 10.1515/znb-2011-0812. [DOI] [Google Scholar]
- 114.Wu X.A., Zhao Y.M., Yu N.J. A novel analgesic pyrazine derivative from the leaves of Croton tiglium L. J. Asian Nat. Prod. Res. 2007;9:437–441. doi: 10.1080/10286020500384781. [DOI] [PubMed] [Google Scholar]
- 115.Barbosa P.S., Abreu A.d.S., Batista E.F., Guilhon G.M.S.P., Müller A.H., Arruda M.S.P., Santos L.S., Arruda A.C., Secco R.S. Glutarimide alkaloids and terpenoids from Croton pullei var. glabrior Lanj. Biochem. Syst. Ecol. 2007;35:887–890. doi: 10.1016/j.bse.2007.04.006. [DOI] [Google Scholar]
- 116.Queiroz M.M.F., Queiroz E.F., Zeraik M.L., Marti G., Favre-Godal Q., Simões-Pires C., Marcourt L., Carrupt P.A., Cuendet M., Paulo M.Q., et al. Antifungals and acetylcholinesterase inhibitors from the stem bark of Croton heliotropiifolius. Phytochem. Lett. 2014;10 doi: 10.1016/j.phytol.2014.08.013. [DOI] [Google Scholar]
- 117.Novello C.R., Marques L.C., Pires M.E., Kutschenco A.P., Nakamura C.V., Nocchi S., Sarragiotto M.H., Mello J.C.P. Bioactive indole alkaloids from Croton echioides. J. Braz. Chem. Soc. 2016;27:2203–2209. [Google Scholar]
- 118.Aderogba M., Ndhlala A.R., Staden J.V. Acetylcholinesterase inhibitors from Croton sylvaticus ethyl acetate leaf extract and their mutagenic effects. Nat. Prod. Commun. 2013;8:795–798. [Google Scholar]
- 119.Athikomkulchai S., Prawat H., Thasana N., Ruangrungsi N., Ruchirawat S. Cox-1, Cox-2 inhibitors and antifungal agents from Croton hutchinsonianus. Chem. Pharm. Bull. 2006;54:262–264. doi: 10.1248/cpb.54.262. [DOI] [PubMed] [Google Scholar]
- 120.Li H.-H., Qi F.-M., Dong L.-L., Fan G.-X., Che J.-M., Guo D.-D., Zhang Z.-X., Fei D.-Q. Cytotoxic and antibacterial pyran-2-one derivatives from Croton crassifolius. Phytochem. Lett. 2014;10:304–308. doi: 10.1016/j.phytol.2014.10.022. [DOI] [Google Scholar]
- 121.Huang W., Wang J., Liang Y., Li Y., Wang G. Pyran-2-one derivatives from the roots of Croton crassifolius. Nat. Prod. Commun. 2016;11:803–804. [PubMed] [Google Scholar]
- 122.Quintyne-Walcott S., Maxwell A.R., Reynolds W.F. Crotogossamide, a cyclic nonapeptide from the latex of Croton gossypifolius. J. Nat. Prod. 2007;70:1374–1376. doi: 10.1021/np078007i. [DOI] [PubMed] [Google Scholar]
- 123.Candido-Bacani Pde M., Figueiredo Pde O., Matos Mde F., Garcez F.R., Garcez W.S. Cytotoxic orbitide from the latex of Croton urucurana. J. Nat. Prod. 2015;78:2754–2760. doi: 10.1021/acs.jnatprod.5b00724. [DOI] [PubMed] [Google Scholar]
- 124.Bracher F., Randau K.P., Lerche H. Crototropone, a new tropone derivative from Croton zehntneri. Fitoterapia. 2008;79:236–237. doi: 10.1016/j.fitote.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 125.Randau K.P., Sproll S., Lerche H., Bracher F. Pernambucone, a new tropone derivative from Croton argyroglossum. Pharmazie. 2009;64:350–351. [PubMed] [Google Scholar]
- 126.Nihei K., Asaka Y., Mine Y., Yamada Y., Iigo M., Yanagisawa T. Musidunin and musiduol, insect antifeedants from Croton jatrophoides. J. Nat. Prod. 2006;69:975–977. doi: 10.1021/np060068d. [DOI] [PubMed] [Google Scholar]
- 127.Zou G.A., Su Z.H., Zhang H.W., Wang Y., Yang J.S., Zou Z.M. Flavonoids from the stems of Croton caudatus Geisel. var. tomentosus Hook. Molecules. 2010;15:1097–1102. doi: 10.3390/molecules15031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahmat N., Said I.M., Latip J., Din L.B., Syah Y.M., Hakim E.H. New prenylated dihydrostilbenes from Croton laevifolius. Nat. Prod. Commun. 2007;2:1137–1140. [Google Scholar]
- 129.Attioua B., Weniger B., Chabert P. Antiplasmodial activity of constituents isolated from Croton lobatus. Pharm. Biol. 2007;45:263–266. doi: 10.1080/13880200701214607. [DOI] [Google Scholar]