Abstract
Herba Siegesbeckiae (HS), derived from the aerial parts of three plants, Siegesbeckia orientalis (SO), S. glabrescens (SG), and S. pubescens (SP), has been used for the treatment of inflammatory diseases in China for centuries. In the present study, hydrodistillation was applied to extract essential oils from dried SO, SG, and SP aerial parts, and chemical composition analysis by gas chromatography–mass spectrometry (GC-MS) led to the identification of a total of 148 compounds (56 in SO, 62 in SG, and 59 in SP). The main components in the essential oils of SO, SG, and SP differed significantly. In vitro anti-inflammatory activity assays showed that SP essential oils (IC50, 0.97 μg/mL) significantly reduced the ability of lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages to release NO, and the SO essential oil (IC50, 14.99 μg/mL) was better than the others at inhibiting the LPS-induced release of cytokine IL-6. Furthermore, the essential oils exhibited antitumor activities (IC50, 37.72–123.16 μg/mL) against Hep3B (liver) and Hela (cervical) cells. Linear regression analysis showed that, caryophyllene oxide peak area percentages showed remarkably high negative correlation coefficients with IC50 values of Hep3B and Hela cytotoxicity, which suggested the contribution of this compound on the cancer cell cytotoxicity of three essential oils. Finally, the ITS1-5.8S-ITS2 region was amplified and sequenced in order to generate genomic reference sequences for each plant. These can be used to identify the origins of the plants, and will assist other research studies related to these three plants.
Keywords: Siegesbeckia orientalis, S. glabrescens, S. pubescens, essential oil composition, GC-MS, anti-inflammatory, antitumor, ITS1-5.8S-ITS2
1. Introduction
Plants produce a wide variety of volatile compounds, including terpenes, alcohols, aldehydes, esters, ethers, ketones, phenols, and oxides, that play important roles in defense, pollinator attraction, signal transduction, and so on. These volatile compounds are also important resources for the pharmaceutical, food, beverages, perfume, and cosmetics industries, and are thus economically significant [1,2,3]. The mixture of plant volatile compounds, which is also known as essential oil, can be extracted using hydrodistillation, steam distillation, supercritical fluid extraction, Soxhlet′s extraction, and ultrasound-assisted extraction [4,5].
Herba Siegesbeckiae (HS), a medicinal material used for the treatment of rheumatic arthritis, malaria, and snake bites in China since centuries ago [6], is derived from the aerial parts of three annual medicinal plants, Siegesbeckia orientalis (SO), S. glabrescens (SG), and S. pubescens (SP), which belong to the genus Siegesbeckia and the family Compositae. The main activities reported for HS extract are anti-inflammatory [7], antiallergic [8], antithrombotic [9], and immunosuppressive [10,11], but other activities have also been identified [12,13]. Recently, it was reported that SP essential oil significantly inhibited the proliferation of hepatocellular carcinoma cells (HepG2, Hep3B, Huh7, SMMC-7721). The 50% proliferation inhibition concentration of the SP essential oils was 42.0–95.2 μg/mL [14]. In the past few decades, through systematic chemical studies, three main categories of compound have been identified in HS: diterpenes, sesquiterpenes, and flavonoids [15,16,17,18]. Pharmacological studies suggested that diterpenoids are the main antirheumatic constituents of HS [19,20]. A series of ent-kaurane and ent-pimarane diterpenoids from HS has been reported [17,18,21,22,23]. However, to the best of our knowledge, a comparative study related to the chemical composition, anti-inflammatory activity, and antitumor activity of essential oils from the three individual plant species has not been reported.
In this study, the hydrodistillation method was used to extract essential oils from dried SO, SG, and SP aerial parts, and chemical composition analysis was carried out using gas chromatography–mass spectrometry (GC-MS). Subsequently, the bioactivities of the oils, including anti-inflammatory and antitumor activities, were evaluated in vitro. Furthermore, in order to provide a reference molecular marker for the plant origin, the ITS1-5.8S-ITS2 genomic regions of the three plants were amplified and sequenced. Our results revealed remarkable differences in the essential oil composition and bioactivities, and in the characteristic ITS sequence, which will facilitate the discrimination of the origins of the three plants and their subsequent utilization.
2. Results and Discussion
2.1. Analysis of Essential Oils
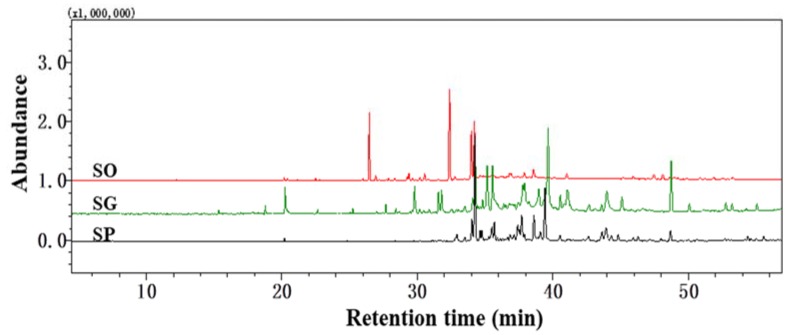
The yield of essential oils from the dried aerial tissue of the three plants was 0.06% (SO), 0.08% (SG), and 0.13% (SP) (w/w). Representative GC-MS chromatograms of the three essential oils are shown in Figure 1. A total of 56 (SO), 62 (SG), and 59 (SP) constituents were identified, representing 98.72% (SO), 93.89% (SG), and 91.35% (SP) of the total peak area. Across the three plants, 148 compounds were identified in total. The retention time, compound name, molecular formula, literature retention indices (RL), experimental retention indices relative to C8-C20 n-alkanes (RI), and peak area percentages are listed in Table 1.
Figure 1.
Representative GC-MS chromatograms of SO (red), SG (green), and SP (black) essential oils.
Table 1.
Chemical composition (peak area percentage) of the essential oils of SO, SG, and SP.
