Abstract
A new aporphine, 3-hydroxyhernandonine (1) and a new lignin, 4′-O-demethyl-7-O-methyldehydropodophyllotoxin (2), have been isolated from the root wood of Hernanadia nymphaeifolia, together with thirteen known compounds (3–15). The structures of these compounds were determined through mass spectrometry (MS) and spectroscopic analyses. The known isolate, 2-O-methyl-7-oxolaetine (3), was first isolated from natural sources. Among the isolated compounds, 3-hydroxyhernandonine (1), 4′-O-demethyl-7-O-methyldehydropodophyllotoxin (2), hernandonine (4), oxohernangerine (5), and oxohernagine (6) displayed inhibition (IC50 values ≤5.72 μg/mL) of superoxide anion production by human neutrophils in response to formyl-l-methionyl-l-leucyl-l-phenylalanine/cytochalasin B (fMLP/CB). In addition, 3-hydroxyhernandonine (1), 4′-O-demethyl-7-O-methyldehydropodophyllotoxin (2), oxohernangerine (5), and oxohernagine (6) suppressed fMLP/CB-induced elastase release with IC50 values ≤5.40 μg/mL.
Keywords: Hernanadia nymphaeifolia, Hernandiaceae, root wood, structure elucidation, aporphine, lignan, anti-inflammatory activity
1. Introduction
Hernanadia nymphaeifolia (Presl) Kubitzki (Hernandiaceae) is an evergreen tree that is distributed in the tropical island shores of the Indian and western Pacific Oceans [1]. Its seed is used as a cathartic [2]. Various aporphines [3,4,5,6,7], isoquinolones [4,5], lignans [4,7,8], benzylisoquinoline [5], steroids [7], and their derivatives were isolated from this species in past studies. Many of these isolates display cytotoxic [4,5,8], vasorelaxing [6], antioxidant [6], and antiplatelet aggregation [7] activities.
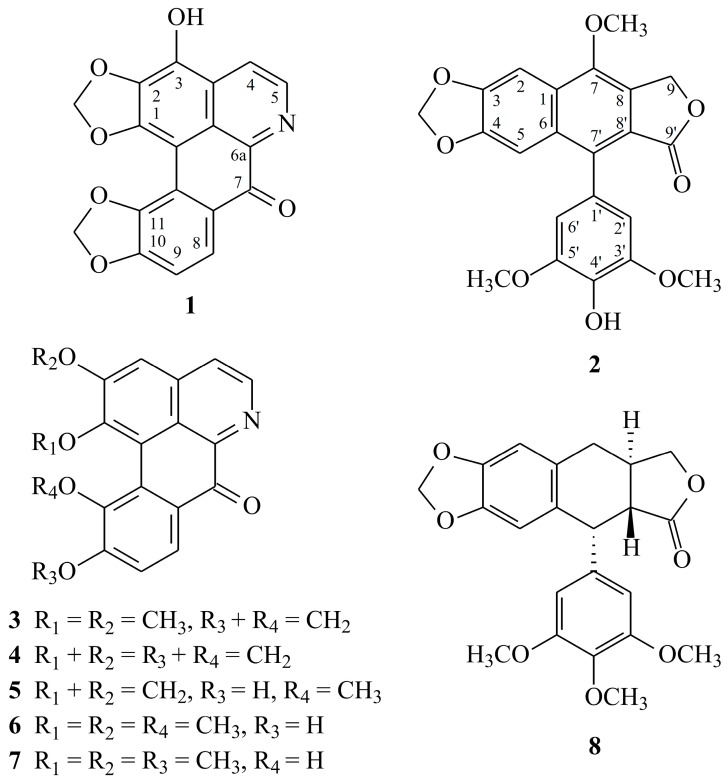
The extensive or inappropriate activation of neutrophils leads to many inflammatory disorders such as chronic obstructive pulmonary disease (COPD), ischemia-reperfusion injury, asthma, rheumatoid arthritis, and metabolic diseases [9,10]. In response to various stimuli, activated neutrophils secrete a series of cytotoxins, such as granule proteases, bioactive lipids, and superoxide anion (O2•–), a precursor of other reactive oxygen species (ROS) [10,11,12]. The inhibition of the abnormal activation of neutrophils by medicines has been recommended as a way to improve inflammatory diseases. In our researches on the anti-inflammatory constituents of Formosan plants, numerous species have been screened for anti-inflammatory activity, and H. nymphaeifolia has been found to be an active species. A new aporphine, 3-hydroxyhernandonine (1), a new lignin, 4′-O-demethyl-7-O-methyldehydropodophyllotoxin (2), and thirteen known compounds (3–15) have been isolated and determined from the root wood of Hernanadia nymphaeifolia, and their structures are described in Figure 1.
Figure 1.
The chemical structures of compounds 1–15 isolated from H. nymphaeifolia.
This article describes the structural elucidation of new compounds 1 and 2 and the inhibitory effects of all isolates on elastase release and superoxide generation by human neutrophils.
2. Results and Discussion
2.1. Isolation and Structural Elucidation
Chromatographic purification of the CH2Cl2-soluble fraction of a MeOH extract of root wood of H. nymphaeifolia through a silica gel column, medium pressure liquid chromatography (MPLC), and preparative thin-layer chromatography (TLC) yielded two new (1 and 2) and thirteen known compounds (3–15) (Figure 1).
The aporphine, 3-hydroxyhernandonine (1), was obtained as yellow needles. The electrospray ionization mass spectrometry (ESI-MS) (Figure S1) afford the quasi-molecular ion [M + Na]+ at m/z 358, implying a molecular formula of C18H9NO6Na, which was confirmed by the high-resolution (HR)-ESI-MS (m/z 358.0325 [M + Na]+, calcd 358.0328) (− 0.84 ppm) (Figure S2) and by the 13C-, 1H-, and distortionless enhancement by polarization transfer (DEPT) NMR data. IR absorptions for OH (3439 cm−1) and conjugated carbonyl (1646 cm−1) functions were observed. The 1H-NMR spectrum (Figure S3) of 1 showed the presence of a hydroxy group at δH 6.52 (1H, s, D2O exchangeable, OH-3), two methylenedioxy groups at δH 6.20 (2H, s, OCH2O-10,11) and 6.28 (2H, s, OCH2O-1,2), and two pairs of AB-doublets at δH 7.08 (1H, d, J = 8.5 Hz, H-9), 8.10 (1H, d, J = 5.0 Hz, H-4), 8.28 (1H, d, J = 8.5 Hz, H-8), and 8.88 (1H, d, J = 5.0 Hz, H-5). The 1H- and 13C-NMR (Figure S4) data of 1 were similar to those of hernandonine [13,14], except that the 3-hydroxy group [δH 6.52 (1H, s, D2O exchangeable)] of 1 replaced H-3 of hernandonine [13,14]. This was supported by HMBC correlations between OH-3 (δH 6.52) and C-2 (δC 139.2), as well as between C-3 (δC 148.4), and C-3a (δC 123.9). The full assignment of 1H- and 13C-NMR resonances was supported by DEPT, 1H–1H COSY (Figure S5), NOESY (Figure 2 and Figure S6), HMBC (Figure 2 and Figure S7), and HSQC (Figure S8) spectral analyses. Based on the above data, the structure of 1 was revealed as 3-hydroxyhernandonine.