| No. | Rt (min) | Compound Name | Molecular Formula | RL | RI | SO | SG | SP |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.14 | (3E)-Hexenol | C6H12O | 855 | 868 | 0.05 | ||
| 2 | 6.59 | Hexanol | C6H14O | 868 | 860 | 0.04 | ||
| 3 | 6.95 | Dibutyl oxide | C8H18O | 878 | 882 | 0.09 | ||
| 4 | 7.49 | Butyl acrylate | C7H12O2 | 893 | 884 | 0.11 | ||
| 5 | 7.96 | Butyl propionate | C7H14O2 | 906 | 894 | 0.02 | ||
| 6 | 11.01 | Nopinene | C10H16 | 992 | 978 | 0.03 | ||
| 7 | 11.28 | Butyl butyrate | C8H16O2 | 1000 | 984 | 0.07 | ||
| 8 | 12.24 | α-Cymene | C10H14 | 1025 | 1027 | 0.21 | ||
| 9 | 12.41 | Clearene | C10H16 | 1029 | 1018 | 0.07 | ||
| 10 | 13.57 | 6-Ethyl-2-methyloctane | C11H24 | 1059 | 1046 | 0.03 | ||
| 11 | 13.61 | 2,4-Dimethyldecane | C12H26 | 1060 | 1086 | 0.03 | ||
| 12 | 15.35 | Linalool | C10H18O | 1105 | 1082 | 0.33 | ||
| 13 | 15.58 | Dehydrocineole | C10H16O | 1111 | 1041 | 0.03 | ||
| 14 | 16.45 | 4-Acetyl-1-methylcyclohexene | C9H14O | 1133 | 1118 | 0.12 | ||
| 15 | 16.96 | Camphor | C10H16O | 1147 | 1121 | 0.07 | ||
| 16 | 17.20 | Mentha-1,5-diene-8-ol | C10H16O | 1153 | 1125 | 0.04 | ||
| 17 | 17.39 | Neryl oxide | C10H16O | 1158 | 1135 | 0.11 | ||
| 18 | 17.78 | 2-Camphanol | C10H18O | 1168 | 1165 | 0.04 | ||
| 19 | 17.83 | Borneol | C10H18O | 1169 | 1165 | 0.16 | ||
| 20 | 17.90 | β-Phellandren-8-ol | C10H16O | 1171 | 1178 | 0.13 | ||
| 21 | 18.20 | 4-terpineol | C10H18O | 1179 | 1174 | 0.01 | 0.07 | |
| 22 | 18.55 | p-Cymen-8-ol | C10H14O | 1188 | 1197 | 0.07 | ||
| 23 | 18.73 | (−)-α-Terpineol | C10H18O | 1192 | 1196 | 0.08 | ||
| 24 | 18.78 | α-terpineol | C10H18O | 1194 | 1194 | 0.71 | 0.04 | |
| 25 | 18.83 | 2-Decanone | C10H20O | 1195 | 1181 | 0.09 | ||
| 26 | 19.16 | 2-Decanol | C10H22O | 1204 | 1218 | 0.11 | ||
| 27 | 20.18 | Nerol | C10H18O | 1233 | 1228 | 0.56 | 2.85 | 0.48 |
| 28 | 20.42 | O-Methylthymol | C11H16O | 1239 | 1231 | 0.42 | ||
| 29 | 20.97 | 2-Hexanoylfuran | C10H14O2 | 1255 | 1265 | 0.04 | ||
| 30 | 21.16 | Geraniol | C10H18O | 1260 | 1256 | 0.27 | 0.07 | |
| 31 | 21.72 | 2,4-Dimethylphenethyl alcohol | C10H14O | 1276 | 1292 | 0.01 | ||
| 32 | 21.76 | Dihydro-4-(3-methyl-2-methylenebutyl)-2(3H)-furanone | C10H16O | 1277 | 1286 | 0.02 | ||
| 33 | 21.98 | 4-Methyldodecane | C13H28 | 1284 | 1269 | 0.03 | ||
| 34 | 22.02 | 4,6-Dimethyldodecane | C13H30 | 1285 | 1285 | 0.04 | ||
| 35 | 22.49 | 2-Undecanone | C11H22O | 1298 | 1295 | 0.58 | ||
| 36 | 22.65 | Thymol | C10H14O | 1302 | 1293 | 0.33 | ||
| 37 | 22.77 | 2-Undecanol | C11H24O | 1306 | 1297 | 0.23 | ||
| 38 | 23.33 | (E,E)-2,4-Decadienal | C10H16O | 1322 | 1321 | 0.03 | ||
| 39 | 24.71 | Hexahydropseudoionone | C13H26O | 1361 | 1341 | 0.05 | ||
| 40 | 24.85 | Eugenol | C10H12O2 | 1365 | 1359 | 0.03 | 0.11 | |
| 41 | 25.05 | Nerol acetate | C12H20O2 | 1370 | 1352 | 0.13 | ||
| 42 | 25.24 | α-Cubebene | C15H24 | 1376 | 1355 | 0.42 | ||
| 43 | 25.98 | 2-Dodecanone | C12H24O | 1397 | 1396 | 0.45 | ||
| 44 | 26.48 | 4,8,8-Trimethyl-2-methylene-4-vinylbicyclo[5.2.0]nonane | C15H24 | 1409 | 1407 | 0.10 | ||
| 45 | 26.93 | cis-Caryophyllene | C15H24 | 1419 | 1425 | 1.30 | ||
| 46 | 27.00 | Caryophyllene | C15H24 | 1420 | 1418 | 14.13 | 0.21 | |
| 47 | 27.19 | 1,4-Dimethoxy-2-tert-butylbenzene | C12H18O2 | 1425 | 1406 | 0.21 | 0.02 | |
| 48 | 27.67 | trans-α-Bergamotene | C15H24 | 1436 | 1430 | 0.89 | ||
| 49 | 27.72 | Aromandendrene | C15H24 | 1437 | 1416 | 0.23 | ||
| 50 | 27.88 | γ-Elemene | C15H24 | 1440 | 1431 | 0.62 | ||
| 51 | 28.34 | cis-α-Bisabolene | C15H24 | 1451 | 1478 | 0.56 | ||
| 52 | 28.37 | α-Farnesene | C15H24 | 1451 | 1458 | 0.12 | ||
| 53 | 28.56 | (E)-β-Famesene | C15H24 | 1456 | 1440 | 0.13 | ||
| 54 | 29.26 | Acoradiene | C15H24 | 1471 | 1474 | 0.61 | ||
| 55 | 29.39 | γ-Muurolene | C15H24 | 1474 | 1483 | 1.42 | 0.28 | |
| 56 | 29.45 | Guaia-1(10),11-diene | C15H24 | 1476 | 1490 | 0.21 | ||
| 57 | 29.57 | 1,5-Cadinadiene | C15H24 | 1479 | 1460 | 0.11 | ||
| 58 | 29.64 | Curcumene | C15H22 | 1480 | 1524 | 0.51 | ||
| 59 | 29.8 | cis-β-Farnesene | C15H24 | 1484 | 1458 | 3.96 | ||
| 60 | 30.15 | γ-Curcumene | C15H24 | 1492 | 1486 | 0.53 | ||
| 61 | 30.18 | β-Funebrene | C15H24 | 1492 | 1479 | 0.15 | ||
| 62 | 30.19 | Elemol | C15H26O | 1492 | 1512 | 0.72 | ||
| 63 | 30.47 | β-Himachalene | C15H24 | 1499 | 1518 | 0.19 | ||
| 64 | 30.54 | α-Bisabolene | C15H24 | 1500 | 1518 | 1.83 | ||
| 65 | 30.73 | Cuparene | C15H22 | 1505 | 1526 | 0.04 | ||
| 66 | 30.79 | β-Bisabolene | C15H24 | 1506 | 1500 | 0.31 | ||
| 67 | 31.12 | Valencen | C15H24 | 1513 | 1504 | 0.29 | ||
| 68 | 31.52 | Cadina-1(10),4-diene | C15H24 | 1523 | 1526 | 0.17 | ||
| 69 | 31.52 | Methyl dodecanoate | C13H26O2 | 1523 | 1535 | 0.31 | ||
| 70 | 31.56 | β-Sesquiphellandrene | C15H24 | 1523 | 1525 | 2.74 | ||
| 71 | 31.78 | 2-penten-1-ol | C15H24O | 1528 | 1504 | 3.14 | ||
| 72 | 32.37 | trans-α-Bisabolene | C15H24 | 1542 | 1540 | 24.41 | ||
| 73 | 32.55 | trans-Farnesene epoxide | C15H24O | 1546 | 1540 | 0.65 | ||
| 74 | 32.76 | (Z)-α-Bisabolene epoxide | C15H24O | 1551 | 1531 | 1.00 | ||
| 75 | 32.89 | 6,10-Dimethyl-3-(1-methylethylidene)cyclodecene | C15H26 | 1553 | 1564 | 0.19 | ||
| 76 | 32.93 | γ-Costol | C15H24O | 1554 | 1542 | 0.94 | ||
| 77 | 33.04 | Sesquirosefuran | C15H22O | 1557 | 1577 | 0.15 | ||
| 78 | 33.09 | Ledol | C15H26O | 1558 | 1566 | 0.16 | ||
| 79 | 33.26 | 6-epi-shyobunol | C15H26O | 1562 | 1555 | 0.09 | ||
| 80 | 33.46 | Globulol | C15H26O | 1566 | 1569 | 0.22 | ||
| 81 | 33.52 | 1,5-Epoxysalvial-4-ene | C15H24O | 1568 | 1560 | 1.07 | 0.99 | |
| 82 | 33.98 | Spathulenol | C15H24O | 1578 | 1577 | 12.46 | 0.87 | 3.44 |
| 83 | 34.21 | Caryophyllene oxide | C15H24O | 1583 | 1582 | 16.88 | 7.18 | 21.89 |
| 84 | 34.50 | 1,2-Dihydronerolidol | C15H28O | 1590 | 1574 | 0.27 | ||
| 85 | 34.63 | 6-(1-methylethyl)-1-naphthalenol | C15H24O | 1593 | 1584 | 1.51 | ||
| 86 | 34.64 | (−)-Globulol | C15H26O | 1593 | 1590 | 0.51 | ||
| 87 | 34.81 | Mintketone | C15H24O | 1597 | 1579 | 1.00 | 1.53 | |
| 88 | 35.13 | Rosifoliol | C15H26O | 1603 | 1598 | 0.33 | ||
| 89 | 35.15 | Cedrol | C15H26O | 1603 | 1583 | 8.35 | ||
| 90 | 35.27 | Copaborneol | C15H26O | 1606 | - | 0.46 | ||
| 91 | 35.45 | Humulene epoxide | C15H24O | 1609 | 1592 | 0.25 | 4.75 | 2.62 |
| 92 | 35.68 | Octahydro-2,2,7a-trimethyl-4-methylene-1,3a-ethano-3aH-inden-5-ol | C15H24O | 1613 | 1599 | 3.33 | ||
| 93 | 35.90 | Allohimachalol | C15H26O | 1617 | - | 0.48 | ||
| 94 | 36.12 | Z-3-Hexadecen-7-yne | C16H28 | 1621 | 1637 | 0.38 | ||
| 95 | 36.30 | Aromadendrane-4,10-diol | C15H26O2 | 1624 | 1619 | 0.26 | ||
| 96 | 36.37 | Agarospirol | C15H26O | 1626 | 1608 | 0.89 | ||
| 97 | 36.45 | Bisabolol | C15H26O | 1627 | 1625 | 0.20 | ||
| 98 | 36.63 | 10,10-Dimethyl-2,6-dimethylenebicyclo[7.2.0]undecan-5-ol | C15H24O | 1630 | 1647 | 0.23 | 0.67 | |
| 99 | 36.78 | α-acorenol | C15H26O | 1633 | 1635 | 1.47 | ||
| 100 | 36.81 | 11,11-Dimethyl-4,8-dimethylenebicyclo[7.2.0]undecan-3-ol | C15H24O | 1633 | 1651 | 0.41 | 1.20 | |
| 101 | 36.92 | Isospathulenol | C15H24O | 1636 | - | 0.34 | ||
| 102 | 37.18 | α-Cadinol | C15H26O | 1640 | 1637 | 0.28 | 1.35 | |
| 103 | 37.40 | Cedrenol | C15H24O | 1644 | 1636 | 1.99 | ||
| 104 | 37.44 | γ-Gurjunenepoxide-(2) | C15H24O | 1645 | 1628 | 0.83 | ||
| 105 | 37.68 | Sesquibenihiol | C15H24O | 1649 | 1653 | 0.14 | 0.30 | |
| 106 | 37.70 | (−)-Spathulenol | C15H24O | 1650 | 1636 | 5.14 | ||
| 107 | 37.88 | Himbaccol | C15H26O | 1653 | 1631 | 1.60 | ||
| 108 | 37.89 | (1S,4R,5S)-1-Methyl-4-(prop-1-en-2-yl)spiro[4.5]dec-7-ene-8-carbaldehyde | C15H22O | 1653 | 1642 | 0.80 | ||
| 109 | 37.90 | Dehydronerolidol | C15H24O | 1653 | 1632 | 6.28 | ||
| 110 | 38.57 | trans-Longipinocarveol | C15H24O | 1665 | 1659 | 2.84 | 5.87 | |
| 111 | 38.79 | Isovalencenol | C15H24O | 1669 | - | 0.28 | ||
| 112 | 38.87 | 3-Isopropyl-6,7-dimethyltricyclo[4.4.0.0(2,8)]decane-9,10-diol | C15H26O2 | 1671 | 1687 | 0.23 | ||
| 113 | 38.96 | 3,7,11-Trimethyl-dodeca-2,4,6,10-tetraenal | C15H22O | 1672 | 1664 | 3.26 | ||
| 114 | 39.07 | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | C15H24O | 1674 | 1690 | 2.02 | ||
| 115 | 39.36 | 1-Isopropyl-4,8-dimethylspiro[4.5]dec-8-en-7-ol | C15H26O | 1680 | 1660 | 0.28 | ||
| 116 | 39.41 | Germacra-4(15),5E,10(14)-trien-1β-ol | C15H24O | 1680 | - | 14.10 | ||
| 117 | 39.64 | 4-(1,5-Dimethylhex-4-enyl)cyclohex-2-enone | C14H22O | 1685 | 1661 | 12.90 | ||