Figure 2.
Key NOESY ( ) and HMBC (
) and HMBC ( ) correlations of 1.
) correlations of 1.
4′-O-Demethyl-7-O-methyldehydropodophyllotoxin (2) was isolated as colorless needles. The ESI-MS (Figure S9) display the sodium adduct ion [M + Na]+ at m/z 433, hinting a molecular formula of C22H18O8, which was supported by the HR-ESI-MS (m/z 433.0898 [M + Na]+, calcd 433.0899) (– 0.23 ppm) (Figure S10). The IR spectrum showed the presence of OH (3452 cm−1) and γ-lactone carbonyl (1764 cm−1) groups. The 1H-NMR spectrum (Figure S11) of 2 showed the presence of three methoxy groups at δH 3.88 (6H, s, OMe-3′ and OMe-5′) and 4.09 (3H, s, OMe-7), a hydroxyl group at δH 5.65 (1H, br s, D2O exchangeable, OH-4′), a methylenedioxy group at δH 6.09 (2H, s, OCH2O), a γ-lactone methylene proton at 5.52 (2H, s, H-9), and four aromatic protons at δH 6.57 (2H, s, H-2′ and H-6′), 7.07 (1H, s, H-5), and 7.57 (1H, s, H-2). The 1H- and 13C-NMR (Figure S12) data of 2 were similar to those of 4′-O-demethyldehydropodophyllotoxin [15], except that the 7-methoxy groups [δH 4.09 (3H, s); δC 59.9 (OMe-7)] of 2 replaced the 7-OH group of 4′-O-demethyldehydropodophyllotoxin [15]. This was supported by NOESY correlations between OMe-7 (δH 4.09) and H-2 (δH 7.57) and by HMBC correlations between OMe-7 (δH 4.09) and C-7 (δC 148.5). According to the above evidence, the structure of 2 was elucidated as 4′-O-demethyl-7-O-methyldehydropodophyllotoxin. This was further affirmed by the 1H–1H-COSY (Figure S13), NOESY (Figure 3 and Figure S14), DEPT, HMBC (Figure 3 and Figure S15), and HSQC (Figure S16) experiments.
Figure 3.
Key NOESY ( ) and HMBC (
) and HMBC ( ) correlations of 2.
) correlations of 2.
2.2. Structure Identification of the Known Isolates
The known isolated compounds were readily confirmed by a comparison of spectroscopic and physical data (IR, UV, 1H-NMR, MS, and [α]D) with the literature values or corresponding authentic samples, and this included five aporphines, 2-O-methyl-7-oxolaetine (3) [16], hernandonine (4) [13,14], oxohernangerine (5) [17], oxohernagine (6) [17], and 7-oxonorisocorydine (7) [18], three lignans, (–)-deoxypodophyllotoxin (8) [19,20], dehydropodophyllotoxin (9) [20,21], (–)-yatein (10) [20], an amide, N-trans-feruloylmethoxytyramine (11) [22], four steroids, a mixture of β-sitostenone (12) [23] and stigmasta-4,22-dien-3-one (13) [23], and mixture of 6 β-hydroxystigmast-4-en-3-one (14) [24,25] and 6 β-hydroxystigmasta-4,22-dien-3-one (15) [24,25].
2.3. Biological Studies
Granule proteases (e.g., cathepsin G, elastase, and proteinase-3) and reactive oxygen species (ROS) (e.g., hydrogen peroxide and superoxide anion (O2•−)) generated by human neutrophils are involved in the pathogenesis of various NMR data [10,11,12,26]. The activities during neutrophil proinflammatory responses to isolates from the root wood of H. nymphaeifolia were assessed by inhibiting fMet-Leu-Phe/cytochalasin B (fMLP/CB)-induced O2•– production and elastase release by human neutrophils. The inhibitory activity data on neutrophil proinflammatory responses are shown in Table 1. Diphenyleneiodonium and phenylmethylsulfonyl fluoride were employed as positive controls for O2•– generation and elastase release, respectively [26]. From the results of our biological assays, the following conclusions can be summarized: (a) 3-hydroxyhernandonine (1), 4′-O-demethyl-7-O-methyldehydropodophyllotoxin (2), hernandonine (4), oxohernangerine (5), and oxohernagine (6) exhibited potent inhibition (IC50 ≤ 5.72 μg/mL) of superoxide anion (O2•–) generation by human neutrophils in response to fMLP/CB; (b) 3-hydroxyhernandonine (1), 4′-O-demethyl-7-O-methyldehydropodophyllotoxin (2), oxohernangerine (5), and oxohernagine (6) exhibited potent inhibition (IC50 ≤ 5.40 μg/mL) of fMLP-induced elastase release; (c) the aporphine alkaloid, 3-hydroxyhernandonine (1) (with a 3-hydroxy group), exhibited more effective inhibition than its analogue, hernandonine (4) (without any substitutant at C-3), against fMLP-induced O2•– generation and elastase release; (d) oxohernagine (6) (with 10-hydroxy and 11-methoxy groups) exhibited more effective inhibition of fMLP-induced O2•– generation and elastase release than its analogue, 7-oxonorisocorydine (7) (with 11-hydroxy and 10-methoxy groups; (e) the lignan compound, 4′-O-demethyl-7-O-methyldehydroodophyllotoxin (2) (with 7-methoxy and 4′-hydroxy groups) exhibited more effective inhibition of fMLP-induced O2•– generation and elastase release than its analogue, dehydropodophyllotoxin (9) (with 7-hydroxy and 4′-methoxy groups); (f) oxohernangerine (5) was the most effective among these compounds, with an IC50 value of 2.65 ± 0.97 μg/mL, against fMLP-induced superoxide anion generation; (g) 3-hydroxyhernandonine (1) was the most effective among the isolates, with an IC50 value of 3.93 ± 0.48 μg/mL against fMLP-induced elastase release.