| 118 | 40.53 | (−)-Isolongifolol, acetate | C17H28O2 | 1701 | 1719 | 1.19 | ||
| 119 | 40.55 | m-Camphorene | C20H32 | 1701 | - | 1.79 | ||
| 120 | 41.03 | Pentadecanal | C15H30O | 1710 | 1701 | 1.65 | 0.19 | |
| 121 | 41.05 | Cuparenal | C15H20O | 1710 | - | 3.46 | ||
| 122 | 41.21 | α-Costol | C15H24O | 1713 | - | 0.19 | ||
| 123 | 42.23 | Platambin-1,6-dione | C15H22O2 | 1731 | 1740 | 0.38 | ||
| 124 | 42.62 | Epicedrol | C15H26O | 1738 | 1743 | 0.81 | ||
| 125 | 43.62 | Ylangenol | C15H24O | 1757 | - | 2.33 | ||
| 126 | 43.98 | Dehydrosaussurea lactone | C15H20O2 | 1763 | 1755 | 5.49 | 4.85 | |
| 127 | 44.32 | Valerenic acid | C15H22O2 | 1769 | 1759 | 1.53 | ||
| 128 | 45.09 | β-Acoradienol | C15H24O | 1783 | - | 2.42 | ||
| 129 | 45.18 | 4a,5-Dimethyl-3-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalen-1-ol | C15H24O | 1785 | - | 0.58 | ||
| 130 | 45.92 | Aristol-1(10)-en-9-ol | C15H24O | 1798 | 1788 | 0.93 | ||
| 131 | 46.27 | Naphthalenemethanol | C15H24O | 1805 | - | 1.16 | ||
| 132 | 46.64 | (1-Methyldecyl)cyclohexane | C17H34 | 1811 | 1790 | 0.35 | ||
| 133 | 47.45 | β-Copaen-4α-ol | C15H24O | 1826 | 1820 | 1.96 | ||
| 134 | 48.04 | Sativene epoxide | C15H24O3 | 1837 | 1812 | 0.13 | ||
| 135 | 48.10 | (+)-Cycloisolongifol-5-ol | C15H24O | 1838 | - | 1.80 | ||
| 136 | 48.62 | 2,15-Hexadecanedione | C16H30O | 1848 | 1864 | 0.66 | ||
| 137 | 48.73 | Phytone | C18H36O | 1850 | 1844 | 7.83 | 2.74 | |
| 138 | 48.94 | 6-Methyl-2-(4-methylcyclohex-3-en-1-yl)hepta-1,5-dien-4-ol | C15H24O | 1854 | 1868 | 1.00 | ||
| 139 | 49.24 | 15-Hydroxy-α-muurolene | C15H24O | 1859 | - | 0.35 | ||
| 140 | 50.06 | Diisobutyl phthalate | C16H22O4 | 1875 | 1888 | 1.09 | 0.29 | |
| 141 | 50.62 | 1-Hexadecanol | C16H34O | 1885 | 1870 | 0.49 | ||
| 142 | 50.87 | 11-Hexadecyn-1-ol | C16H30O | 1890 | 1872 | 0.44 | ||
| 143 | 51.21 | Z,Z,Z-4,6,9-Nonadecatriene | C19H34 | 1896 | 1934 | 0.32 | ||
| 144 | 51.87 | Geranyl-α-terpinene | C20H32 | 1908 | 1962 | 0.84 | ||
| 145 | 52.75 | Farnesyl acetone | C18H30O | 1924 | 1909 | 1.17 | 0.59 | |
| 146 | 53.16 | Methyl isoheptadecanoate | C18H36O2 | 1932 | 1909 | 0.19 | ||
| 147 | 54.25 | Isophytol | C20H40O | 1952 | 1939 | 0.26 | 0.36 | |
| 148 | 55.04 | Butyl isobutyl phthalate | C16H22O4 | 1967 | 1973 | 0.99 | 0.49 | |
| Sum | 98.72 | 93.89 | 91.35 |
RI: Experimental retention indices relative to C8-C20n-alkanes; RL: Literature retention indices. Compounds shared in common in two or three essential oils are in bold.
As shown in Figure 1 and Table 1, the composition of the three essential oils differed significantly. The essential oil of SO was dominated by hydrocarbon sesquiterpenes (45.93%), oxygenated sesquiterpenes (44.99%), and oxygenated monoterpenes (1.53%), among which trans-α-bisabolene (24.41%), caryophyllene oxide (16.88%), caryophyllene (14.13%), and spathulenolspathulenol (12.46%) were found to be the main components. The SG essential oil mainly contained oxygenated sesquiterpenes (65.51%), hydrocarbon sesquiterpenes (9.79%), oxygenated diterpenes (9.26%), and oxygenated monoterpenes (4.90%), among which 4-(1,5-dimethylhex-4-enyl)cyclohex-2-enone (12.90%), cedrol (8.35%), phytone (7.83%), caryophyllene oxide (7.18%), dehydronerolidol (6.28%), and dehydrosaussurea lactone (5.49%) were the main components. The essential oil of SP was dominated by oxygenated sesquiterpenes (83.46%), oxygenated diterpenes (3.69%), and hydrocarbon sesquiterpenes (0.94%), with caryophyllene oxide (21.89%), germacra-4(15),5E,10(14)-trien-1β-ol (14.10%), trans-longipinocarveol (5.87%), (−)-spathulenol (5.14%), and dehydrosaussurea lactone (4.85%) as the main components.
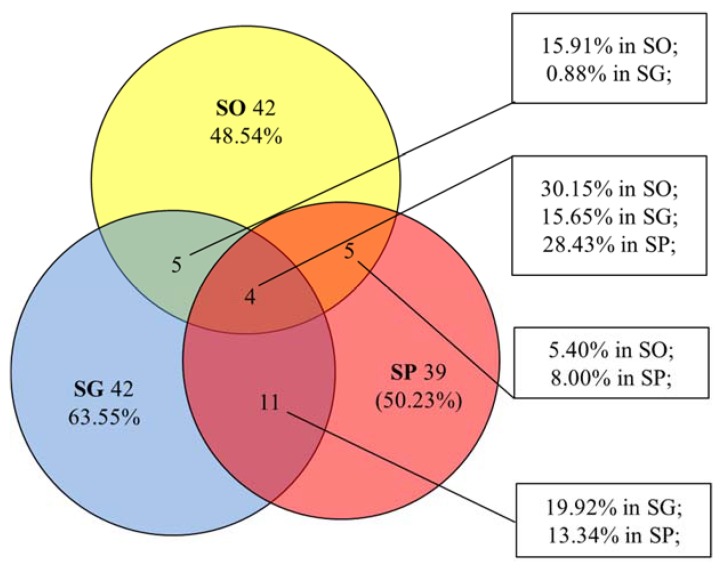
Only four chemical components were in common among all three species, namely nerol, spathulenol, caryophyllene oxide, and humulene epoxide. These accounted for 30.15%, 15.65%, and 28.43% of the total peak area in SO, SG, and SP essential oils, respectively (Figure 2). The essential oils of SO and SG shared 5 chemical components, as did the essential oils of SO and SP (Figure 2). SG and SP shared eleven identical components, as shown in Figure 2.
Figure 2.
Venn diagram of essential oil compositions of SO, SG, and SP.