Table 1.
Inhibitory effects of compounds 1–15 from the root wood of H. nymphaeifolia on superoxide radical anion generation and elastase release by human neutrophils in response to fMet-Leu-Phe/cytochalasin B a.
| Compounds | Superoxide anion | Elastase |
|---|---|---|
| IC50 [µg/mL] b or (Inh %) c | ||
| 3-Hydroxyhernandonine (1) | 4.09 ± 0.44 *** | 3.93 ± 0.48 *** |
| 4′-O-Demethyl-7-O- methyldehydro-podophyllotoxin (2) |
5.72 ± 0.42 *** | 5.40 ± 0.40 *** |
| 2-O-Methyl-7-oxolaetine (3) | 7.37 ± 0.46 *** | 6.82 ± 0.09 *** |
| Hernandonine (4) | 4.41 ± 0.76 *** | (45.76 ± 6.92) *** |
| Oxohernangerine (5) | 2.65 ± 0.97 *** | 4.82 ± 0.39 *** |
| Oxohernagine (6) | 2.86 ± 0.85 *** | 4.87 ± 0.27 *** |
| 7-Oxonorisocorydine (7) | 6.62 ± 0.28 *** | 6.58 ± 0.08 *** |
| (–)-Deoxypodophyllotoxin (8) | (38.95 ± 4.83) ** | (33.76 ± 3.82) |
| Dehydropodophyllotoxin (9) | (43.91 ± 3.86) *** | 9.53 ± 0.84 *** |
| (–)-Yatein (10) | (42.36 ± 3.41) * | (36.74 ± 3.05) ** |
| N-trans-Feruloylmethoxytyramine (11) | 6.26 ± 0.65 *** | 7.03 ± 0.21 *** |
| Mixture of β-sitostenone (12) and stigmasta-4,22-dien-3-one (13) | (24.71 ± 2.67) | (29.15 ± 2.89) |
| Mixture of 6β-hydroxystigmast-4-en-3-one (14) and 6β-hydroxystigmasta-4,22-dien-3-one (15) | (16.74 ± 2.66) ** | 7.91 ± 1.20 ** |
| Diphenyleneiodonium d | 0.55 ± 0.22 *** | – |
| Phenylmethylsulfonyl fluoride d | – | 34.5 ± 5.3 *** |
a Results are displayed as averages ± SEM (n = 4). b Concentration necessary for 50% inhibition (IC50). If IC50 value of tested compound was <10 μg/mL, it was presented as IC50 [μg/mL]. c Percentage of inhibition (Inh %) at 10 μg/mL. If IC50 value of tested compound was ≥10 μg/mL, it was displayed as (Inh %) at 10 μg/mL. d Diphenyleneiodonium and phenylmethylsulfonyl were employed as positive controls for superoxide anion (O2•–) production and elastase release, respectively. * p < 0.05 compared with the control. ** p < 0.01 compared with the control. *** p < 0.001 compared with the control.
3. Experimental Section
3.1. General Procedures
Melting points were determined on a Yanaco micromelting point apparatus and were uncorrected. Ultraviolet (UV) spectra were measured on a Jasco UV-240 spectrophotometer. Optical rotations were acquired using a Jasco DIP-370 polarimeter (Japan Spectroscopic Corporation, Tokyo, Japan) in CHCl3. Infrared (IR) spectra (KBr or neat) were recorded on a Perkin Elmer 2000 FT-IR spectrometer (Perkin Elmer Corporation, Norwalk, CT, USA). Nuclear magnetic resonance (NMR) spectra, including correlation spectroscopy (COSY), nuclear overhauser effect spectrometry (NOESY), heteronuclear multiple-bond correlation (HMBC) experiments, and heteronuclear single-quantum coherence (HSQC), were obtained using a Varian Inova 500 spectrometer (Varian Inc., Palo Alto, CA, USA) operating at 500 MHz (1H) and 125 MHz (13C), respectively, with chemical shifts given in ppm (δ) and applying tetramethylsilane (TMS) as an internal standard. Electrospray ionization (ESI) and high-resolution electrospray ionization (HRESI)-mass spectra were recorded on a VG Platform Electrospray ESI/MS mass spectrometer (Fison, Villeurbanne, France) or a Bruker APEX II (Bruker, Bremen, Germany). Silica gel (70–230, 230–400 mesh, Merck) was used for column chromatography (CC). Silica gel 60 F-254 (Merck, Darmstadt, Germany) was employed for thin-layer chromatography (TLC) and preparative thin-layer chromatography (PTLC).
3.2. Plant Material
The root wood of Hernanadia nymphaeifolia (Presl) Kubitzki (Hernandiaceae) was collected from Mudan Township, Pingtung County, Taiwan, in August 2008 and identified by Prof. I.-S. Chen. A voucher specimen (Chen 5521) was deposited in the Herbarium of School of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan.
3.3. Extraction and Isolation
The dried root wood (5.1 kg) of H. nymphaeifolia was sliced and extracted three times with MeOH (40 L each) for three days. The extract was concentrated under reduced pressure at 35 °C, and the residue (386 g) was partitioned between CH2Cl2 and H2O (1:1) to provide the CH2Cl2-soluble fraction (fraction A; 87 g). Fraction A (87 g) was purified by CC (3.9 kg of SiO2, 70–230 mesh; CH2Cl2/MeOH gradient) to produce 12 fractions: A1–A12. Fraction A3 (7.5 g) was subjected to CC (340 g of SiO2, 230–400 mesh; CH2Cl2/acetone 30:1–0:1, 900 mL fractions) to give 11 subfractions: A3-1–A3-11. Fraction A3-4 (340 mg) was purified by MPLC (silica column, CH2Cl2/acetone 8:1–0:1) to produce eight subfractions (each 250 mL, A3-4-1–A3-4-8). Fraction A3-4-4 (46 mg) was purified by preparative TLC (silica gel, CHCl3/MeOH, 10:1) to obtain a mixture of β-sitostenone (12) and stigmasta-4,22-dien-3-one (13) (8.5 mg). Fraction A5 (6.9 g) was subjected to CC (365 g of SiO2, 230–400 mesh; CH2Cl2/MeOH 15:1–0:1, 950 mL fractions) to form ten subfractions: A5-1–A5-10. Fraction A5-3 (625 mg) was purified by CC (28 g of SiO2, 230–400 mesh, CHCl3/acetone (7:1–0:1), 250 mL fractions) to give nine subfractions: A5-3-1–A5-3-9. Fraction A5-3-5 (88 mg) was further purified by preparative TLC (SiO2; CH2Cl2/acetone 8:1) to yield a mixture of 6β-hydroxystigmast-4-en-3-one (14) and 6β-hydroxystigmasta-4,22-dien-3-one (15) (3.7 mg). Fraction A7 (7.3 g) was subjected to CC (330 g of SiO2, 230–400 mesh; CHCl3/MeOH 10:1–0:1, 800 mL fractions) to give nine subfractions: A7-1–A7-9. A part (142 mg) of fraction A7-2 was further purified by preparative TLC (SiO2; CH2Cl2/MeOH 15:1) to form (–)-deoxypodophyllotoxin (8) (7.2 mg). A part (133 mg) of fraction A7-3 was further purified by preparative TLC (SiO2; CHCl3/MeOH 12:1) to yield (–)-yatein (10) (5.1 mg). A part (136 mg) of fraction A7-4 was further purified by preparative TLC (SiO2; CHCl3/MeOH 9:1) to obtain 2-O-methyl-7-oxolaetine (3) (5.3 mg). A part (118 mg) of fraction A7-6 was further purified by preparative TLC (SiO2; CH2Cl2/MeOH 5:1) to produce 7-oxonorisocorydine (7) (6.5 mg). Fraction A7-7 (650 mg) was purified by MPLC (silica column, CH2Cl2/MeOH 9:1–0:1) to form seven subfractions (each 170 ml, A7-7-1–A7-7-7). A part (112 mg) of fraction A7-7-4 was further purified by preparative TLC (SiO2; CHCl3/acetone 5:1) to form 4′-O-demethyl-7-O-methyldehydropodophyllotoxin (2) (5.5 mg). A part (125 mg) of fraction A7-7-5 was purified by preparative TLC (SiO2; CH2Cl2/acetone 4:1) to obtain dehydropodophyllotoxin (9) (6.9 mg). A part (122 mg) of fraction A7-8 was purified by preparative TLC (SiO2; CH2Cl2/EtOAc 2:1) to yield N-trans-feruloylmethoxytyramine (11) (4.9 mg). Fraction A8 (7.2 g) was subjected to CC (325 g of SiO2, 230–400 mesh; CH2Cl2/MeOH 8:1–0:1, 850 mL fractions) to give ten subfractions: A8-1–A8-10. Fraction A8-2 (530 mg) was purified by MPLC (silica column, CHCl3/MeOH 7:1–0:1) to form six subfractions (each 180 ml, A8-2-1–A8-2-6). Fraction A8-2-4 (83 mg) was further purified by preparative TLC (SiO2; CH2Cl2/acetone 6:1) to obtain hernandonine (4) (8.2 mg). Fraction A8-5 (135 mg) was further purified by preparative TLC (SiO2; CH2Cl2/MeOH 4:1) to obtain oxohernagine (6) (7.1 mg). Fraction A8-6 (135 mg) was further purified by preparative TLC (SiO2; CHCl3/MeOH 3:1) to yield oxohernangerine (5) (6.5 mg). Fraction A9 (6.4 g) was subjected to CC (290 g of SiO2, 230–400 mesh; CHCl3/MeOH 6:1–0:1, 1 L fractions) to obtain eight subfractions: A9-1–A9-8. A part (142 mg) of fraction A9-3 was purified by preparative TLC (SiO2; CHCl3/MeOH 5:1) to obtain 3-hydroxyhernandonine (1) (4.5 mg). A part (105 mg) of fraction A9-5 was purified by preparative TLC (SiO2; CH2Cl2/MeOH 4:1) to obtain oxohernangerine (5) (5.9 mg).
3-Hydroxyhernandonine (1): yellow needles; m.p. 268–270 °C (MeOH); UV (MeOH): λmax (log ε) = 220 (3.90), 268 (3.79), 284 (3.78), 343 (3.46), 362 (3.47) nm; IR (KBr): υmax = 3315 (OH), 1652 (C=O), 1060, 969 (OCH2O) cm−1; 1H-NMR (CDCl3, 500 MHz): δ 6.20 (2H, s, OCH2O-10,11), 6.28 (2H, s, OCH2O-1,2), 6.52 (1H, s, D2O exchangeable, OH-3), 7.08 (1H, d, J = 8.5 Hz, H-9), 8.10 (1H, d, J = 5.0 Hz, H-4), 8.28 (1H, d, J = 8.5 Hz, H-8), 8.88 (1H, d, J = 5.0 Hz, H-5); 13C-NMR (CDCl3, 125 MHz): δ 101.3 (OCH2O-1,2), 101.7 (OCH2O-10,11), 108.6 (C-9), 114.9 (C-11b), 118.7 (C-8), 119.1 (C-4), 122.3 (C-11a), 122.9 (C-11c), 123.9 (C-3a), 127.6 (C-7a), 139.2 (C-2), 145.2 (C-5), 145.8 (C-11), 148.4 (C-3), 149.6 (C-1), 150.4 (C-10), 157.3 (C-6a), 182.5 (C-7); ESI-MS: m/z = 358 [M + Na]+; HR-ESI-MS: m/z = 358.0325 [M + Na]+ (calcd for C18H9NO6Na, 358.0328).
4′-O-Demethyl-7-O-methyldehydropodophyllotoxin (2): colorless needles; m.p. 273–275 °C (MeOH); UV (MeOH): λmax (log ε) = 223 (4.47), 262 (4.58), 321 (3.98), 355 (3.69) nm; IR (KBr): υmax = 3452 (OH), 1764 (C=O) cm−1; 1H-NMR (CDCl3, 500 MHz): δ 3.88 (6H, s, OMe-3′ and OMe-5′), 4.09 (3H, s, OMe-7), 5.52 (2H, s, H-9), 5.65 (1H, br s, D2O exchangeable, OH-4′), 6.09 (2H, s, OCH2O), 6.57 (2H, s, H-2′, and H-6′), 7.07 (1H, s, H-5), 7.57 (1H, s, H-2); 13C-NMR (CDCl3, 125 MHz): δ 56.0 (OMe-3′), 56.0 (OMe-5′), 59.9 (OMe-7), 66.4 (C-9), 98.4 (C-2), 101.8 (OCH2O), 103.9 (C-5), 107.7 (C-2′), 107.7 (C-6′), 119.5 (C-8′), 125.8 (C-8), 127.8 (C-6), 130.4 (C-1), 132.2 (C-1′), 133.6 (C-4′), 137.7 (C-7′), 148.5 (C-7), 148.9 (C-4), 148.9 (C-3′), 148.9 (C-5′), 150.0 (C-3), 169.3 (C-9′); ESI-MS: m/z = 433 [M + Na]+; HR-ESI-MS: m/z = 433.0898 [M + Na]+ (calcd for C22H18O8Na, 433.0899).