2.2. Anti-Inflammatory Activity
According to traditional use and recently published results, anti-inflammatory [7], antiallergic [8], antithrombotic [9], and immunosuppressive [10,11] effects are reported as the main activities of HS. Diterpenoids were the main antirheumatic constituents of HS [19], of which kirenol was the main chemical constituent. Pharmacological studies suggested that kirenol could inhibit paw swelling, boost up the proliferation of splenic lymphocyte of adjuvant arthritis rats, regulate T lymphocyte subpopulation ratio, and inhibit specific immune response of normal mice [20]. As we know, NO plays an important role in the inflammatory, immune responses, and in thrombotic pathogenesis processes. Inhibition of excess NO production has been used as an assay in the screening of anti-inflammatory, antithrombotic, and immunosuppressive drugs. Therefore, in this study, we treated the mouse macrophage cell line RAW264.7 with lipopolysaccharide (LPS) to induce an inflammatory response and we investigated the inhibitory effects of the three essential oils on NO production by the activated cells. Before the anti-inflammatory activity assay, the cytotoxicity of the three essential oils was evaluated by MTT assay. Our results suggested that the three essential oils had no obvious cytotoxic effects on the RAW264.7 cell line when the concentration was lower than 200 μg/mL (shown in Figure S1).
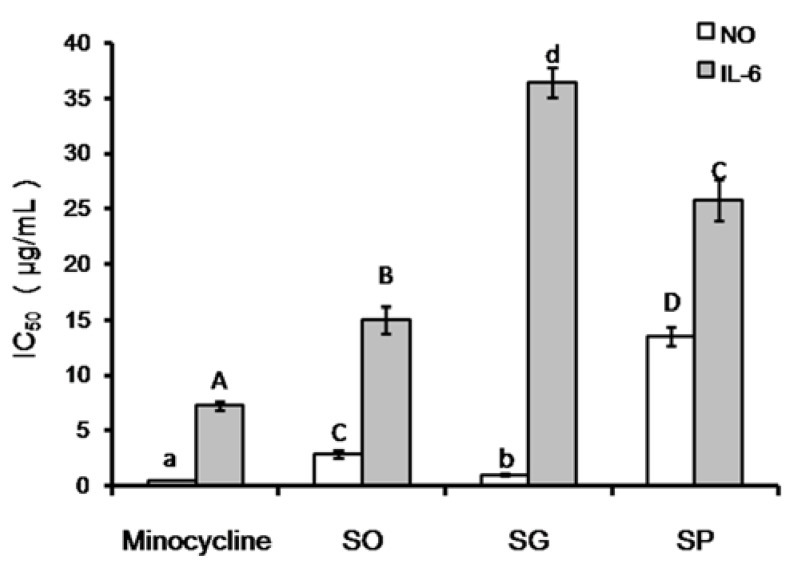
As shown in Figure 3, the positive control minocycline showed the strongest NO inhibition activity with IC50 = 0.37 μg/mL, which is significantly lower than SG oil (p < 0.05), SO oil (p < 0.01), and SP oil (p < 0.01). Among the three essential oils, SG oil showed the lowest IC50 of 0.97 μg/mL, which is significantly lower (p < 0.01) than SO oil (IC50 = 2.83 μg/mL) and SP oil (IC50 = 13.48 μg/mL). Even though the SG oil showed weaker NO inhibition activity than minocycline, it still has the strongest NO inhibition effect of any crude essential oil as far as we know.
Figure 3.
Anti-inflammatory effect of the SO, SG, and SP essential oils. A–D, p < 0.01; a,b,d, p < 0.05.
Inflammatory cells can release a variety of inflammatory cytokines which participate in the regulation of the body’s innate immune response and directly kill target cells or mediate apoptosis, thus promoting the repair of damaged tissues. One of these cytokines is IL-6, a critical component of the inflammatory mediator network which plays an important role in the inflammatory response. The ability of the essential oils to inhibit the release of IL-6 was tested in LPS-treated RAW264.7 cells. As indicated in Figure 3, the ability of SO essential oil (IC50, 14.99 μg/mL) to inhibit the release of cytokine IL-6 was significantly weaker (p < 0.01) than that of the positive control (minocycline) (IC50, 7.21 μg/mL), but stronger (p < 0.01) than that of SP (IC50, 25.76 μg/mL) and SG (IC50, 36.41 μg/mL) essential oils.
NO and cytokine IL-6 are important indicators of the cellular inflammatory response. Our results demonstrated that the three essential oils showed significant anti-inflammatory activity. In summary, the three essential oils significantly inhibited the LPS-induced secretion of NO and cytokine IL-6 by the macrophage cell line RAW264.7. These results provide important clues for the identification of possible candidate compounds from the three essential oils, especially SG and SO essential oils.
2.3. Antitumor Activity
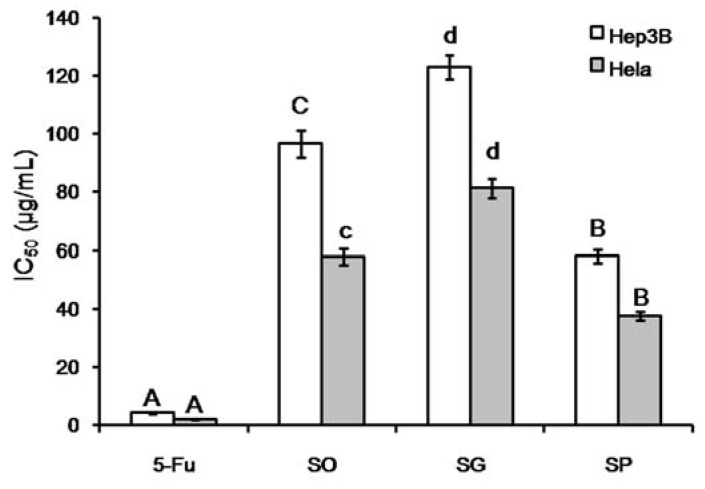
The in vitro antitumor activities of the essential oils were evaluated using two cancer cell lines, Hep3B (liver) and Hela (cervical). The cytotoxicity was expressed as IC50 values, as shown in Figure 4. The IC50 of the positive control 5-Fu (4.19 μg/mL in Hep3B, 2.08 μg/mL in Hela) was significantly lower than the three essential oils tested (p < 0.01). Among the three essential oils, SP exhibited the strongest cytotoxicity (38.10 μg/mL for Hep3B, 37.72 μg/mL for Hela). These results suggest that essential oils from the three plants might be used as a natural source of compounds for anticancer compound screening.
Figure 4.
Cytotoxicity of the essential oils of SO, SG, and SP against Hep3B and Hela cell lines. A–C, p < 0.01; c,d, p < 0.05.
A recent study [24] showed that SO ethanol extract significantly inhibited the proliferation of RL 95-2 human endometrial cancer cells. Furthermore, SO ethanol extract was effective against A549 (lung cancer), HepG2 (hepatpma), MDA-MB-231 (breast cancer), and especially on LNCaP (prostate cancer) cell lines. At the same time, it was reported that caryophyllene oxide (IC50 value = 14.90 μg/mL) and caryophyllene (IC50 value = 33.20 μg/mL) were mainly responsible for most cytotoxic activity of SO ethanol extract against RL 95-2 cells. As mentioned previously in Section 2.1, there are only four common compounds in the three essential oils, and caryophyllene oxide is one of the four common components in the three essential oils.