2-O-Methyl-7-oxolaetine (3): yellow needles; m.p. > 300 °C (MeOH); UV (MeOH): λmax (log ε) = 221 (4.49), 266 (4.33), 362 (4.00), 427 (3.95) nm; IR (KBr): υmax = 1652 (C=O) cm−1; 1H-NMR (CDCl3, 400 MHz): δ 3.93 (3H, s, OMe-1), 6.21 (2H, s, OCH2O-10,11), 7.08 (1H, d, J = 8.4 Hz, H-9), 7.21 (1H, s, H-3), 7.77 (1H, d, J = 5.2 Hz, H-4), 8.24 (1H, d, J = 8.4 Hz, H-8), 8.86 (1H, d, J = 5.2 Hz, H-5); ESI-MS: m/z = 358 [M + Na]+; HR-ESI-MS: m/z = 358.0692 [M + Na]+ (calcd for C19H13O5Na, 358.0691).
Hernandonine (4): yellow needles; m.p. > 350 °C (CH2Cl2-MeOH); UV (MeOH): λmax (log ε) = 222 (4.50), 265 (4.34), 295 (sh, 3.90), 312 (sh, 3.63), 364 (4.02), 428 (3.97) nm; IR (KBr): υmax = 1651 (C=O), 1062, 971 (OCH2O) cm−1; 1H-NMR (CDCl3, 500 MHz): δ 6.20 (2H, s, OCH2O-10,11), 6.28 (2H, s, OCH2O-1,2), 7.08 (1H, d, J = 8.5 Hz, H-9), 7.21 (1H, s, H-3), 7.74 (1H, d, J = 5.0 Hz, H-4), 8.29 (1H, d, J = 8.5 Hz, H-8), 8.85 (1H, d, J = 5.0 Hz, H-5); ESI-MS: m/z = 342 [M + Na]+.
Oxohernangerine (5): yellow prisms; m.p. 257–258 °C (MeOH); UV (MeOH): λmax (log ε) = 211 (4.55), 252 (4.46), 268 (sh, 4.42), 316 (3.87), 362 (4.04), 408 (4.02), 477 (3.55) nm; IR (KBr): υmax = 3415 (OH), 1642 (C=O) cm−1; 1H-NMR (CDCl3, 400 MHz): δ 3.68 (3H, s, OMe-11), 6.32 (2H, s, OCH2O-1,2), 7.24 (1H, d, J = 8.4 Hz, H-9), 7.27 (1H, s, H-3), 7.76 (1H, d, J = 5.2 Hz, H-4), 8.37 (1H, d, J = 8.4 Hz, H-8), 8.86 (1H, d, J = 5.2 Hz, H-5); ESI-MS: m/z = 344 [M + Na]+.
Oxohernagine (6): yellow prisms; m.p. 253–255 °C (MeOH); UV (MeOH): λmax (log ε) = 213 (4.51), 274 (4.41), 361 (3.95), 403 (3.92) nm; IR (KBr): υmax = 3424 (OH), 1650 (C=O) cm−1; 1H-NMR (CDCl3, 400 MHz): δ 3.54 (3H, s, OMe-1), 3.76 (3H, s, OMe-11), 4.11 (3H, s, OMe-2), 7.21 (1H, s, H-3), 7.22 (1H, d, J = 8.4 Hz, H-9), 7.77 (1H, d, J = 5.2 Hz, H-4), 8.31 (1H, d, J = 8.4 Hz, H-8), 8.86 (1H, d, J = 5.2 Hz, H-5); ESI-MS: m/z = 360 [M + Na]+.
7-Oxonorisocorydine (7): yellow needles; m.p. 250–252 °C (EtOAc); UV (MeOH): λmax (log ε) = 212 (4.49), 273 (4.40), 362 (3.94), 403 (3.90) nm; IR (KBr): υmax = 3385 (OH), 1653 (C=O) cm−1; 1H-NMR (CDCl3, 400 MHz): δ 3.53 (3H, s, OMe-1), 4.03 (3H, s, OMe-10), 4.08 (3H, s, OMe-2), 7.14 (1H, d, J = 8.4 Hz, H-9), 7.23 (1H, s, H-3), 7.77 (1H, d, J = 5.2 Hz, H-4), 8.28 (1H, d, J = 8.4 Hz, H-8), 8.86 (1H, d, J = 5.2 Hz, H-5); ESI-MS: m/z = 360 [M + Na]+.
(–)-Deoxypodophyllotoxin (8): colorless needles; m.p. 168–170 °C (MeOH); UV (MeOH): λmax (log ε) = 212 (4.62), 291 (3.68) nm; IR (KBr): υmax = 1765 (C=O), 1581, 1502, 1474 (aromatic ring C=C stretch), 1032, 941 (OCH2O) cm−1; 1H-NMR (CDCl3, 500 MHz): δ 2.73 (3H, m, H-7, H-8, and H-8′), 3.07 (1H, m, H-7), 3.75 (6H, s, OMe-3′, and OMe-5′), 3.80 (3H, s, OMe-4′), 3.92 (1H, m, H-9), 4.46 (1H, m, H-9), 4.60 (1H, d, J = 3.5 Hz, H-7′), 5.93, 5.95 (each 1H, each d, J = 1.5 Hz, OCH2O), 6.34 (2H, s, H-2′, and H-6′), 6.52 (1H, s, H-5), 6.67 (1H, s, H-2); ESI-MS: m/z = 421 [M + Na]+.
Dehydropodophyllotoxin (9): colorless needles; m.p. 264–266 °C (CH2Cl2-MeOH); UV (MeOH): λmax (log ε) = 262 (4.57), 311 (3.95), 321 (3.97) nm; IR (KBr): υmax = 3421 (OH), 1762 (C=O) cm−1; 1H-NMR (CDCl3, 500 MHz): δ 3.83 (6H, s, OMe-3′, and OMe-5′), 3.95 (3H, s, OMe-4′), 5.37 (2H, br s, H-9), 5.68 (1H, br s, D2O exchangeable, OH-7), 6.10 (2H, s, OCH2O), 6.52 (2H, s, H-2′, and H-6′), 7.09 (1H, s, H-5), 7.49 (1H, s, H-2); ESI-MS: m/z = 433 [M + Na]+.
(–)-Yatein (10): yellowish solid (MeOH); UV (MeOH): λmax (log ε) = 212 (4.35), 230 (sh, 3.93), 287 (3.47) nm; IR (KBr): υmax = 1764 (C=O), 1591, 1502, 1488 (aromatic ring C=C stretch), 1037, 925 (OCH2O) cm−1; 1H-NMR (CDCl3, 400 MHz): δ 2.49 (1H, m, H-8), 2.53 (1H, m, H-7α), 2.58 (1H, m, H-8′), 2.62 (1H, dd, J = 13.2, 6.4 Hz, H-7β), 2.89 (1H, dd, J = 14.0, 6.2 Hz, H-7′α), 2.93 (1H, dd, J = 14.0, 5.2 Hz, H-7′β), 3.82 (6H, s, OMe-3′, and OMe-5′), 3.83 (3H, s, OMe-4′), 3.88 (1H, dd, J = 9.2, 7.6 Hz, H-9β), 4.18 (1H, dd, J = 9.2, 7.2 Hz, H-9α), 5.93, 5.94 (each 1H, each d, J = 1.2 Hz, OCH2O), 6.36 (2H, s, H-2′, and H-6′), 6.46 (1H, d, J = 1.6 Hz, H-2), 6.47 (1H, dd, J = 7.6, 1.6 Hz, H-6), 6.69 (1H, d, J = 7.6 Hz, H-5); ESI-MS: m/z = 423 [M + Na]+.
N-trans-Feruloylmethoxytyramine (11): white needles; m.p. 112–114 °C (CHCl3-MeOH); UV (MeOH): λmax (log ε) = 221 (3.61), 290 (2.86), 319 (3.34) nm; IR (KBr): υmax = 3362 (OH), 1652 (C=O) cm−1; 1H-NMR (CDCl3, 400 MHz): δ 2.82 (2H, t, J = 6.8 Hz, H-11), 3.62 (2H, q, J = 6.8 Hz, H-10), 3.88 (3H, s, OMe-14), 3.92 (3H, s, OMe-3), 5.52 (1H, br t, J = 6.8 Hz, D2O exchangeable, NH), 5.53 (1H, s, D2O exchangeable, OH), 5.79 (1H, s, D2O exchangeable, OH), 6.16 (1H, d, J = 15.6 Hz, H-8), 6.71 (1H, dd, J = 8.0, 1.6 Hz, H-17), 6.73 (1H, d, J = 1.6 Hz, H-13), 6.87 (1H, d, J = 8. Hz, H-16), 6.90 (1H, d, J = 8.4 Hz, H-5), 6.97 (1H, d, J = 1.6 Hz, H-2), 7.04 (1H, dd, J = 8.4, 1.6 Hz, H-5), 7.53 (1H, d, J = 15.6 Hz, H-7); ESI-MS: m/z = 366 [M + Na]+.
Mixture of β-Sitostenone (12) and stigmasta-4,22-dien-3-one (13): colorless needles; m.p. 88–90 °C (MeOH); [α = +85.8° (c 0.18, CHCl3); UV (MeOH): λmax (log ε) = 242 (4.21); IR (KBr): υmax = 1685 (C=O) cm−1; 1H-NMR (CDCl3, 400 MHz) of 12: δ 0.70 (3H, s, H-18), 0.81 (3H, d, J = 6.8 Hz, H-27), 0.83 (3H, d, J = 6.8 Hz, H-26), 0.86 (3H, t, J = 7.2 Hz, H-29), 0.92 (3H, d, J = 6.4 Hz, H-21), 1.18 (3H, s, H-19), 5.71 (1H, s, H-4); 1H-NMR (CDCl3, 400 MHz) of 13: δ 0.72 (3H, s, H-18), 0.79 (3H, d, J = 6.8 Hz, H-27), 0.82 (3H, t, J = 7.2 Hz, H-29), 0.83 (3H, d, J = 6.8 Hz, H-26), 1.02 (3H, d, J = 6.8 Hz, H-21), 1.18 (3H, s, H-19), 5.02 (1H, dd, J = 15.2, 8.8 Hz, H-23), 5.14 (1H, dd, J = 15.2, 8.8 Hz, H-22), 5.71 (1H, s, H-4); ESI-MS of 12: m/z = 435 [M + Na]+; ESI-MS of 13: m/z = 433 [M + Na]+.
Mixture of6β-Hydroxystigmast-4-en-3-one (14) and 6β-hydroxystigmasta-4,22-dien-3-one (15): colorless needles; m.p. 208–209 °C (CH2Cl2-MeOH); [α = + 29.7° (c 0.17, CHCl3); UV (MeOH): λmax (log ε) = 235 (4.11) nm; IR (KBr): υmax = 3412 (OH), 1679 (C=O) cm−1; 1H-NMR (CDCl3, 400 MHz) of 14: δ 0.74 (3H, s, H-18), 0.81 (3H, d, J = 6.8 Hz, H-27), 0.84 (3H, d, J = 7.2 Hz, H-26), 0.87 (3H, t, J = 7.2 Hz, H-29), 0.92 (3H, d, J = 6.4 Hz, H-21), 1.38 (3H, s, H-19), 4.35 (1H, br s, H-6), 5.82 (1H, s, H-4); 1H-NMR (CDCl3, 500 MHz) of 15: δ 0.76 (3H, s, H-18), 0.80 (3H, d, J = 6.8 Hz, H-27), 0.81 (3H, d, J = 6.8 Hz, H-26), 0.85 (3H, t, J = 7.2 Hz, H-29), 1.02 (3H, d, J = 6.8 Hz, H-21), 1.38 (3H, s, H-19), 4.35 (1H, br s, H-6), 5.03 (1H, dd, J = 15.2, 8.6 Hz, H-23), 5.15 (1H, dd, J = 15.2, 8.6 Hz, H-22), 5.82 (1H, s, H-4); ESI-MS of 14: m/z = 451 [M + Na]+; ESI-MS of 15: m/z = 449 [M + Na]+.
3.4. Biological Assay
The effect of the isolates on the neutrophil proinflammatory response was assessed by detecting the inhibition of elastase release and O2•− generation in fMLP/CB-activated neutrophils in a concentration-dependent manner.