In order to find out the possible relationships between common compounds and two kinds of activities, a linear regression analysis was carried out between percentages and IC50 values. Our results showed that (Figure S2) only caryophyllene oxide peak area percentages showed remarkably strong negative correlation coefficients with IC50 of NO inhibition (R2 = 0.959), Hep3B (R2 = 0.918), and Hela (R2 = 0.980) cytotoxicity. These results suggested the possible important contribution of caryophyllene oxide on the anticancer activity and anti-inflammatory effects of Herba Siegesbeckiae essential oil.
2.4. ITS1-5.8S-ITS2 Sequence
Due to the similarity in morphological characteristics of dried and chopped commercial materials, it is difficult to distinguish the three plants from each other. Therefore, it is important to provide a reliable molecular marker for the identification of the plant origin and for comparison with other research studies. It has been accepted that genomic ribosomal DNA sequences, especially ITS1-5.8S-ITS2, can be used for the interspecific identification of plant origin. Therefore, the full ITS1-5.8S-ITS2 sequence was amplified from genomic DNA of the three plants and sequenced. The accession numbers in Genbank are MH701787 (SO), MH701847 (SG), and MH701848 (SP). Multiple alignment of the ITS1-5.8S-ITS2 sequence from the three plants was carried out using DNAMAN 9.0 software. As shown in Figure S3, across the total sequence of 730–732 bp, the three plants shared 99.27% similarity with each other. There are 16 single nucleotide polyphorism (SNP), which can be used for the development of specific Polymerase chain reaction (PCR) assays to distinguish the origins of samples of these three plants.
3. Conclusions
In this study, hydrodistillation was used to extract essential oils from the dried aerial parts of SO, SG, and SP, and their chemical compositions and bioactivities were analyzed and compared. A total of 56, 62, and 59 constituents were identified in SO, SG, and SP essential oils, respectively, by GC-MS. There was a marked difference between the components of the three essential oils. The essential oils of SO, SG, and SP exhibited various levels of anti-inflammatory and antitumor activity in vitro. The essential oil of SP (IC50, 0.97 μg/mL) significantly reduced NO release by LPS-induced RAW264.7 macrophages, and the essential oil of SO was the most effective at reducing the release of cytokine IL-6. The antitumor activities of the essential oil from SP were much better than SG and SO. Therefore, the essential oils of SO, SG, and SP were significantly different from each other in terms of phytochemical composition, but with similar bioactivities with various activity levels. Among four common compounds in three essential oils, caryophyllene oxide peak area percentages showed remarkably high negative correlation coefficients with IC50 values of Hep3B and Hela cytotoxicity, which suggested the contribution of this compound on the cancer cell cytotoxicity of three essential oils. Finally, in order to provide detailed information about the origin of the plants used in the present work, the complete ITS1-5.8S-ITS2 region was amplified from plant genomic DNA and sequenced. This sequence can be used by other researchers to confirm whether their plant specimens have the same origin as ours or not.
4. Materials and Methods
4.1. Materials
The flowering SP and SG plant materials were collected in October 2017 from Jinzhai, Anhui province, China. The flowering SO plant material was collected in April 2018 from Zhanzhou, Hainan province, China. Voucher specimens were deposited at the Herbarium of Shenyang Pharmaceutical University (Shenyang, China). The voucher numbers were SYPU-P-201710-007 (SP), SYPU-P-201710-008 (SG), and SYPU-P-201804-025 (SO). They were identified by Associate Professor Jia Lingyun in Shenyang Pharmaceutical University (Shenyang, China). The fresh plants were air-dried and ground into a fine powder which was passed through a 20 mesh and stored in sealed plastic bags at −20 °C for future use. RAW264.7, a mouse macrophage cell line, was purchased from the Cell Bank of the Shanghai Institute of Cell Biology and Biochemistry, Chinese Academy of Sciences (Shanghai, China). The cancer cells lines Hep3B (liver) and Hela (cervical) were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
4.2. Reagents
He (purity > 99.999%) and N2 (purity > 99.999%) were supplied by Shenyang Qianzhen Chemical Gas (Shenyang, China). Hexane (HPLC grade) and anhydrous sodium sulfate (analytical grade) were purchased from Shandong Yuwang Chemical Group (Shandong, China). Plant Genomic DNA Extraction Kits, 2X Taq master premix, and GoldView dye were purchased from Kangweishiji Bio. (Beijing, China). Agarose G-10 powder was purchased from Biowest (Hongkong, China). Synthesis of primers ITS1 and ITS4 as well as Sanger sequencing were carried out at Genewiz Bio. Ltd. (Suzhou, China). DMSO and 1% penicillin-streptomycin antibiotics were purchased from Sigma (biology grade, Burlington, VT, USA). RPMI 1640, Eagle’s Minimum Essential Medium, and 10% fetal bovine serum were purchased from Gibco (Waltham, MA, USA). Nitric Oxide Assay Kit was purchased from Beyotime Institute of Biotechnology (Jiangsu, China). ELISA kits were purchased from Shanghai Joyee Biotechnics Co., Ltd. (Shanghai, China). Minocycline was purchased from Dalian Meilun Biotech Co., Ltd. (Dalian, China). 5-Fluorouracil was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China).
4.3. DNA Extraction, ITS Amplification, Electrophoresis and Sequencing
Dried leaf tissues were ground into a fine powder. Then, DNA was extracted using a Plant Genomic DNA Extraction Kit following the manufacturer′s instructions. Extracted DNA (1 μL) was used as the template for PCR amplification, with 25 μL 2X Taq master premix, 3 μL 10 μM ITS1 primer (5′-TCCGTAGGTGAACCTGCGG-3′), 3 μL 10 μM ITS4 primer (5′-TCCTCCGCTT ATTGATATGC-3′), and 18 μL double-distilled H2O. The amplification conditions were as follows: 94 °C for 5 min (1 cycle); 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 40 s (30 cycles); 72 °C for 10 min (1 cycle). PCR product (5 μL) was applied to a 1% agarose gel containing GoldView dye and electrophoresis was carried out for 15 min at 150 V followed by observation under UV light in a Tenon gel imaging system. After confirmation of a strong, single amplified band of about 700 bp, the PCR product was sent for Sanger sequencing using ITS1 and ITS4 as sequencing primers. Sequences were confirmed by comparing them with the original sequencing chromatogram and were connected using Seqman software (Madison, MI, USA).
4.4. Essential Oil Extraction
100 g powder of dried aerial parts were added together with 1 L distilled water into a 2 L round-bottomed bottle, which was connected to a Clevenger-type apparatus with tap water for cooling. The hydrodistillation was continued for 4 h to extract the essential oils. The obtained essential oil was collected from the side arm and dried with anhydrous sodium sulphate, then sealed in a headspace bottle and stored at 4 °C until it was tested.