3.4.1. Mensuration of Human Neutrophils
Human neutrophils from the venous blood of adult, healthy volunteers (20–27 years old) were isolated by a standard pattern of dextran sedimentation before centrifugation in a Ficoll Hypaque gradient and hypotonic lysis of the erythrocytes [27]. The purified neutrophils had >98% viable cells, as detected by the trypan blue exclusion method [28], were resuspended in a calcium (Ca2+)-free HBSS buffer at pH 7.4 and were kept at 4 °C prior to use.
3.4.2. Mensuration of Superoxide Anion (O2•−) Generation
The assay for the measurement of O2•− generation was based on the superoxide dismutase (SOD)-inhibitable reduction of ferricytochrome c [29,30]. In short, after supplementation with 1 mM Ca2+ and 0.5 mg/mL ferricytochrome c, neutrophils (6 × 105/mL) were equilibrated at 37 °C for 2 min and incubated with varied concentrations (10–0.01 μg/mL) of either DMSO (as a control) or tested compounds 1–15 (purity ≥ 98%) for 5 min. Cells were incubated with cytochalasin B (1 μg/mL) for 3 min before they were activated with 100 nM formyl-l-methionyl-l-leucyl-l-phenylalanine for 10 min. Changes in absorbance with the reduction of ferricytochrome c at 550 nm were constantly detected in a double-beam, six-cell positioner spectrophotometer with continuous stirring (Hitachi U-3010, Tokyo, Japan). Calculations were founded on differences in the reactions with and without SOD (100 U/mL) divided by the extinction coefficient for the reduction of ferricytochrome c (ε = 21.1/mM/10 mm).
3.4.3. Mensuration of Elastase Release
The degranulation of azurophilic granules was measured by determining elastase release as reported previously [30,31]. Assays were carried out by applying MeO-Suc-Ala-Ala-Pro-Val- p-nitroanilide as the elastase substrate. In brief, after supplementation with MeO-Suc-Ala-Ala-Pro-Val- p-nitroanilide (100 μM), neutrophils (6 × 105/mL) were equilibrated at 37 °C for 2 min and incubated with tested compounds for 5 min. Cells were treated with fMLP (100 nM)/CB (0.5 μg/mL), and the changes in absorbance at 405 nm were detected constantly in order to measure elastase release. The results were displayed as the percent of elastase release in the fMLP/CB-activated, drug-free control system.
3.4.4. Statistical Analysis
Results are represented as mean ± SEM, and comparisons were done by applying student’s t-test. A probability of 0.05 or less was deemed significant. The software SigmaPlot was employed for the statistical analysis.
4. Conclusions
Fifteen compounds, including a new aporphine, 3-hydroxyhernandonine (1), and a new lignin, 4′-O-demethyl-7-O-methyldehydropodophyllotoxin (2), were isolated from the resinous wood of the root wood of H. nymphaeifolia. The structures of these isolates were elucidated according to spectroscopic data. Granule proteases (e.g., cathepsin G, elastase) and reactive oxygen species (ROS) [e.g., hydrogen peroxide, superoxide anion (O2•−)] generated by human neutrophils gave rise to the pathogenesis of inflammatory diseases. The effects of the isolated compounds on proinflammatory responses were assessed by inhibiting fMLP/CB-induced elastase release and O2•− generation by neutrophils. The results of anti-inflammatory assays reveal that compounds 1–7 and 11 can obviously inhibit fMLP-induced elastase release and/or O2•− generation. Oxohernangerine (5) and 3-hydroxyhernandonine (1) were the most effective among the isolated compounds, with IC50 values of 2.65 ± 0.97 and 3.93 ± 0.48 μg/mL, respectively, against fMLP-induced O2•– generation and elastase release. Our research indicates H. nymphaeifolia and its isolated compounds (especially 1–7 and 11) are worth further study and may be expectantly developed as candidates for the prevention or treatment of diverse inflammatory diseases.
Acknowledgments
This research was supported by grants from the Ministry of Science and Technology, Taiwan (No. MOST 106-2320-B-010-033-MY3 and MOST 105-2320-B-010-040), awarded to J.-J. Chen. This work was also supported by the grants from Chang Gung Memorial Hospital (CMRPD1B0281~3, CMRPF1D0442~3, CMRPF 1F0011~3, CMRPF1F0061~3 and BMRP450). We are grateful to Ih-Sheng Chen for unselfishly providing us with plant material (root wood of H. nymphaeifolia).
Supplementary Materials
Supplementary materials are available online, Figures S1–S8: MS, 1D, and 2D-NMR spectra for 3-hydroxyhernandonine (1), Figures S9–S16: MS, 1D, and 2D-NMR spectra for 4′-O-demethyl-7-O-methyldehydropodophyllotoxin (2).
Author Contributions
C.-Y.W. and J.-J.C. performed the isolation and structure elucidation of the constituents and manuscript writing. C.-Y.W., S.-W.W., J.-W.Y., T.-L.H., M.-J.C., P.-J.S., T.-H.C., and J.-J.C. conducted the bioassay and analyzed the data. J.-J.C. planned, designed, and organized all of the research of this study and the preparation of the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Yang Y.P., Lu S.Y. Flora of Taiwan. 2nd ed. Volume 2. Editorial Committee of the Flora of Taiwan; Taipei, Taiwan: 1996. Hernandiaceae; pp. 500–503. [Google Scholar]
- 2.Kan W.S. Manual of Medicinal Plants in Taiwan. National Research Institute of Chinese Medicine; Taipei, Taiwan: 1970. pp. 178–179. [Google Scholar]
- 3.Chen J.J., Tsai I.L., Ishikawa T., Wang C.J., Chen I.S. Alkaloids from trunk bark of Hernandia nymphaeifolia. Phytochemistry. 1996;42:1479–1484. doi: 10.1016/0031-9422(96)00123-9. [DOI] [Google Scholar]
- 4.Chen J.J., Ishikawa T., Duh C.Y., Tsai I.L., Chen I.S. New dimeric aporphine alkaloids and cytotoxic constituents of Hernandia nymphaeifolia. Planta Med. 1996;62:528–533. doi: 10.1055/s-2006-957963. [DOI] [PubMed] [Google Scholar]
- 5.Chen I.S., Chen J.J., Duh C.Y., Tsai I.L., Chang C.T. New aporphine alkaloids and cytotoxic constituents of Hernandia nymphaeifolia. Planta Med. 1997;63:154–157. doi: 10.1055/s-2006-957634. [DOI] [PubMed] [Google Scholar]
- 6.Chen J.J., Chang Y.L., Teng C.M., Chen I.S. Vasorelaxing and antioxidant constituents from Hernandia nymphaeifolia. Planta Med. 2001;67:593–598. doi: 10.1055/s-2001-17348. [DOI] [PubMed] [Google Scholar]
- 7.Chen J.J., Chang Y.L., Teng C.M., Chen I.S. Anti-platelet aggregation alkaloids and lignans from Hernandia nymphaeifolia. Planta Med. 2000;66:251–256. doi: 10.1055/s-2000-8562. [DOI] [PubMed] [Google Scholar]
- 8.Chen I.S., Chen J.J., Duh C.Y., Tsai I.L. New cytotoxic lignans from Formosan Hernandia nymphaeifolia. Phytochemistry. 1997;45:991–996. doi: 10.1016/S0031-9422(97)00064-2. [DOI] [PubMed] [Google Scholar]
- 9.Ennis M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep. 2003;3:159–165. doi: 10.1007/s11882-003-0029-2. [DOI] [PubMed] [Google Scholar]
- 10.Borregaard N. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 1998;41:401–413. doi: 10.1111/j.1600-0609.1988.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 11.Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Inverstig. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 12.Roos D., van Bruggen R., Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–1315. doi: 10.1016/j.micinf.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa H., Ueda F., Ito M., Ishii H., Hagniwa J. Alkaloids of Hernandia ovigera. IV. Constituents of Hernandia ovigera collected in the Bonin Islands. Yakugaku Zasshi. 1972;92:150–154. doi: 10.1248/yakushi1947.92.2_150. [DOI] [PubMed] [Google Scholar]
- 14.Yang T.H., Liu S.C., Lin T.S., Yang L.M. Studies on the constituents of the root-bark of Hernandia ovigera L. III. J. Chin. Chem. Soc. 1976;23:29–34. doi: 10.1002/jccs.197600005. [DOI] [Google Scholar]
- 15.Atta-ur-Rahman, Ashraf M., Choudhary M.I., Habib-ur-Rehman, Kazmi M.H. Antifungal aryltetralin lignans from leaves of Podophyllum hexandrum. Phytochemistry. 1995;40:427–431. doi: 10.1016/0031-9422(95)00195-D. [DOI] [Google Scholar]
- 16.Orito K., Uchiito S., Satoh Y., Tatsuzawa T., Harada R., Tokuda M. Aryl radical cyclizations of 1-(2′-bromobenzyl) isoquinolines with AIBN-Bu3SnH: Formation of aporphines and indolo[2,1-a]isoquino-lines. Org. Lett. 2000;2:307–310. doi: 10.1021/ol990360v. [DOI] [PubMed] [Google Scholar]
- 17.Chen J.J., Tsai I.L., Chen I.S. New oxoaporphine alkaloids from Hernandia nymphaeifolia. J. Nat. Prod. 1996;59:156–158. doi: 10.1021/np960034d. [DOI] [Google Scholar]
- 18.Kametani T., Nitadori R., Terasawa H., Takahashi K., Ihara M., Fukumoto K. Studies on the syntheses of heterocyclic compounds–DCXCIII: A total synthesis of atheroline by photolysis. Tetrahedron. 1977;33:1069–1071. doi: 10.1016/0040-4020(77)80227-5. [DOI] [Google Scholar]
- 19.Yamaguchi H., Arimoto M., Yamamoto K., Numata A. Studies on the constituents of the seeds of Hernandia ovigera L. Yakugaku Zasshi. 1979;99:674–677. doi: 10.1248/yakushi1947.99.6_674. [DOI] [PubMed] [Google Scholar]
- 20.Tanoguchi M., Arimoto M., Saika H., Yamaguchi H. Studies on the constituents of the seeds of Hernandia ovigera L. VI. Isolation and structural determination of three lignans. Chem. Pharm. Bull. 1987;35:4162–4168. doi: 10.1248/cpb.35.4162. [DOI] [Google Scholar]
- 21.Ito C., Matsui T., Wu T.S., Furukawa H. Isolation of 6,7-demethyl-enedesoxypodophyllotoxin from Hernandia ovigera. Chem. Pharm. Bull. 1992;40:1318–1321. doi: 10.1248/cpb.40.1318. [DOI] [Google Scholar]
- 22.Chen C.Y., Wang Y.D., Wang H.M. Chemical constituents from the roots of Synsepalum dulcificum. Chem. Nat. Compd. 2010;46:46–448. doi: 10.1007/s10600-010-9639-9. [DOI] [Google Scholar]
- 23.Chen C.Y., Chang F.R., Wu Y.C. The constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 1997;44:313–319. doi: 10.1002/jccs.199700047. [DOI] [Google Scholar]
- 24.Sun X.B., Zhao P.H., Xu Y.J., Sun L.M., Cao M.A., Yuan C.S. Chemical constituents from the roots of Polygonum bistorta. Chem. Nat. Compd. 2007;43:563–566. doi: 10.1007/s10600-007-0193-z. [DOI] [Google Scholar]
- 25.Ayyad S.N. A new cytotoxic stigmastane steroid from Pistia stratiotes. Pharmazie. 2002;57:212–214. [PubMed] [Google Scholar]
- 26.Chen C.H., Hwang T.L., Chen L.C., Chang T.H., Wei C.S., Chen J.J. Isoflavones and anti-inflammatory constituents from the fruits of Psoralea corylifolia. Phytochemistry. 2017;143:186–193. doi: 10.1016/j.phytochem.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Inverstig. 1968;97:77–89. [PubMed] [Google Scholar]
- 28.Jauregui H.O., Hayner N.T., Driscoll J.L., Williams-Holland R., Lipsky M.H., Galletti P.M. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes-freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro. 1981;17:1100–1110. doi: 10.1007/BF02618612. [DOI] [PubMed] [Google Scholar]
- 29.Babior B.M., Kipnes R.S., Curnutte J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Inverstig. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang T.L., Leu Y.L., Kao S.H., Tang M.C., Chang H.L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radic. Biol. Med. 2006;41:1433–1441. doi: 10.1016/j.freeradbiomed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Chen J.J., Ting C.W., Wu Y.C., Hwang T.L., Cheng M.J., Sung P.J., Wang T.C., Chen J.F. New labdane-type diterpenoids and anti-inflammatory constituents from Hedychium coronarium. Int. J. Mol. Sci. 2013;14:13063–13077. doi: 10.3390/ijms140713063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.