4.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
The GC-MS analysis was carried out on a Shimadzu (TQ-8040) series GC-MS system (Tokyo, Japan) equipped with an AOC-20i autosampler. The columns used an HP-5 capillary column (30 m × 0.32 mm, i.d. 0.25 μm) with a stationary phase of 5% diphenyl/95% dimethylpolysiloxane. Helium was used as the carrier gas. The column temperature was maintained at 40 °C for 3 min and then programmed to increase as follows: rising from 40 °C to 130 °C at a rate of 4 °C/min and holding for 2 min; rising from 130 °C to 150 °C at a rate of 2 °C/min and holding for 3 min; rising from 150 °C to 180 °C at a rate of 2 °C/min and holding for 3 min; and finally rising from 180 °C to 210 °C at a rate of 5 °C/min and holding for 5 min. The ionization energy was 70 eV with a scan time of 0.3 s and a mass range of 45–500 AMU. The identification of the compounds was based on the comparison of their retention times and mass spectra with those from the NIST14 and NIST14s (National Institute of Standards and Technologies, Mass Spectra Libraries, Gaithersburg, MD, USA) [25].
4.6. Anti-Inflammatory Activities
4.6.1. Cell Line and Cell Culture
RAW264.7, a mouse macrophage cell line, was grown in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin in a 37 °C humidified incubator containing 5% CO2.
4.6.2. Cell Viability Assay
The MTT assay was used to evaluate the effect of essential oils on cell viability [26]. RAW264.7 cells were seeded in 96-well plates at a density of 5 × 104 cells/well. After overnight growth, cells were treated with different concentrations of essential oils for 1 h, then incubated in the presence or absence of LPS (100 ng/mL) for the next 24 h. Then, 20 μL of MTT solution (5 mg/mL) was added and the cells were cultured for a further 4 h. After that, the supernatant was carefully removed and then the resulting formed azan crystals were dissolved in 100 µL DMSO with horizontal shaking. The absorbance at 490 nm (ref. 570 nm) [24] was measured with a microplate reader.
4.6.3. Measurement of NO and IL-6 Release
RAW264.7 cells were seeded into 96-well plates at a density of 8 × 104 cells/mL and cultured overnight. After pretreatment with different concentrations of essential oils (0.2–200 µg/mL) or the positive control (minocycline) for 1 h, the cells were stimulated with LPS (100 ng/mL) for 24 h. The concentration of NO in the conditioned culture medium was examined with the Nitric Oxide Assay Kit and the release of IL-6 in the supernatants was assayed using ELISA kits according to the manufacturer’s instructions [27,28]. The concentrations were calculated from standard curves.
4.7. Antitumor Assay
4.7.1. Cell Lines and Cell Culture
The cancer cells lines Hep3B (liver) and Hela (cervical) were grown in media supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin antibiotics and maintained in a CO2 incubator at 37 °C in 5% CO2. The medium was changed every 2 days until the cells reached confluence, at which point they were subcultured.
4.7.2. Cytotoxicity Assay
The essential oils from HS were evaluated for their cytotoxic activities against cells using the MTT cell viability assay with 5-fluorouracil as the positive control. First, cellular suspensions (1 × 105 cells/mL) were cultured in 96-well plates and exposed to different concentrations of the essential oils (1–200 μg/mL) using 3 wells for each concentration. The plates were incubated at 37 °C in 5% CO2 for 48 h [29]. Then, 200 μL MTT solution was added to each well and the plates were cultured for 4 h. The absorbance of each well was measured at 490 nm using a microplate reader. The data were expressed as the mean percentage of viable cells as compared to the respective control cultures treated with the solvent. The half maximal growth inhibitory concentration (IC50 values) was calculated from the standard curves of the dose-dependent cytotoxicity of the essential oils.
4.8. Statistical Analysis
All activity assay experiments were repeated three times and the data were represented as mean ± SD. Significance analysis was carried out using the t-test in Microsoft Excel 2007. Letters above the columns in Figure 2 and Figure 3 represent significance (A–D: p < 0.01; a,b,d: p < 0.05)
Acknowledgments
The authors also appreciate the authentication of the plant samples by Jia Lingyun, Shenyang Pharmaceutical University.
Supplementary Materials
The following are available online at http://www.mdpi.com/1420-3049/23/9/2185/s1, Figure S1: Effects of SO, SG and SP essential oil on cell viability of RAW264.7. Figure S2: Linear regression analysis of caryophyllene oxide peak area percentages and IC50 of Hep3B and Hela cytotoxicity. Figure S3: Multiple alignment of the ITS1-5.8S-ITS2 sequence of SO, SG and SP.
Author Contributions
X.G., G.H., and J.J. conceived and designed the experiments; X.G. collected the plant material, extracted essential oil, and carried out statistical analysis of all data, and with the help of S.F., analyzed the components using GC-MS. The anti-inflammatory activity assay was carried out by J.W. and the antitumor activity was assayed by L.H. X.G。 and G.H. conducted the ITS sequence amplification and wrote the manuscript.
Funding
This research was funded by Liaoning Provincial Engineering Center of Endangered and Daodi Medicinal Plant Resources Utilization in Shenyang Pharmaceutical University.
Conflicts of Interest
There is no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Bajpaia V.K., Baekb K.H. Biological efficacy and application of essential oils in foods—A review. J. Essent. Oil Bear. Plants. 2016;19:1–19. doi: 10.1080/0972060X.2014.935033. [DOI] [Google Scholar]
- 2.Ghayempour S., Montazer M. Micro/nanoencapsulation of essential oils and fragrances: Focus on perfumed, antimicrobial, mosquito-repellent and medical textiles. J. Microencapsul. 2016;33:497–510. doi: 10.1080/02652048.2016.1216187. [DOI] [PubMed] [Google Scholar]
- 3.Huang X.W., Feng Y.C., Huang Y., Li H.L. Potential cosmetic application of essential oil extracted from Litsea cubeba fruits from China. J. Essent. Oil Res. 2013;25:112–119. doi: 10.1080/10412905.2012.755479. [DOI] [Google Scholar]
- 4.Chang K.M., Choi E.M., Kim G.H. Chemical constituents of Chrysanthemum indicum L. flower oil and effect on osteoblastic MC3T3-E1 cells. Food Sci. Biotechnol. 2010;19:815–819. doi: 10.1007/s10068-010-0114-y. [DOI] [Google Scholar]
- 5.Wang L.H., Chen Y.S., Song Y.T., Chen Y., Liu X.S. GC-MS of volatile components of Schisandra chinensis obtained by supercritical fluid and conventional extraction. J. Sep. Sci. 2008;31:3238–3245. doi: 10.1002/jssc.200800341. [DOI] [PubMed] [Google Scholar]
- 6.State Pharmacopoeia Commission of the PRC . Pharmacopoeia of the People’s Republic of China. 1st ed. People′s Medical Publishing House; Beijing, China: 2015. p. 368. [Google Scholar]
- 7.Wang J.P., Zhou Y.M., Ye Y.J., Shang X.M., Cai Y.L., Xiong C.M., Wu Y.X., Xu H.X. Topical anti-inflammatory and analgesic activity of kirenol isolated from Siegesbeckia orientalis. J. Ethnopharmacol. 2011;137:1089–1094. doi: 10.1016/j.jep.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Kim H.M., Kim C.Y., Kwon M.H., Shin T.Y., Lee E.J. Suppression of anaphylactic reaction in mice by Siegesbeckiae pubescens. Arch. Pharm. Res. 1997;20:122–127. doi: 10.1007/BF02973998. [DOI] [PubMed] [Google Scholar]
- 9.Wang J.P., Xu H.X., Wu Y.X., Ye Y.J., Ruan J.L., Xiong C.M., Cai Y.L. Ent-16β,17-dihydroxy-kauran-19-oic acid, a kaurane diterpene acid from Siegesbeckia pubescens, presents antiplatelet and antithrombotic effects in rats. Phytomedicine. 2011;18:873–878. doi: 10.1016/j.phymed.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Sun H.X., Wang H. Immunosuppressive activity of the ethanol extract of Siegesbeckia orientalis on the immune responses to ovalbumin in mice. Chem. Biodivers. 2006;3:754–761. doi: 10.1002/cbdv.200690077. [DOI] [PubMed] [Google Scholar]
- 11.Hwang W.J., Park E.J., Jang C.H., Han S.W., Oh G.J., Kim N.S., Kim H.M. Inhibitory effect of immunoglobulin E production by jin-deuk-chal (Siegesbeckia orientalis) Immunopharmacol. Immunotoxicol. 2001;23:555–563. doi: 10.1081/IPH-100108601. [DOI] [PubMed] [Google Scholar]
- 12.Dong X.Y., Chen M., Jing W., Huang D.X., Shen S.M., Li H.T. Studies on antifertility constituents of Siegesbeckiae glabrescens. Acta Pharmacol. Sin. 1989;24:833–836. [PubMed] [Google Scholar]
- 13.Su J.D., Osawa T., Namiki M. Screening for antioxidative activity of crude drugs. Agric. Biol. Chem. 1986;50:199–203. [Google Scholar]
- 14.Lv D., Guo K.W., Xu C., Huang M., Zheng S.J., Ma X.H., Pan L.H., Wang Q., Yang X.Z. Essential oil from Siegesbeckia pubescens induces apoptosis through the mitochondrial pathway in human HepG2 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017;37:87–92. doi: 10.1007/s11596-017-1699-7. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q., Li H., Lee S.Y., Lee H.J., Ryu J.H. New cytotoxic Sesquiterpenoids from Siegesbeckia glabrescens. Molecules. 2015;20:2850–2856. doi: 10.3390/molecules20022850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng L.F., Xu J.W., Xu L., Zhu L.L., Liu H. Separation and identification of flavonoids from Siegesbeckia glabrescens. Chin. J. Exp. Trad. Med. Form. 2017;23:74–77. [Google Scholar]
- 17.Liu K., Roder E. Diterpenes from Siegesbeckia glabrescens. Planta Med. 1991;57:395–396. doi: 10.1055/s-2006-960129. [DOI] [PubMed] [Google Scholar]
- 18.Wang J.B., Duan H.Q., Wang Y., Pan B., Gao C., Gai C.Y., Wu Q., Fu H.Z. ent-Strobane and ent-Pimarane Diterpenoids from Siegesbeckia pubescens. J. Nat. Prod. 2017;80:19–29. doi: 10.1021/acs.jnatprod.6b00150. [DOI] [PubMed] [Google Scholar]
- 19.Hu H.H., Tang L.X., Li X.M. Experimental research of effect of crude and processed Herba siegesbeckiae on anti-inflammation and anti-rheumatism. J. Chin. Mater. Med. 2004;29:542–545. [PubMed] [Google Scholar]
- 20.Xin H.L., Bi J., Liu M., Lin W.H., Qian R.Q. Experimental study on anti-inflammatory and immuno-regulating effect of kirenol. Chin. Tradit. Herb. Drugs. 2005;36:866–869. [Google Scholar]
- 21.Wang F., Cheng X.L., Li Y.J., Shi S., Liu J.K. ent-Pimarane diterpenoids from Siegesbeckia orientalis and structure revision of a related compound. J. Nat. Prod. 2009;72:2005–2008. doi: 10.1021/np900449r. [DOI] [PubMed] [Google Scholar]
- 22.Xiong J., Ma Y.B., Xu Y.L. Diterpenoids from Siegesbeckia pubescens. Phytochemistry. 1992;31:917–921. doi: 10.1016/0031-9422(92)80039-H. [DOI] [Google Scholar]
- 23.Xiang Y., Zhang H., Fan C.Q., Yue J.M. Novel diterpenoids and diterpenoid glycosides from Siegesbeckia orientalis. J. Nat. Prod. 2004;67:1517–1521. doi: 10.1021/np0400407. [DOI] [PubMed] [Google Scholar]
- 24.Chang C.C., Hsu H.F., Huang K.H., Wu J.W., Kuo S.M., Ling X.H., Houng J.Y. Anti-prolifertive effects of Siegesbeckia orientalis ethanol extract on human endometrial RL-95 cancer cells. Molecules. 2014;19:19980–19994. doi: 10.3390/molecules191219980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NIST: National Institute of Standards and Technologies, Mass Spectra Libraries. [(accessed on 15 July 2018)]; Available online: http://www.sisweb.com/software/nist-gc-library.htm.
- 26.Kwon O.K., Lee M.Y., Yuk J.E., Oh S.R., Chin Y.W., Lee H.K., Ahn K.S. Anti-inflammatory effects of methanol extracts of the root of Lilium lancifolium on LPS-stimulated raw264.7 cells. J. Ethnopharnacol. 2010;130:28–34. doi: 10.1016/j.jep.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.M., Jung H.A., Choi J.S., Lee N.G. Identification of anti-inflammatory target genes of Rhizoma coptidis extract in lipopolysaccharide-stimulated RAW264.7 murine macrophage-like cells. J. Ethnopharmacol. 2010;130:354–362. doi: 10.1016/j.jep.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Yan T., Yu X.Y., Sun X.D., Meng D.L., Jia J.M. A new steroidal saponin, furotrilliumoside from Trillium tschonoskii inhibits lipopolysaccharide-induced inflammation in Raw264.7 cells by targeting PI3K/Akt, MARK and Nrf2/HO-1 pathways. Fitoterapia. 2016;115:37–45. doi: 10.1016/j.fitote.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 29.He Z.F., Zhang H., Miao H.W., Li Z.J., Zhou J.J., Yan X.Y., Cai X.J. 1-o-acetylbritannilactone (ABL) inhibits angiogenesis and lung cancer cell growth through regulating VEGF-Src-FAK signaling. Biochem. Biophy. Res. Commun. 2015;464:422–427. doi: 10.1016/j.bbrc.2015.06.126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.